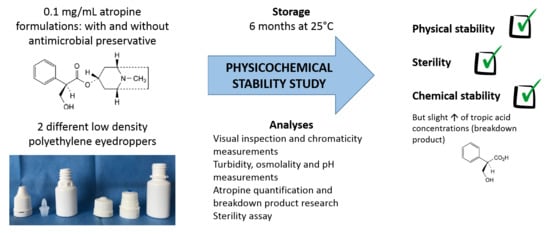
Stability of Ophthalmic Atropine Solutions for Child Myopia Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Storage of Atropine Solution Formulations
- -
- atropine solution with an antimicrobial preservative (cetrimide) for use with ethylene oxide sterilized white opaque LDPE squeezable multidose eyedroppers (reference VPLA25B10; Laboratoire CAT®, Lorris, France)
- -
- atropine solution without the preservative for use within gamma-sterilized white opaque LDPE squeezable multidose eyedropper (reference 10002134) equipped with sterility preserving Novelia® caps (reference 20050772; Nemera, La Verpillère, Cedex France).
2.2. Study Design
2.2.1. Stability of 0.1 mg/mL Atropine in Unopened Multidose Eyedroppers
2.2.2. Evaluation of Atropine Concentrations in Eye Drops during Simulated Use
2.3. Analyses Performed on the Atropine Solutions
2.3.1. Visual Inspection
2.3.2. Chromaticity Analysis
2.3.3. Atropine Quantification and BPs Research
Chemicals and Instrumentation
Method Validation
2.3.4. Osmolality, pH, and Turbidity Measurements
2.3.5. Sterility Assay
2.4. Data Analysis—Acceptability Criteria
3. Results
3.1. Atropine Quantification and Breakdown Products (BP) Research
3.2. Stability of Atropine in Unopened Multidose Eyedroppers
3.2.1. Physical Stability
3.2.2. Chemical Stability
3.2.3. Sterility Assay
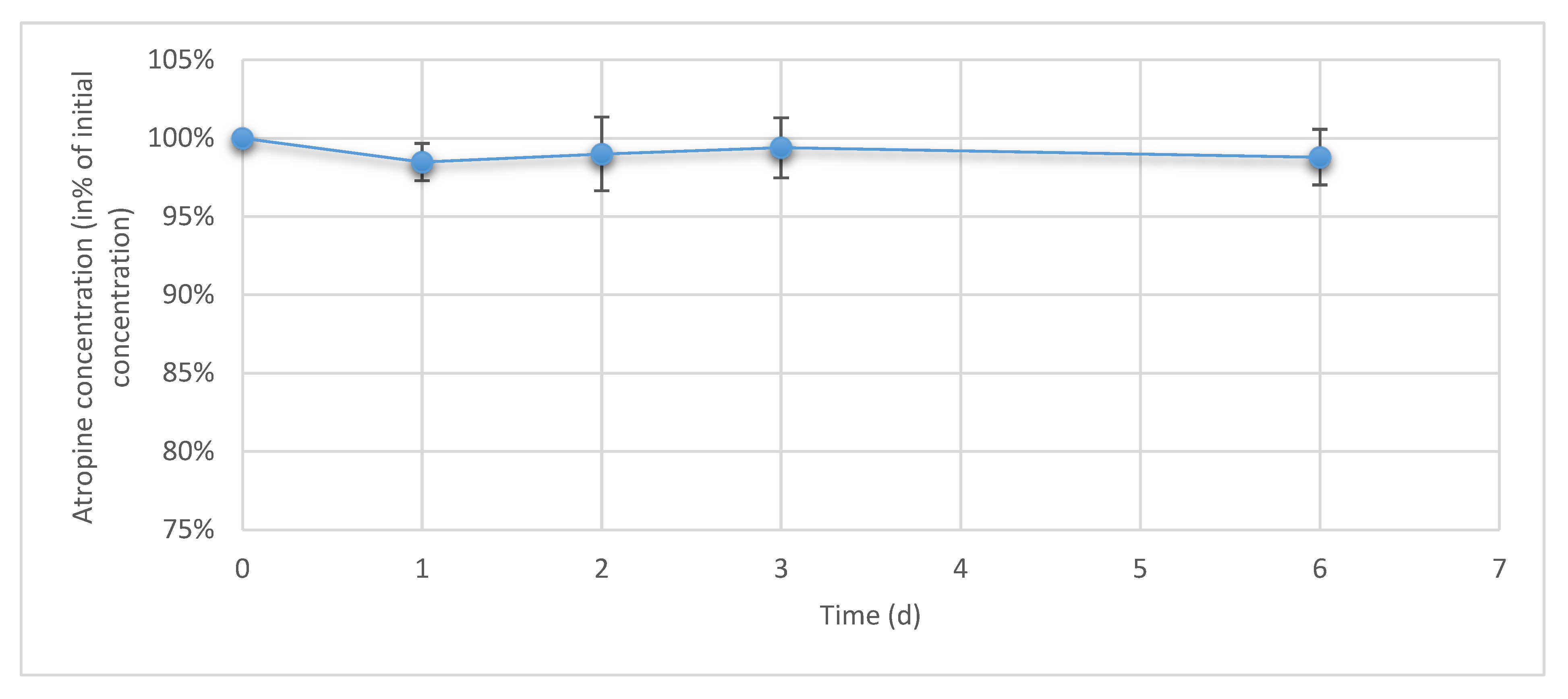
3.3. Atropine Concentrations in Eye Drops During Simulated Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.M.; Verhoeven, V.J.M.; Cumberland, P.; Bertelsen, G.; Wolfram, C.; Buitendijk, G.H.S.; Hofman, A.; van Duijn, C.M.; Vingerling, J.R.; Kuijpers, R.W.A.M.; et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E3) Consortium. Eur. J. Epidemiol. 2015, 30, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Theophanous, C.; Modjtahedi, B.S.; Batech, M.; Marlin, D.S.; Luong, T.Q.; Fong, D.S. Myopia prevalence and risk factors in children. Clin. Ophthalmol. 2018, 12, 1581–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.-Y.; Shih, Y.-F.; Lin, L.L.K.; Hsiao, C.K.; Wang, I.-J. Myopia among schoolchildren in East Asia and Singapore. Surv. Ophthalmol. 2017, 62, 677–697. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Long, Y.; Wang, J.; Li, Q.; Zhang, Q. Prevalence of myopia and associated risk factors among primary students in Chongqing: Multilevel modeling. BMC Ophthalmol. 2020, 20, 146. [Google Scholar] [CrossRef] [Green Version]
- Dirani, M.; Crowston, J.G.; Wong, T.Y. From reading books to increased smart device screen time. Br. J. Ophthalmol. 2019, 103, 1–2. [Google Scholar] [CrossRef]
- Wen, L.; Cao, Y.; Cheng, Q.; Li, X.; Pan, L.; Li, L.; Zhu, H.; Lan, W.; Yang, Z. Objectively measured near work, outdoor exposure and myopia in children. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Nickels, S.; Hopf, S.; Pfeiffer, N.; Schuster, A.K. Myopia is associated with education: Results from NHANES 1999–2008. PLoS ONE 2019, 14, e0211196. [Google Scholar] [CrossRef] [Green Version]
- Congdon, N.; Burnett, A.; Frick, K. The impact of uncorrected myopia on individuals and society. Commun. Eye Health 2019, 32, 7–8. [Google Scholar]
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential lost productivity resulting from the global burden of myopia: Systematic review, meta-analysis, and modeling. Ophthalmology 2019, 126, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Spillmann, L. Stopping the rise of myopia in Asia. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, B.S.; Ferris, F.L.; Hunter, D.G.; Fong, D.S. Public health burden and potential interventions for myopia. Ophthalmology 2018, 125, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, K.; Ryce, A. Laser Refractive Surgery for Vision Correction: A Review of Clinical Effectiveness and Cost-Effectiveness; CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Sankaridurg, P.; Conrad, F.; Tran, H.; Zhu, J. Controlling Progression of Myopia: Optical and pharmaceutical strategies. Asia Pac. J. Ophthalmol. 2018, 7, 405–414. [Google Scholar] [CrossRef]
- Upadhyay, A.; Beuerman, R.W. Biological Mechanisms of Atropine Control of Myopia. Eye Contact Lens 2020, 46, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.-H.; Balakrishnan, V.; Chan, Y.-H.; Tong, L.; Ling, Y.; Quah, B.-L.; Tan, D. Atropine for the treatment of childhood myopia. Ophthalmology 2006, 113, 2285–2291. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.-H.; Cheung, Y.-B.; Wong, W.-L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef]
- Chia, A.; Lu, Q.-S.; Tan, D. Five-Year Clinical Trial on Atropine for the treatment of myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology 2016, 123, 391–399. [Google Scholar] [CrossRef]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Logan, N.; Strang, N.; Saunders, K.; Allen, P.M.; Weir, R.; Doherty, P.; Adams, C.; Gardner, E.; Hogg, R.; et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: A multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)-study protocol. Br. J. Ophthalmol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, M.; Serafino, M.; Villani, E.; Tagliabue, E.; Luccarelli, S.; Bonsignore, F.; Nucci, P. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 2019, 97, e1136–e1140. [Google Scholar] [CrossRef]
- Akers, M.J. Formulation and Stability of Solutions. Int. J. Pharm. Compd. 2016, 20, 137–141. [Google Scholar] [PubMed]
- Vigneron, J.; D’Huart, E.; Demoré, B. Stability studies in oncology: A marketing tool for pharmaceutical companies, a scientific mission for hospital pharmacists. Eur. J. Oncol. Pharm. 2019, 2, e12. [Google Scholar] [CrossRef]
- Driver, R.P.; Brula, J.M.; Bezouska, C.A. The stability of atropine sulfate solutions stored in plastic syringes in the operating room. Anesth. Analg. 1999, 89, 1056. [Google Scholar] [CrossRef] [PubMed]
- Farenq, P.O.; Jobard, M.; Cros, C.; Bezia, C.; Brandely-Piat, M.-L.; Batista, R. Physical, Chemical and Microbiological Stability Study of 0.1 mg mL−1 Atropine Eye Drops. In Proceedings of the 22th European GERPAC Conference, Hyères, France, 2–4 October 2019. [Google Scholar]
- Saito, J.; Imaizumi, H.; Yamatani, A. Physical, chemical, and microbiological stability study of diluted atropine eye drops. J. Pharm. Health Care Sci. 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennell, P.; Delaborde, L.; Wasiak, M.; Jouannet, M.; Feschet-Chassot, E.; Chiambaretta, F.; Sautou, V. Stability of an ophthalmic micellar formulation of cyclosporine A in unopened multidose eyedroppers and in simulated use conditions. Eur. J. Pharm. Sci. 2017, 100, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, Y.; Chennell, P.; Wasiak, M.; Jouannet, M.; Sautou, V. Stability of an ophthalmic formulation of polyhexamethylene biguanide in gamma-sterilized and ethylene oxide sterilized low density polyethylene multidose eyedroppers. PeerJ 2018, 6. [Google Scholar] [CrossRef]
- Roche, M.; Lannoy, D.; Bourdon, F.; Danel, C.; Labalette, P.; Berneron, C.; Simon, N.; Odou, P. Stability of frozen 1% voriconazole eye-drops in both glass and innovative containers. Eur. J. Pharm. Sci. 2020, 141, 105102. [Google Scholar] [CrossRef]
- Ghiglioni, D.G.; Martino, P.A.; Bruschi, G.; Vitali, D.; Osnaghi, S.; Corti, M.G.; Beretta, G. Stability and safety traits of novel cyclosporine a and tacrolimus ophthalmic galenic formulations involved in vernal keratoconjunctivitis treatment by a high-resolution mass spectrometry approach. Pharmaceutics 2020, 12, 378. [Google Scholar] [CrossRef]
- Velpandian, T. Preservatives for topical ocular drug formulations. In Pharmacology of Ocular Therapeutics; Velpandian, T., Ed.; Springer International Publishing: Cham, Germany, 2016; pp. 419–430. ISBN 978-3-319-25498-2. [Google Scholar]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.-W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial stability of pharmaceutical and cosmetic products. AAPS Pharm. Sci. Tech. 2018, 19, 60–78. [Google Scholar] [CrossRef]
- European Pharmacopeia. Edition 10.2 Atropine Sulfate Monography; United States Pharmacopeia: New York, NY, USA, 2020. [Google Scholar]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part I. J. Pharm. Biomed. Anal. 2004, 36, 579–586. [Google Scholar] [CrossRef]
- Hubert, P.H.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.H.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal—Part III. J. Pharm. Biomed. Anal. 2007, 45, 82–96. [Google Scholar] [CrossRef]
- International Conference of Harmonization (ICH) Quality Guidelines. Guidelines for Stability Q1A to Q1f. Available online: http://www.ich.org/products/guidelines/%20quality/article/quality-guidelines.html (accessed on 18 July 2016).
- French Society of Clinical Pharmacy (SFPC); Evaluation and Research Group on Protection in Controlled Atmospher (GERPAC). Methodological Guidelines for Stability Studies of Hospital Pharmaceutical Preparations; SFPC: Paris, France, 2013. [Google Scholar]
- Kirchhoff, C.; Bitar, Y.; Ebel, S.; Holzgrabe, U. Analysis of atropine, its degradation products and related substances of natural origin by means of reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2004, 1046, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Schier, J.G.; Ravikumar, P.R.; Nelson, L.S.; Heller, M.B.; Howland, M.A.; Hoffman, R.S. Preparing for Chemical Terrorism: Stability of Injectable Atropine Sulfate. Acad. Emerg. Med. 2004, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Chemicalize-Instant Cheminformatics Solutions. Available online: https://chemicalize.com/app/calculation/tropic%20acid (accessed on 7 June 2020).
- Choudhury, A.K.R. Principles of Colour and Appearance Measurement: Object Appearance, Colour Perception and Instrumental Measurement; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-85709-924-2. [Google Scholar]
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef]
- Connors, K.A.; Amidon, G.L.; Stella, V.J. Chemical Stability of Pharmaceuticals: A Handbook for Pharmacists, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1986; ISBN 978-0-471-87955-8. [Google Scholar]
- American Society of Health-System Pharmacists. Handbook on Injectable Drugs, 20th ed.; ASHP: Bethesda, MA, USA, 2018; ISBN 978-1-58528-615-7. [Google Scholar]
- European Pharmacopeia. Monography 2.6.1 Sterility; United States Pharmacopeia: New York, NY, USA, 2020. [Google Scholar]
- Crauste-Manciet, S.; Krämer, I.; Lagarce, F.; Sautou, V.; Beaney, A.; Smith, J.; Fenton-May, V.; Hecq, J.-D.; Sadeghipour, F.; Brun, P.L. GERPAC Consensus Conference—Guidance on the Assignment of Microbiological Shelf-life for Hospital Pharmacy Aseptic Preparations. Pharm. Technol. Hosp. Pharm. 2020, 5. [Google Scholar] [CrossRef]
- Nemera. A Sorption Study between Ophthalmic Drugs and Multi Dose Eyedroppers in Simulated Use Conditions. Pharm. Technol. Hosp. Pharm. 2017, 2, 181–191. [Google Scholar] [CrossRef]Ophthalmic-Preservative-free multidose eyedropper; Nemera. Drug Deliv. Mag. 2018, 82, 16–20.
- Le Basle, Y.; Chennell, P.; Sautou, V. A Sorption Study between Ophthalmic Drugs and Multi Dose Eyedroppers in Simulated Use Conditions. Pharm. Technol. Hosp. Pharm. 2017, 2, 181–191. [Google Scholar] [CrossRef]
- Coroi, M.C.; Bungau, S.; Tit, M. Preservatives from The Eye Drops and the Ocular Surface. Rom. J. Ophthalmol. 2015, 59, 2–5. [Google Scholar]
- Ramli, N.; Supramaniam, G.; Samsudin, A.; Juana, A.; Zahari, M.; Choo, M.M. Ocular surface disease in glaucoma: Effect of polypharmacy and preservatives. Optom. Vis. Sci. 2015, 92, e222–e226. [Google Scholar] [CrossRef] [Green Version]
- Steven, D.W.; Alaghband, P.; Lim, K.S. Preservatives in glaucoma medication. Br. J. Ophthalmol. 2018, 102, 1497–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koželj, G.; Perharič, L.; Stanovnik, L.; Prosen, H. Simple validated LC–MS/MS method for the determination of atropine and scopolamine in plasma for clinical and forensic toxicological purposes. J. Pharm. Biomed. Anal. 2014, 96, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am. J. Hosp. Pharm. 1983, 40, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. USP <1150> Pharmaceutical Stability; USP 43-NF38; United States Pharmacopeia: New York, NY, USA, 2018.
- Association of Southeast Asian Nations. Asean Guidelines on Stability Study of Drug Product. Update Review. In Proceedings of the 9th ACCSQ-PPWg Meeting, Manila, Philliphines, 21–24 February 2005. [Google Scholar]
- Bardin, C.; Astier, A.; Vulto, A.; Sewell, G.; Vigneron, J.; Trittler, R.; Daouphars, M.; Paul, M.; Trojniak, M.; Pinguet, F. Guidelines for the practical stability studies of anticancer drugs: A European consensus conference. Eur. J. Hosp. Pharm. 2012, 19, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, M.J.; Hundt, H.K.; Müller, F.O. The metabolism of atropine in man. J. Pharm. Pharmacol. 1986, 38, 781–784. [Google Scholar] [CrossRef]
- Wang, L.Z.; Syn, N.; Li, S.; Barathi, V.A.; Tong, L.; Neo, J.; Beuerman, R.W.; Zhou, L. The penetration and distribution of topical atropine in animal ocular tissues. Acta Ophthalmol. 2019, 97, e238–e247. [Google Scholar] [CrossRef]
- Argikar, U.A.; Dumouchel, J.L.; Kramlinger, V.M.; Cirello, A.L.; Gunduz, M.; Dunne, C.E.; Sohal, B. Do We Need to Study Metabolism and Distribution in the Eye: Why, When, and Are We There Yet? J. Pharm. Sci. 2017, 106, 2276–2281. [Google Scholar] [CrossRef] [Green Version]







| Chemical Components | Formulation (mg) | |
|---|---|---|
| Without Preservative | With Preservative | |
| Atropine sulphate (batch 18276508, exp. 31/01/2021, Inresa, France) | 100 | 100 |
| Natrium dihydrogenophosphate dihydrate (NaH2PO4) (batch 190298040, exp. 30/11/2021, Inresa, France) | 7800 | 7800 |
| Dinatrium monohydrogenophosphate dodecahydrate (Na2HPO4) (batch 18129611, exp. 30/04/2023, Inresa, France) | 4480 | 4480 |
| Cetrimide (batch 16F08-B01-334049, exp. 05/2020, Fagron, Netherlands) | 100 | |
| Sodium chloride (NaCl) 0.9% (Versylene®; Fresenius Kabi France, Louviers, France) | q.s 1000 mL | q.s 1000 mL |
| Time (min) | Mobile Phase (%) | |
|---|---|---|
| A | B | |
| 0 | 95 | 5 |
| 2 | 95 | 5 |
| 20 | 70 | 30 |
| 21 | 95 | 5 |
| 25 | 95 | 5 |
| Impurity Retention Times | ||
|---|---|---|
| Experimental Absolute Retention Time (min) | Relative Retention Time | |
| Atropine | 9.7 | 1 |
| Impurity A | 16.2 | 1.7 |
| Impurity B | 9.3 | 0.9 |
| Impurity C | 2.5 | 0.3 |
| Impurity D | 7.6 | 0.8 |
| Impurity E | 7.1 | 0.7 |
| Impurity F | 8.0 | 0.8 |
| Impurity G | 10.8 | 1.1 |
| Impurity H | 9.3 | 0.9 |
| Turbidity (FNU) | |||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 8 | Day 15 | Day 30 | Day 60 | Day 90 | Day 180 | |
| Atropine solution with preservative conditioned in LDPE CAT® eyedroppers | 0.33 | 0.31 | 0.27 | 0.78 | 0.78 | 0.43 | 0.93 |
| Atropine solution without preservative conditioned LDPE NOVELIA® eyedroppers | 0.32 | 0.31 | 0.26 | 0.54 | 0.44 | 0.34 | 0.64 |
| Day 0 | Day 8 | Day 15 | Day 30 | Day 60 | Day 90 | Day 180 | ||
|---|---|---|---|---|---|---|---|---|
| Atropine solution with preservative conditioned in LDPE CAT® eyedroppers | pH | 6.10 ± 0.01 | 6.11 ± 0.01 | 6.13 ± 0.02 | 6.13 ± 0.01 | 6.13 ± 0.02 | 6.21 ± 0.04 | 6.12 ± 0.01 |
| Osmolality (mOsm/kg) | 412 ± 16 | 400 ± 6 | 403 ± 14 | 393 ± 14 | 400 ± 5 | 413 ± 11 | 418 ± 23 | |
| Atropine solution without preservative conditioned LDPE NOVELIA® eyedroppers | pH | 6.10 ± 0.01 | 6.11 ± 0.01 | 6.13 ± 0.01 | 6.14 ± 0.02 | 6.13 ± 0.01 | 6.21 ± 0.01 | 6.09 ± 0.01 |
| Osmolality (mOsm/kg) | 399 ± 2 | 401 ± 6 | 409 ± 6 | 405 ± 2 | 415 ± 15 | 408 ± 10 | 405 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berton, B.; Chennell, P.; Yessaad, M.; Bouattour, Y.; Jouannet, M.; Wasiak, M.; Sautou, V. Stability of Ophthalmic Atropine Solutions for Child Myopia Control. Pharmaceutics 2020, 12, 781. https://doi.org/10.3390/pharmaceutics12080781
Berton B, Chennell P, Yessaad M, Bouattour Y, Jouannet M, Wasiak M, Sautou V. Stability of Ophthalmic Atropine Solutions for Child Myopia Control. Pharmaceutics. 2020; 12(8):781. https://doi.org/10.3390/pharmaceutics12080781
Chicago/Turabian StyleBerton, Baptiste, Philip Chennell, Mouloud Yessaad, Yassine Bouattour, Mireille Jouannet, Mathieu Wasiak, and Valérie Sautou. 2020. "Stability of Ophthalmic Atropine Solutions for Child Myopia Control" Pharmaceutics 12, no. 8: 781. https://doi.org/10.3390/pharmaceutics12080781
APA StyleBerton, B., Chennell, P., Yessaad, M., Bouattour, Y., Jouannet, M., Wasiak, M., & Sautou, V. (2020). Stability of Ophthalmic Atropine Solutions for Child Myopia Control. Pharmaceutics, 12(8), 781. https://doi.org/10.3390/pharmaceutics12080781