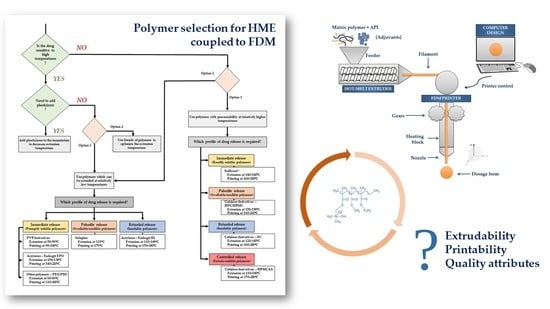
Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics
Abstract
:Table of Contents
- Introduction
- Hot-Melt Extrusion
- Fused Deposition Modelling
- Polymers Used in Hot-Melt Extrusion and Fused Deposition Modelling FDM
- 4.1.
- Polyvinyl Alcohol
- 4.2.
- Polyvinylpyrrolidone
- 4.3.
- Cellulose-Derived Polymers
- 4.3.1.
- Ethylcellulose
- 4.3.2.
- Hydroxypropylcellulose
- 4.3.3.
- Hydroxypropylmethylcellulose
- 4.3.4.
- Hydroxypropylmethylcellulose Acetate Succinate
- 4.4.
- Acrylates
- 4.4.1.
- Eudragit® E PO
- 4.4.2.
- Eudragit® RL
- 4.4.3.
- Eudragit® RS
- 4.4.4.
- Eudragit® L
- 4.5.
- Other Polymers
- 4.6.
- Commercial Polymer Blends
- Characterization of Filaments and 3D Dosage Forms Produced by HME-Coupled with FDM
- 5.1.
- Rheologic Properties
- 5.2.
- Mechanical Properties
- 5.3.
- Thermal Properties and Other Characterization at Molecular Level Techniques
- 5.4.
- In Vitro and In Vivo Characterization
- 5.5.
- Other Properties
- Final Remarks
1. Introduction
2. Hot-Melt Extrusion
3. Fused Deposition Modelling
4. Polymers Used in Hot-Melt Extrusion and Fused Deposition Modelling
4.1. Polyvinyl Alcohol
4.2. Polyvinylpyrrolidone
4.3. Cellulose-Derived Polymers
4.3.1. Ethylcellulose
4.3.2. Hydroxypropylcellulose
4.3.3. Hydroxypropylmethylcellulose
4.3.4. Hydroxypropylmethylcellulose Acetate Succinate
4.4. Acrylates
4.4.1. Eudragit® E PO
4.4.2. Eudragit® RL
4.4.3. Eudragit® RS
4.4.4. Eudragit® L
4.5. Other Polymers
4.6. Commercial Polymer Blends
5. Characterization of Filaments and 3D Dosage Forms Produced by HME-Coupled with FDM
5.1. Rheologic Properties
5.2. Mechanical Properties
5.3. Thermal Properties and Other Characterization at Molecular Level Techniques
5.4. In Vitro and In Vivo Characterization
5.5. Other Properties
6. Final Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 4-ASA | 4-Aminosalicylic acid |
| 5-ASA | 5-Aminosalicylic acid |
| API | Active pharmaceutical ingredient |
| ASD | Amorphous solid dispersion |
| ATM | Atomic force microscopy |
| DMA | Dynamic mechanical analysis |
| DSC | Differential scanning calorimetry |
| EC | Ethyl cellulose |
| EVA | Ethylene vinyl acetate |
| FDM | Fused deposition modelling |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| HEC | Hydroxyethyl cellulose |
| HME | Hot-melt extrusion |
| HPC | Hydroxypropyl cellulose |
| HPLC | High-pressure liquid chromatography |
| HPMC | Hydroxypropyl methylcellulose |
| HPMCAS | Hydroxypropyl methylcellulose acetate succinate |
| MC | Methyl cellulose |
| MDSC | Modulated differential scanning calorimetry |
| MW | Molecular weight |
| NIR | Near infrared spectroscopy |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene oxide) |
| PCL | Polyvinyl caprolactam |
| PCLa | Polycaprolactone |
| PLA | Polylactic acid |
| PLM | Polarized light microscopy |
| PVA | Polyvinyl alcohol |
| PVAc | Polyvinyl acetate |
| PVP | Polyvinylpyrrolidone |
| SEM | Scanning electron microscopy |
| ssNMR | Solid state nuclear magnetic resonance |
| Tg | Glass transition temperature |
| Tm | Melting point |
| TBP | Tribasic phosphate sodium |
| TCP | Tri-calcium phosphate |
| TEC | Triethyl citrate |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| XRPD | X-Ray powder diffraction |
References
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef]
- Genina, N.; Boetker, J.P.; Colombo, S.; Harmankaya, N.; Rantanen, J.; Bohr, A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. J. Control. Release 2017, 268, 40–48. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Strohmeier, D.M.; Schrödl, N.; Voura, C.; Gruber, M.; Khinast, J.G.; Zimmer, A. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int. J. Pharm. 2011, 420, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Fuh, J.Y.H.; Lu, W.F. 3D printing and 3D bioprinting in pediatrics. Bioengineering 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.; Castellano, J.M.; Onuma, O.K. Putting polypills into practice: Challenges and lessons learned. Lancet 2017, 389, 1066–1074. [Google Scholar] [CrossRef]
- Huffman, M.D.; Xavier, D.; Perel, P. Uses of polypills for cardiovascular disease and evidence to date. Lancet 2017, 389, 1055–1065. [Google Scholar] [CrossRef]
- Hsiao, W.K.; Lorber, B.; Reitsamer, H.; Khinast, J. 3D printing of oral drugs: A new reality or hype? Expert Opin. Drug Deliv. 2018, 15, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Comparison of fused-filament fabrication to direct compression and injection molding in the manufacture of oral tablets. Int. J. Pharm. 2019, 558, 328–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursan, I.D.; Chiu, L.; Pierce, A. Three-dimensional drug printing: A structured review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From Theory to application in pharmaceutical formulation. AAPS Pharmscitech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, S. Application of hot melt extrusion in pharmaceutical 3D printing. J. Bioequiv. Availab. 2018, 10, 54–57. [Google Scholar] [CrossRef]
- Araújo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The digital pharmacies era: How 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Snick, B.V.; Boone, M.N.; Hellemans, T.; Hoorebeke, L.V.; Remon, J.P.; Vervaet, C.; et al. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D printing: Design and qualification of a single step print and fill capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Gholizadeh, H.; Lu, J.; Seyfoddin, A. Review: Application of fused deposition modelling (FDM) method of 3D printing in drug delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Preis, M. Printed drug-delivery systems for improved patient treatment. Trends Pharmacol. Sci. 2016, 37, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Fernández-Ferreiro, A.; Majeed, A.; Gomez-Lado, N.; Awad, A.; Luaces-Rodríguez, A.; Gaisford, S.; Aguiar, P.; Basit, A.W. PET/CT imaging of 3D printed devices in the gastrointestinal tract of rodents. Int. J. Pharm. 2018, 536, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef]
- Bruce, L.D.; Shah, N.H.; Waseem Malick, A.; Infeld, M.H.; McGinity, J.W. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. Eur. J. Pharm. Biopharm. 2005, 59, 85–97. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Warsi, M.H.; Yusuf, M.; Al Robaian, M.; Khan, M.; Alsaab, H.; Muheem, A.; Khan, S. 3D printing methods for pharmaceutical manufacturing: Opportunity and challenges. Curr. Pharm. Des. 2018, 25, 4949–4956. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material considerations for fused-filament fabrication of solid dosage forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saviano, M.; Aquino, R.P.; Del Gaudio, P.; Sansone, F.; Russo, P. Poly(vinyl alcohol) 3D printed tablets: The effect of polymer particle size on drug loading and process efficiency. Int. J. Pharm. 2019, 561, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a simple mechanical screening method for predicting the feedability of a pharmaceutical FDM 3D printing filament. Pharm. Res. 2018, 35, 151. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2004, 58, 157–162. [Google Scholar] [CrossRef]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.J.; Yang, W.; Vo, A.Q.; Feng, X. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 49–57. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D printing of medicines: Engineering novel oral devices with unique design and drug release characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient- centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Fabrication of floating capsule-in- 3D-printed devices as gastro-retentive delivery systems of amoxicillin. J. Drug Deliv. Sci. Technol. 2020, 55, 101393. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Szafraniec-Szczęsny, J.; Czech, A.; Gawlak, K.; Knapik-Kowalczuk, J.; Leszczyński, B.; Wróbel, A.; Paluch, M.; Jachowicz, R. Speed it up, slow it down…An issue of bicalutamide release from 3D printed tablets. Eur. J. Pharm. Sci. 2020, 143, 105196. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D printing of multi-compartmental PVA capsules for drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686. [Google Scholar] [CrossRef]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appro- priate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef]
- Ilyés, K.; Balogh, A.; Casian, T.; Igricz, T.; Borbás, E.; Démuth, B.; Vass, P.; Menyhárt, L.; Kovács, N.K.; Marosi, G.; et al. 3D floating tablets: Appropriate 3D design from the perspective of different in vitro dissolution testing methodologies. Int. J. Pharm. 2019, 567. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A Lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wen, H.; Jia, D.; Guan, X.; Pan, H.; Yang, Y.; Yu, S.; Zhu, Z.; Xiang, R.; Pan, W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017, 525, 5–11. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: Screening polymers for drug release, drug-polymer miscibility and printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T.M. Development of 3D printed tablets by fused deposition modeling using polyvinyl alcohol as a polymeric matrix for rapid drug release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D printed “starmix” drug loaded dosage forms for paediatric applications. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.I.; Ishikawa, T.; Iwao, Y.; Itai, S.; Kondo, H. Fabrication of zero-order sustained-release floating tablets via fused depositing modeling 3D printer. Chem. Pharm. Bull. 2019, 67, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. Eur. J. Pharm. Sci. 2016, 90, 47–52. [Google Scholar] [CrossRef]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef]
- Van Nguyen, H.; Nguyen, V.H.; Lee, B.-J. Dual release and molecular mechanism of bilayered aceclofenac tablet using polymer mixture. Int. J. Pharm. 2016, 515, 233–244. [Google Scholar] [CrossRef]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Korte, C.; Quodbach, J. 3D-printed network structures as controlled-release drug delivery systems: Dose adjustment, API release analysis and prediction. AAPS Pharmscitech 2018, 19, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Qinna, N.; Cieszynska, M.; Forbes, R.T.; Alhnan, M.A. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur. J. Pharm. Biopharm. 2018, 128, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sarabu, S.; Bandari, S.; Kallakunta, V.R.; Tiwari, R.; Patil, H.; Repka, M.A. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: Part II. Expert Opin. Drug Deliv. 2019, 16, 567–582. [Google Scholar] [CrossRef]
- Flory, P. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576. [Google Scholar] [CrossRef]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, thermal, and mechanical properties of polymers. Biosurfaces 2015, 329–344. [Google Scholar] [CrossRef]
- Kolter, K.; Karl, M.; Gryczke, A. Hot-Melt Extrusion with BASF Pharma Polymers: Extrusion Compendium, 2nd ed.; BASF: Ludwigshafen am Rhein, Germany, 2012; ISBN 978-3-00039415-7. [Google Scholar]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef]
- Shahriar, B.B.; France, C.; Valerie, N.; Arthur, C.; Christian, G. Toward improvement of the properties of parts manufactured by FFF (fused filament fabrication) through understanding the influence of temperature and rheological behaviour on the coalescence phenomenon. In AIP Conference Procceedings; AIP Publishing: College Park, MD, USA, 2017; Volume 1896, p. 040008. [Google Scholar] [CrossRef] [Green Version]
- Mehuys, E.; Remon, J.P.; Vervaet, C. Production of enteric capsules by means of hot-melt extrusion. Eur. J. Pharm. Sci. 2005, 24, 207–212. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Iftimi, L.D.; Edinger, M.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Edible solid foams as porous substrates for inkjet-printable pharmaceuticals. Eur. J. Pharm. Biopharm. 2019, 136, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery–A review. Int. J. Pharm. 2020, 579. [Google Scholar] [CrossRef] [PubMed]
- Cameron, G.G.; Ingram, M.D.; Qureshi, M.Y.; Gearing, H.M.; Costa, L.; Camino, G.; Giuria, V.P. The thermal degradation of poly(Ethylene Oxide) and its complex with NaCNS. Eur. Polym. J. 1989, 25, 779–784. [Google Scholar] [CrossRef]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D printing in the formulation of multilayered fast dissolving oral films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Pharmacopeial Convention Inc. United States Pharmacopeia; USP43 NF38; United States Pharmacopeial Convention Inc.: Bethesda, MD, USA, 2019. [Google Scholar]
- Council of Europe. European Pharmacopeia, 10th ed.; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D printing factors important for the fabrication of polyvinylalcohol filament-based tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, R.; Manchanda, S.; Das, P.; Velu, V.; Malipeddi, H.; Pabreja, K.; Pinto, T.D.J.A.; Gupta, G.; Dua, K. Poly(vinylpyrrolidone) . In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing-Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–272. ISBN 9780081017517. [Google Scholar]
- Kollidon VA 64 Fine Pharmaceuticals. BASF: Ludwigshafen am Rhein, Germany. Available online: https://pharmaceutical.basf.com/global/en/drug-formulation/products/kollidon-va64-fine.html (accessed on 1 June 2020).
- BASF. Soluble Kollidon® Grades; BASF: Ludwigshafen am Rhein, Germany, 2013; pp. 1–12. [Google Scholar]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Gómez-Carracedo, A.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; Concheiro, A. Chemical structure and glass transition temperature of non-ionic cellulose ethers. J. Therm. Anal. Calorim. 2003, 73, 587–596. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 317–349. ISBN 9780128104606. [Google Scholar]
- Picker-Freyer, K.M.; Dürig, T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS Pharmscitech 2007, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Karkri, M. Thermal Conductivity of Biocomposite Materials. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–153. [Google Scholar]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: AffinisolTM HPMC HME Polymers. AAPS Pharmscitech 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press-Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–223. ISBN 9780128024478. [Google Scholar]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Palugan, L.; Gazzaniga, A. Gastroresistant capsular device prepared by injection molding. Int. J. Pharm. 2013, 440, 264–272. [Google Scholar] [CrossRef]
- Dong, Z.; Choi, D.S. Hydroxypropyl Methylcellulose Acetate Succinate: Potential Drug—Excipient Incompatibility. AAPS Pharmscitech 2008, 9, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Tanno, F.; Nishiyama, Y.; Kokubo, H.; Obara, S. Evaluation of Hypromellose Acetate Succinate (HPMCAS) as a Carrier in Solid Dispersions. Drug Dev. Ind. Pharm. 2004, 30, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.V.; Kevadiya, B.D.; Bajaj, H.C. Controlled release formulation of ranitidine-containing montmorillonite and Eudragit® E-100. Drug Dev. Ind. Pharm. 2010, 36, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.A.; Konan-Kouakou, Y.N.; Allémann, E.; Doelker, E.; Quintanar-Guerrero, D.; Fessi, H.; Gurny, R. Preparation of surfactant-free nanoparticles of methacrylic acid copolymers used for film coating. AAPS Pharmscitech 2006, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdsakundee, N.; Mahattanadul, S.; Wiwattanapatapee, R. Development and evaluation of gastroretentive raft forming systems incorporating curcumin-Eudragit® EPO solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Biopharm. 2015, 94, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Azs, B.A.L.; Farkas, A.; Marosi, G.Y.O. Comparison of Electrospun and Extruded Soluplus R -Based Solid. J. Pharm. Sci. 2012, 101, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, D.; Yu, L.; Fransson, E.; Gómez, A.; Primetzhofer, D.; Amassian, A.; Campoy-Quiles, M.; Müller, C. A Solution-Doped Polymer Semiconductor:Insulator Blend for Thermoelectrics. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Cardoso, A.; Viana, M.; Lins, V. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Solanki, N.G.; Kathawala, M.; Serajuddin, A.T.M. Effects of Surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate Solid Dispersion Prepared by Hot Melt Extrusion III: Tableting of Extrudates and Drug Release From Tablets. J. Pharm. Sci. 2019, 108, 3859–3869. [Google Scholar] [CrossRef]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Saerens, L.; Dierickx, L.; Lenain, B.; Vervaet, C.; Remon, J.P.; Beer, D.T. Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur. J. Pharm. Biopharm. 2011, 77, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int. J. Pharm. 2015, 494, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.F.; Duarte, F.M.; Covas, J.A. Estimation of filament temperature and adhesion development in fused deposition techniques. J. Mater. Process. Technol. 2017, 245, 167–179. [Google Scholar] [CrossRef]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of processing conditions on the bonding quality of FDM polymer filaments. Rapid Prototyp. J. 2008, 14, 72–80. [Google Scholar] [CrossRef]
- Konta, A.; García-Piña, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Lewis, J.A.; Gratson, G.M. Direct writing in three dimensions. Mater. Today 2004, 7, 32–39. [Google Scholar] [CrossRef]
- Elbadawi, M. Rheological and Mechanical Investigation into the Effect of Different Molecular Weight Poly(ethylene glycol)s on Polycaprolactone-Ciprofloxacin Filaments. ACS Omega 2019, 4, 5412–5423. [Google Scholar] [CrossRef]
- Tanner, R.I.; Keentok, M. Shear Fracture in Cone-Plate Rheometry. J. Rheol. 1983, 27, 47–57. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Lee, Y.W.; Jung, W.K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A. Plastic Properties and Testing. In Introduction to Plastics Engineering; Elsevier: Amsterdam, The Netherlands, 2018; p. 262. ISBN 9780323395007. [Google Scholar]
- Venkataraman, N.; Rangarajan, S.; Matthewson, M.J.; Harper, B.; Safari, A.; Danforth, S.C.; Wu, G.; Langrana, N.; Guceri, S.; Yardimci, A. Feedstock material property—Process relationships in fused deposition of ceramics (FDC). Rapid Prototyp. J. 2000, 6, 244–252. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Characterization of amorphous solid dispersions: An update. J. Drug Deliv. Sci. Technol. 2019, 50, 113–124. [Google Scholar] [CrossRef]
- Bellehumeur, C.; Li, L.; Sun, Q.; Gu, P. Modeling of bond formation between polymer filaments in the fused deposition modeling process. J. Manuf. Process. 2004, 6, 170–178. [Google Scholar] [CrossRef]
- Rösler, J.; Harders, H.; Bäker, M. Mechanical behaviour of polymers. In Mechanical Behaviour of Engineering Materials; Springer: Berlin, Germany, 2007; pp. 257–294. [Google Scholar]
- Ebnesajjad, S. Surface and Material Characterization Techniques. In Surface Treatment of Materials for Adhesion Bonding; Elsevier: Amsterdam, The Netherlands, 2014; pp. 39–75. [Google Scholar]
- Vo, C.L.N.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Messimer, S.L.; Patterson, A.E.; Muna, N.; Deshpande, A.P.; Rocha Pereira, T. Characterization and Processing Behavior of Heated Aluminum-Polycarbonate Composite Build Plates for the FDM Additive Manufacturing Process. J. Manuf. Mater. Process. 2018, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Heidemann, H.M.; Dotto, M.E.R.; Laurindo, J.B.; Carciofi, B.A.M.; Costa, C. Cold plasma treatment to improve the adhesion of cassava starch films onto PCL and PLA surface. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123739. [Google Scholar] [CrossRef]
- Azman Mohammad Taib, M.N.; Julkapli, N.M. 4—Dimensional stability of natural fiber-based and hybrid composites. In Woodhead Publishing Series in Composites Science and Engineering; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing-Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–79. [Google Scholar]
- Vakili, H.; Kolakovic, R.; Genina, N.; Marmion, M.; Salo, H.; Ihalainen, P.; Peltonen, J.; Sandler, N. Hyperspectral imaging in quality control of inkjet printed personalised dosage forms. Int. J. Pharm. 2015, 483, 244–249. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Boparai, K.S.; Singh, R. Development of rapid tooling using fused deposition modeling. Addit. Manuf. Emerg. Mater. 2018, 251–277. [Google Scholar] [CrossRef]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef] [PubMed]
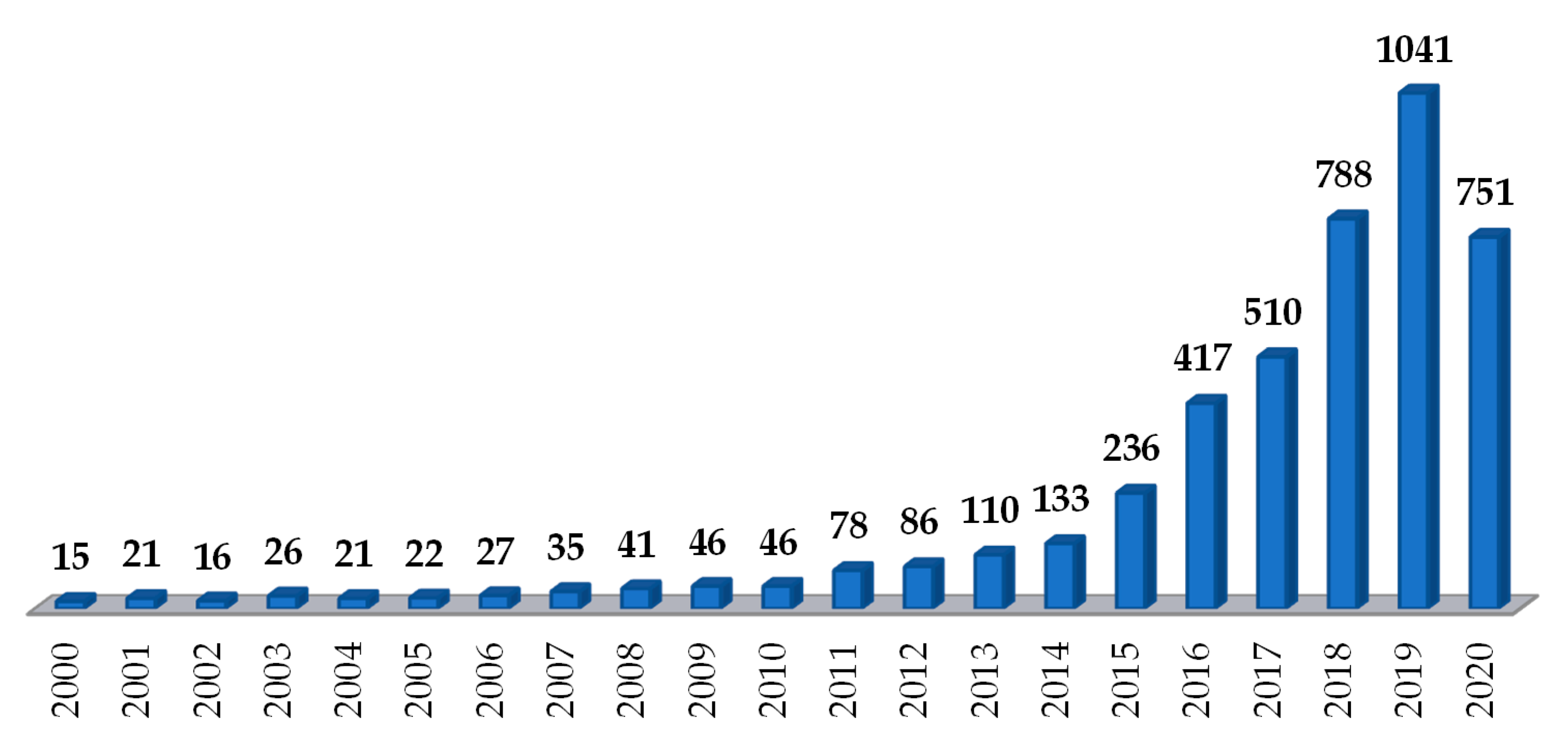








| Drug | BCS Class 1 | Therapeutic Class | Characteristics | Works Reporting HME Coupled to FDM | ||
|---|---|---|---|---|---|---|
| Molecular Weight (g/mol) | Melting Point (°C) | Solubility at 37 °C (mg/mL) | ||||
| 4-Aminosalicylic acid | II | Antitubercular agent | 153.14 | 150 | 2.0 | [47] |
| 5-Aminosalicylic acid | IV | Anti-inflammatory | 153.135 | 275 | 0.1 | [4,29,45] |
| Acetaminophen | I | Anti-inflammatory | 151.16 | 170 | 218.0 | [4,42,48,49,50] |
| Amlodipine besylate | II | Calcium channel blocker | 408.88 | 198 | 0.207 | [51] |
| Amoxicillin | IV | Antibiotic | 365.40 | 194 | 0.958 | [52] |
| Aripiprazole | IV | Atypical antipsychotic | 448.38 | 140 | 0.00777 | [53] |
| Ascorbic acid | I | Vitamin/Antioxidant | 176.12 | 191 | 330.0 | [54] |
| Baclofen | III | Gamma-aminobutyric acid agonist | 213.66 | 207 | 0.712 | [55] |
| Bicalutamide | II | Non-steroidal anti-androgen | 430.37 | 192 | 0.00928 | [53] |
| Budesonide | II | Corticosteroid | 430.50 | 226 | 0.0457 | [4,50,56] |
| Caffeine | II | Bronchodilator or vasodilator | 194.19 | 234 | 11.0 | [50] |
| Captopril | I | Angiotensin-converting-enzyme inhibitor | 217.29 | 427 | 160.0 | [29] |
| Carvedilol | II | Antihypertensive | 442.90 | 114.5 | 0.00444 | [57] |
| Ciprofloxacin | IV | Antibiotic | 318.10 | 581.8 | 1.35 | [43] |
| Diclofenac sodium | II | Anti-inflammatory | 430.50 | 285 | 0.00482 | [56] |
| Domperidone | II | Antiemetic | 425.91 | 242 | 0.986 | [13] |
| Dronedarone hydrochloride | II | Antiarrhythmic | 556.76 | 140 | 0.00201 | [54] |
| Dypyridamole | II | Antiplatelet drug | 504.63 | 163 | 0.922 | [58] |
| Felodipine | II | Antihypertensive | 384.26 | 471 | 19.7 | [42] |
| Glimepiride | II | Antidiabetic | 490.62 | 207 | 0.0384 | [59] |
| Glipizide | II | Antidiabetic | 445.53 | 200 | 0.0164 | [60] |
| Haloperidol | II | Antipsychotic | 375.90 | 148 | 0.00446 | [61,62] |
| Hydrochlorothiazide | II | Diuretic | 297.74 | 267 | 2.24 | [59] |
| Ibuprofen | II | Anti-inflammatory | 206.29 | 319 | 0.021 | [42,63] |
| Indapamide | II | Antihypertensive | 365.84 | 161 | 0.0342 | [51] |
| Indomethacin | II | Anti-inflammatory | 357.80 | 162 | 0.937 | [64,65] |
| Itraconazole | II | Antifungal agent | 705.64 | 166 | 0.00964 | [66] |
| Lisinopril dihydrate | III | Antihypertensive | 405.49 | 148 | 0.216 | [51] |
| Metformin | II | Antidiabetic | 129.16 | 172 | 1.38 | [59] |
| Nitrofurantoin | II | Antibiotic | 238.16 | 225 | 0.415 | [67] |
| Pantoprazole | II | Proton pump inhibitor | 383.37 | 155 | 0.431 | [68] |
| Prednisolone | I | Corticosteroid | 360.44 | 230 | 0.24 | [7,29] |
| Quinine | I | Antimalarial | 324.42 | 57 | 0.334 | [69] |
| Ramipril | II | Antihypertensive | 416.50 | 109 | 0.035 | [47] |
| Rosuvastatin calcium | II | Antihyperlipidemic | 481.54 | 155 | 0.0886 | [51] |
| Theophylline | I | Bronchodilator | 180.16 | 270 | 5.5 | [7,26,29,56,58,70,71,72,73] |
| Warfarin | I | Anticoagulant | 308.32 | 161 | 0.0472 | [74] |
| Chemical Name of the Polymer | Trade Name | FDA Approved | Biodegradable Polymer | Thermal Properties 1 | References | ||
|---|---|---|---|---|---|---|---|
| Tm (°C) | Tg (°C) | Degradation Temperature (°C) | |||||
| Polyvinyl alcohol (PVA) | --- | Yes | Yes | --- | 85 | --- | [3,27,77,82] |
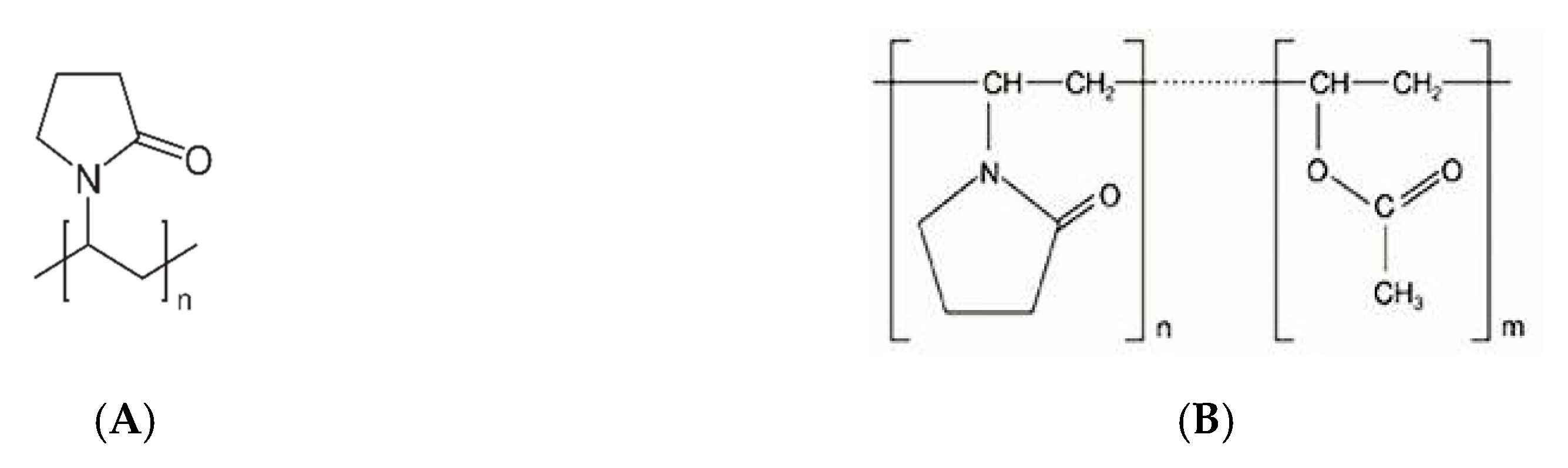
| Poly(vinylpyrrolidone) (PVP) | |||||||
| (MW 7000–11,000) | Kollidon® 17 PF | Yes | Yes | --- | 140 | 217 | [56,58,68] |
| (MW 2000–3000) | Kollidon® 12 PF | Yes | Yes | --- | 72 | 196 | |
| Poly(vinylpyrrolidone)/vinyl acetate (PVP/VA) (MW 45,000–70,000) | Kollidon® VA64 | Yes | Yes | --- | 105 | 270 | [78] |
| Poly(vinyl caprolactam-covinylacetate-ethylene glycol (MW 90,000–140,000) | Soluplus® | Yes | Yes | --- | 72 | 278 | [4,42] |
| Hydroxypropyl cellulose (HPC) (MW 95,000) | Klucel® LF | Yes | Yes | --- | 111 | 227 | [13,36,83] |
| Hydroxypropylmethyl cellulose (HPMC) | [84,85] [76,82] | ||||||
| (MW 25,000) | Methocel™ K100LV | Yes | Yes | 168 | 147 | 259 | |
| (MW 150,000) | Methocel™ K100M | Yes | Yes | 173 | 96 | 259 | |
| Hydroxypropyl methyl cellulose acetate succinate (HPMCAS) | Affinisol™ | Yes | Yes | --- | 115 | <250 | [25,35,65,86,87] |
| Poly ethylene oxide (PEO) | --- | Yes | Yes | 100 | −67 | 340 | [88] |
| Poly(butyl methacrylate-co-(2-demethylamino ethyl) methacrylate-co-methyl methacrylate) 1:2:1 | Eudragit E PO® | Yes | Yes | 189–193 | 52 | 250 | [29,42] |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonio ethyl methacrylate chloride) 1:2:0.2 | Eudragit RL® | Yes | Yes | --- | 63 | 166 | [5,18,25] |
| Poly(methacrylic acid-co-methyl methacrylate) 1:1 | Eudragit L® | Yes | Yes | --- | 111 | 176 | [77] |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonioethylmethacrylate chloride) 1:2:0.1 | Eudragit RS® | Yes | Yes | --- | 64 | 170 | [77] |
| Poly(e-caprolactone) (PCLa) | --- | Yes | Yes | 60 | --- | 310 | [5] |
| Ethylene vinyl acetate (EVA) | --- | Yes | Yes | 70 | 91 | 300 | [64] |
| Ethylcellulose | [77] | ||||||
| (4 cPs) | Ethocel® 4P | 168 | 128 | 200 | |||
| (7 cPs) | Ethocel® 7P | Yes | Yes | 168 | 128 | 205 | |
| (10 cPs) | Ethocel® 10P | 172 | 132 | 205 | |||
| Polyvinyl alcohol;polyethylene glycol graft copolymer (PVA:PEG) | Kollicoat® IR | Yes | Yes | --- | 208 | 200 | [77] |
| Main Matrix-Former Polymer | Polymer (% w/w) | Drug (% w/w) | Other Components | Polymer Trade Name (Manufacturer) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Plasticizer (% w/w) | Lubricant/Filler (% w/w) | Other (% w/w) | |||||
| ALCOHOL-DERIVED POLYMERS | |||||||
| Polyvinyl alcohol (PVA) | 65–90 | Ciprofloxacin (10–35) | Dibutyl sebacate (0.20) | --- | --- | Natural PVA (Ultimaker, Utrecht, The Netherlands) | [43] |
| 95 | Budesonide (5) | --- | --- | --- | Commercial filament (Makerbot Inc., New York, NY, USA) | [89] | |
| 100 | Fluorescein | --- | --- | --- | Commercial filament (Makerbot Inc.) | [27] | |
| 100 | Curcumin | --- | --- | --- | Commercial filament (---) | [17] | |
| 56–70 | Lisinopril dihyd. (20) Amlodipine besylate (10) Indapamide (5) Rosuvastatin Calcium (20) | Sorbitol (23–30) Titanium dioxide (1) | --- | --- | PVA (Parteck MXP) | [51] | |
| 90, 95 | Acetaminophen or Caffeine (5,10) | --- | --- | --- | PVA (Makerbot Inc.) | [50] | |
| 80 | Baclofen (10) | Sorbitol (10) | --- | --- | PVA (Parteck MXP) | [55] | |
| 84 | Hydrochlorotiazide (6) | Mannitol (10) | --- | --- | Mowiol 4–88 (Sigma Aldrich, St. Louis, MO, USA) | [40] | |
| 97.8, 95.2 | Glipizide (2.2, 4.8) | --- | --- | --- | --- | [60] | |
| 67.5 | Felodipine (10) | Tween 80 (22.5) | --- | --- | PVA MW 18,000–25,000 (Colorcon, Lower Salford Township, PA, USA) | [42] | |
| 90, 80, 70 | Carvedilol (10 or 20) Haloperidol (10 or 20) | Sorbitol (10) | --- | --- | PVA (Parteck MXP) | [62] | |
| 98.8 | Aripiprazole (1.2) | --- | --- | --- | Poval 4–88 (Kuraray, Kurashiki, Japan) | [90] | |
| 63 or 73.8 | Acetaminophen (20) | --- | --- | Disintegrant: Sodium starch glycolate (7) Croscarmellose (7) | PVA MW 89,000–98,000 (Sigma Aldrich) | [91] | |
| POLY-(VINYLPYRROLIDONE) AND RELATED POLYMERS | |||||||
| Poly-(vinylpyrrolidone) (PVP) | 50 | Theophylline (10) | Triethyl citrate (12.5) | Talc (27.5) | --- | PVP MW 40,000 (Sigma-Aldrich) | [58] |
| 50 | Dipyridamole (10) | Triethyl citrate (12.5) | Talc (27.5) | --- | |||
| 32.5 | Ramipril (3) | PEG 1500 (20) Mannitol (10) | Magnesium Carbonate (2) | Polymer: Kollidon® VA64 (32.5) | Kollidon® 12PF (BASF, Ludwigshafen, Germany) | [47] | |
| 45–75 | Pantoprazole (10–30) | Triethyl citrate (15–25) | --- | --- | PVP K12 (Carl Roth, Karlsruhe, Germany) | [68] | |
| 50 | Theophylline (10) | Triethyl citrate (12.5) | Talc (27.5) | --- | PVP MW 40,000 (Sigma-Aldrich) | [56] | |
| Theophylline (10) | Triethyl citrate (12.5) | Tribasic Phosphate Sodium (27.5) | --- | ||||
| Budesonide (2.3) | Triethyl citrate (12.5) | Talc (35.2) | --- | ||||
| 45.5 | Diclofenac sodium (20) | Triethyl citrate (17.5) | Talc (17) | --- | |||
| Poly(vinylpyrrolidone)/vinyl acetate (PVP/VA) | 65 | 4-ASA or Ramipril (3) | PEG 1500 (20) Mannitol (10) | Magnesium Carbonate (2) | --- | Kollidon® VA64 (BASF) | [47] |
| 39 | Ramipril (3) | PEG 1500 (20) Mannitol (10) | Magnesium Carbonate (2) | Polymer: Kollidon® 12PF (26) | |||
| 32.5 | Ramipril (3) | PEG 1500 (20) Mannitol (10) | Magnesium Carbonate (2) | Polymer: Kollidon® 12PF (32.5) | |||
| 65 | Pantoprazole (10) | Triethyl citrate (25) | --- | --- | Kollidon® VA64 (BASF) | [68] | |
| CELLULOSE-DERIVED POLYMERS | |||||||
| Ethylcellulose (EC) | 90 | --- | Triethyl citrate (10) | --- | --- | Ethocel™ (Dow, Midland, MI, USA) | [25] |
| 100 | --- | --- | --- | --- | Aqualon™ EC N14 (Ashland Inc., Wilmington, NC, USA) | [86] | |
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | Aqualon™ (Ashland Inc.) | [4] | |
| 35 | Acetaminophen (30) | --- | --- | Polymer: HPMC (35) or Soluplus® (35) | |||
| 50 | Acetaminophen (30) | --- | --- | Polymer: Eudragit® L100 (15) Disintegrant: Kollidon® CL-F (5) | |||
| 60 | Ibuprofen (20) | --- | --- | Release Modifiers: Sodium Alginate (20) or PVA (20) or Xanthan Gum (20) | EC Std.10 No.PD416124 (Colorcon) | [63] | |
| 50–70 | Ibuprofen (20) | --- | --- | Release Modifier: HPMC (10–30) | |||
| 57–63 | Ibuprofen (16–24) | --- | --- | Release modifier: HPMC (19–21) | |||
| 75 | --- | Methylparaben (20) | Magnesium Stearate (5) | --- | Aqualon N7 (Ashland Inc.) | [35] | |
| 60 | Quinine (5) | Triacetin (35%) | --- | --- | --- | [69] | |
| Hydroxypropyl cellulose (HPC) | 100 | --- | --- | --- | --- | Klucel® LF (Ashland Inc.) | [25] |
| 100 | --- | --- | --- | --- | Klucel™ HPC EF (Ashland Inc.) Klucel™ HPC HF (Ashland Inc.) | [86] | |
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 100 | --- | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | Klucel™ HPC EF (Ashland Inc.)Klucel™ HPC LF (Ashland Inc.) | [4] | |
| 35 | Acetaminophen (30) | --- | --- | Polymer: HPMC (35) or EC N14 (35) | |||
| 19.5 | Acetaminophen (30) | --- | --- | Polymer: HPMC E5 (45.5) Disintegrant: Kollidon® CL-F (5) | |||
| 73.75 | --- | Mannitol (21.25) | Magnesium Stearate (5) | Colorants: Candurin Orange Amber (1) Candurin Gold Sheen (1) Food Colouring (5) | Klucel® ELF (Ashland Inc.) | [91] | |
| 46 | Theophylline (50) | Triacetin (4) | --- | --- | HPLC SSL (Nisso Chemical Europe, Düsseldorf, Germany) | [26] | |
| 45 | Theophylline (50) | Triacetin (5) | --- | --- | HPLC SSL (Nisso Chemical Europe) | [36] | |
| 45 | Theophylline (45) | Triacetin (5) | --- | Disintegrant (5): Croscarmellose Sodium (Ac-Di-Sol®) or Sodium Starch Glycolate (Primojel® and Explotab®) or Crospovidone (Polyplasdone™ XL-10) | |||
| 90–100 | Acetaminophen (trace amounts) | PEG (0–10) | --- | --- | Klucel® LF (Ashland Inc.) | [83] | |
| 90 | Domperidone (10) | --- | --- | --- | KlucelTM EXF (Ashland Inc.) | [13] | |
| 80 | Domperidone (10) | --- | --- | Opalescent agent: Barium Sulphate (10) | |||
| 65 | Itraconazole (20) | --- | --- | Solubility enhancer: PVP (15) | HPC-SL (Nippon Soda Co. Ltd., Tokyo, Japan) | [66] | |
| 100 | --- | --- | --- | --- | Klucel LF (Ashland Inc.) | [71] | |
| 99.5 | --- | --- | --- | Polymer: EC (0.5) | |||
| 73.75 | --- | Mannitol (21.25) | Magnesium Stearate (5) | --- | Klucel® EF (Ashland Inc.) | [35] | |
| Hydroxypropyl methylcellulose (HPMC) | 95 | --- | PEG 400 (5) | --- | --- | Affinisol™15cP (Dow) | [25] |
| 100 | --- | --- | --- | --- | Benecel™ HPMC E5 (Ashland Inc.) Benecel™ HPMC K100M (Ashland Inc.) | [86] | |
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 100 | --- | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | Benecel™ HPMC E5 (Ashland Inc.) | [4] | |
| 35 | Acetaminophen (30) | --- | --- | Polymer: (35) EC N14 or HPC EF or HPC LF or Soluplus® or Eudragit® L100 | |||
| 45.5 | Acetaminophen (30) | --- | --- | Polymer: (19.5) EC N14 or HPC EF or HPC LFor Soluplus® or Eudragit® L100 | |||
| 50 | Acetaminophen (30) | --- | --- | Polymer: (19.5) EC N14 or HPC EF or HPC LF or Soluplus® or Eudragit® L100 | |||
| 70 | Acetaminophen (10) | --- | --- | Solubilizer: Soluplus® (20) | Benecel™ HPMC E5 (Ashland Inc.) | [48] | |
| 60 | Carvedilol (20) | Kolliphor TPGS (5) | --- | Polymer: udragit PO (15) | Affinisol™ 15cP (Dow) | [57] | |
| 95 | Acetaminophen (trace amounts) | PEG 400 (5) | --- | --- | Affinisol™ 15cP (Dow) | [34] | |
| 70–90 | Haloperidol (10–30) | --- | --- | --- | Affinisol™ 15cP (Dow) | [61] | |
| 20, 40 | Nitrofurantoin (5) | --- | --- | Polymer: PLA (55, 75) | Affinisol™ 15cP (Dow) | [67] | |
| Hydroxypropyl methylcellulose acetate succinate (HPMCAS) | 95 | --- | PEG 8000 (5) | --- | --- | AQUOT®-LG (Shin-Etsu, Tokyo, Japan) | [25] |
| 100 | --- | --- | --- | --- | AquaSolve™ HPMCAS LG (Ashland Inc.) AquaSolve™ HPMCAS HG (Ashland Inc.) | [86] | |
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 100 | --- | --- | --- | --- | |||
| 70 | Acetaminophen (30) | --- | --- | --- | |||
| 80 | Acetaminophen (trace amounts) | PEG 8000 (20) | --- | --- | AQUOT®-LG (Shin-Etsu) | [34] | |
| 40,75 | Acetaminophen (5,50) | Methylparaben (5,15) | Magnesium Stearate (5) | --- | AQUOT®-HG (Shin-Etsu) | [49] | |
| 70 | --- | Methylparaben (15) | Magnesium Stearate (5) Talc (10) | --- | Aquasolve™-LG (Ashland Inc.) | [35] | |
| 60 | Indomethacin (20) | PEG 6000 (20) | --- | --- | AQUOT®-AS-MF (Shin-Etsu) | [65] | |
| ACRYLATES | |||||||
| Poly(butyl methacrylate-co-(2-demethylaminoeethyl) methacrylate-co-methyl methacrylate) 1:2:1 | 50–55.56 | Felodipine (10) | PEG 4000 (15) | --- | Polymer: PEO (15) Solubility Enhancer: Tween 80 (10) | Eudragit® E PO (Evonik Industries, Essen, Germany) | [42] |
| 100 | --- | --- | --- | --- | Eudragit® E PO (Evonik Industries) | [44] | |
| 55.5 | --- | Tween 80 (11.1) | Polymer: PEG or PEO (16.7) | Eudragit® E PO (Evonik Industries) | [44] | ||
| 46 | 5-ASA, Captopril, Prednisolone or Theophylline (12.5) | Triethyl citrate (6.5) | --- | Disintegrant: Tri-calcium phosphate (37.5) | Eudragit® E PO (Evonik Industries) | [29] | |
| 46.5 | Warfarin (1) | Triethyl citrate (3) | --- | Disintegrant: Tri-calcium Phosphate (49) | Eudragit® E PO (Evonik Industries) | [74] | |
| 46.5 | Theophyline (50) | Triethyl citrate (3.5) | --- | --- | Eudragit® E (Evonik Industries) | [26] | |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonio ethyl methacrylate chloride) 1:2:0.2 | 59.6–69.6 | Theophyline (30) | Stearic acid (3.5–7) or PEG 4000 (5–10) | Anhyd. colloidal silica (0.4) | Polymer: HPC (84.68) | Eudragit® RL PO (Evonik Industries) | [72] |
| 62.6 | Theophyline (30) | Stearic acid (7) PEG 4000 (10) | Anhyd. colloidal silica (0.4) | --- | Eudragit® RL PO (Evonik Industries) | [18] | |
| 64 | --- | Triethyl citrate (6) Mannitol (20) | PEG 6000 (10) Microcrystalline cellulose (20) | --- | Eudragit® RL 100 (Evonik Industries) | [5] | |
| 35–40 | Glimepiride (2) Metformin (50) | PEG 400 (5–7) Triethyl citrate (5) Citric acid Monohydrate (10–15) Mannitol (15) | Calcium stearate (3) | Polymer: PLA (10) | Eudragit® RL PO (Evonik Industries) | [59] | |
| 45 | Theophyline (50) | Triethyl citrate (5) | --- | --- | Eudragit® RL 100 (Evonik Industries) | [26] | |
| 85 | --- | Triethyl citrate (15) | --- | --- | Eudragit® RL 100 (Evonik Industries) | [25] | |
| Ethyl Prop-2-enoate; methyl 2-methylprop-2-enoate;Trimethyl-[2-(2-methylprop-2-enoyloxy)ethyl] azanium; chloride | 95 | Quinine (5) | --- | --- | --- | Eudragit® R (Evonik Industries) | [69] |
| Poly(methacrylic acid-co-methyl methacrylate) 1:1 | 80 | --- | Triethyl citrate (20) | --- | --- | Eudragit® L (Evonik Industries) | [25] |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonio ethyl methacrylate chloride) 1:2:0.1 | 42.5 | Theophyline (50) | Triethyl citrate (7.5) | --- | --- | Eudragit® RS 100 (Evonik Industries) | [26] |
| OTHER POLYMERS | |||||||
| Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (PCL: PVA: PEG) | 90 | --- | PEG 400 (10) | --- | --- | Soluplus® (BASF) | [25] |
| 50 | Felodipine (10) | Tween 80 + PEG 4000 + PEO WSR (15:10:15) | --- | --- | Soluplus® (BASF) | [42] | |
| 100 | --- | --- | --- | --- | Soluplus® (BASF) | [44] | |
| 90 | --- | --- | --- | Polymer: PEG (10) | Soluplus® (BASF) | [44] | |
| 80 | --- | Tween 80 (20) | --- | --- | Soluplus® (BASF) | [44] | |
| Poly(e-caprolactone) (PCLa) | 64 | --- | Mannitol (20) | PEG 6000 (10) Microcrystalline cellulose (20) | --- | CapaTM 6506 (Triiso, Cardiff, UK) | [5] |
| 95, 85, 70 | Indomethacin (5, 15, 30) | --- | --- | --- | Capa™ 6500 (Triiso) | [92] | |
| 95 | Quinine (5) | --- | --- | --- | PCLa MW 14,000 (Sigma Aldrich) | [69] | |
| Polyvinyl alcohol/polyethylene glycol graft copolymer (PVA: PEG) | 88 | --- | Glycerol (12) | --- | --- | Kollicoat® IR (BASF) | [25] |
| 88 | Acetaminophen (trace amounts) | Glycerol (12) | --- | --- | Kollicoat® IR (BASF) | [34] | |
| 45 | --- | Methylparaben (20) Mannitol (20) | Magnesium Stearate (5) Talc (10) | --- | Kollicoat® IR (BASF) | [35] | |
| 97.5 | Bicalutamide (3.5) | --- | --- | --- | Kollicoat® IR (BASF) | [53] | |
| 90 | Haloperidol (10) | --- | --- | --- | Kollicoat® IR (BASF) | [61] | |
| Polyethylene oxide (PEO) | 58 | Acetaminophen (20–40) Ibuprofen (20–40) | --- | Sodium Lauril Sulfate (2) | Disintegrant: Starch (20) Sodium starch glycolate (7) Croscarmellose (2) | PEO 100K (Sigma-Aldrich) | [93] |
| 40–58 | Ibuprofen (20–40) | --- | Starch (18–20) Sodium Lauril Sulfate (1–2) | --- | PEO 200K (Sigma-Aldrich) | ||
| 35 | Theophylline (30) | PEG 6K (35) | --- | --- | PEO 300K (Sigma-Aldrich) | [73] | |
| 35 | Theophylline (30) | PEG 6K (35) | --- | --- | PEO 600K (Sigma-Aldrich) | ||
| Ethylene vinyl Acetate (EVA) | 85, 95 | Indomethacin (5, 15) | --- | --- | --- | ATEVA 1070, 1075A, 1081G, 1241, 1641, 1821A, 1850A, 1880A, 2821A, 3325A (Celanese, Irving, TX, USA) | [64] |
| Matrix Polymer | HME Process | Extruder Model (Manufacturer) | FDM Process | Printer Model (Manufacturer) | Dosage Form Printed | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extrusion Temperature (°C) | Screw Speed (rpm) | Torque (N/cm) | Nozzle Size (mm) | Printing Temperature (°C) | Extrusion Speed (mm/s) | Travelling Speed (mm/s) | Infill (%) | Nozzle Size (mm) | Layer Height (mm) | |||||
| ALCOHOL-DERIVED POLYMERS | ||||||||||||||
| Polyvinyl alcohol (PVA) | --- | --- | --- | --- | --- | 220 | 90 | 150 | 0, 10, 25, 50 90, 100 | --- | 0.20 | Replicator 2 (MakerBot Ind., New York, NY, USA) | IM or ER tablets depending on infill | [27] |
| --- | --- | --- | --- | --- | 140–250 | 20 | --- | 0–100 | --- | 0.80 | FDM- 200W, (NinjaBot) | ER tablets | [17] | |
| 160–175 | 30–60 | 8 | 0.30 | Noztek Touch HT, (Noztek, Shoreham-by-Sea, UK) | 195 | 8 | 150 | 100 | 0.4 | 0.30 | Ultimaker 3 (Ultimaker) | CR tablets | [43] | |
| 90 | 35 | --- | 1.70 | Haake™ MiniCTW (Thermo Fisher, Waltham, MA, USA) | 150 | --- | --- | 100 | --- | 0.50 | MarketBot 2 (MakerBot Ind.) | MR tablets | [51] | |
| 180 | 15 | --- | 1.75 | Noztek, (Noztek) | 200 | 90 | 150 | 100 | --- | 0.2 | Replicator 2 (MakerBot Ind.) | MR capsules | [50] | |
| 160 | 200 | --- | 1.55 | Twin Screw Extruder (Thermo Scientific) | 170 | --- | --- | 30, 65, 100 | --- | --- | --- (MakerBot Ind.) | IR minicaplets | [55] | |
| 190 | 23 | 0.7 | 1.50 | Filabot Original (Filabot, Inc., Boston, MA, USA) | 90 | 70 | 90 | 100 | --- | 0.2 | Replicator 2 (MakerBot Ind.) | SR tablets | [40] | |
| --- | --- | --- | --- | --- | 25 | 0.0075 | 10 | 50 | --- | 0.45 | MAMII (Fochif Mech. Tech., Shanghai, China) | SR tablets | [60] | |
| 100–130 | 23 | 8 | 1.75 | Haake™ MiniCTW (Thermo Fisher) | 150 | --- | --- | 100 | 0.4 | 0.20 | Replicator 2 (MakerBot Ind.) | ER disks | [42] | |
| 170–190 | 200 | --- | 1.5 | Twin Screw Extruder (Thermo Scientific) | 210 | 50 | 150 | 60, 100 | 0.4 | 0.05 | Replicator 2 (MakerBot Ind.) | IR tablets | [62] | |
| 172 | --- | --- | 1.75 | Noztek, (Noztek) | 190 | 5 | --- | 40 | 0.3 | 0.15 | --- | IR films | [90] | |
| 130 | 100 | 1.6 | Noztek, (Noztek) | 190 | 90 | 150 | 100 | 0.4 | 0.2 | Wanhao Duplicator 4 (Jinhua) | IR films | [93] | ||
| POLY-(VINYLPYRROLIDONE) AND RELATED POLYMERS | ||||||||||||||
| Poly-(vinylpyrrolidone) (PVP) | 90 | --- | 40 | --- | Haake™ MiniCTW (Thermo Fisher) | 160 60 | 90 | 150 | 100 | 0.4 | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | [58] |
| 65 | 15 | --- | 1.30 | Noztec Pro Hot Melt (Noztec, Bentley, Australia) | 90 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | [47] | |
| 47–60 | 4.5–7.5 | --- | 2.80 | --- | 79–87 | 60 | --- | 50,90, 100 | 0.35 | 0.1 | Multirap M420 (Multec GmbH, Illmensee, Germany) | IR tablets | [68] | |
| 90 | --- | 40 | 1.25 | Haake™ MiniCTW (Thermo Fisher) | 110 40 | 12 | 50 | 100 | --- | 0.2 | --- | IR tablet core | [56] | |
| Poly(vinylpyrrolidone)/vinyl acetate (PVP/VA) | 70 | 15 | --- | 1.30 | Noztec Pro Hot Melt (Noztec) | 90 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | [47] |
| 65 | 15 | --- | 1.30 | Noztec Pro Hot Melt (Noztec) | 90 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | ||
| 65 | 15 | --- | 1.30 | Noztec Pro Hot Melt (Noztec) | 90 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | ||
| 54–56 | 4.5–7.5 | --- | 2.80 | --- | 85 | 60 | --- | 100 | 0.35 | 0.1 | Multirap M420 (Multec GmbH) | IR tablets | [68] | |
| CELLULOSE-DERIVED POLYMERS | ||||||||||||||
| Ethylcellulose(EC) | 160 | 100 | 100 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | ER disks | [25] |
| 150 | 50 | 72 | --- | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research, Prague, Czech Republic) | ER tablets | [86] | |
| 140–160 | 50 | --- | 2.00 | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | ER tablets | [4] | |
| 100–120 | 60 | --- | 2.00 | Haake™ MiniCTW, (Thermo Fisher) | 170–186 | 7.5–75 | 100 | 15–25 | 0.4 | 0.10–0.30 | JG Aurora A3 (JG Aurora, Shenzhen, China) | ER tablets | [63] | |
| 120 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 160 0 | 3 | 25 | 100 | --- | 0.10 | Replicator 2 (MakerBot Ind.) | ER capsular devices | [35] | |
| 59 | 0.94 | --- | 3.00 | --- | 145 | 144 | 40 | 100 | 0.35 | 0.10 | Multirap M420 (Multec GmbH). | IR tablets | [69] | |
| Hydroxypropyl cellulose (HPC) | 165 | 80 | 40 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 180 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | CR disks | [25] |
| 140–170 | 50 | 55–120 | --- | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | ER tablets | [86] | |
| 140–160 | 50 | --- | 2.00 | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | ER tablets | [4] | |
| 130 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 140 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | CR caplets | [91] | |
| 125–110 | --- | 60 | 1.50 | Haake™ MiniCTW (Thermo Fisher) | 160 60 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | [26] | |
| 120 | 80 | 80 | 1.70 | Haake™ MiniCTW (Thermo Fisher) | --- | 70 | 150 | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | IR tablets | [36] | |
| 150–165 | 50–60 | --- | 1.75 | Haake™ MiniLab II (Thermo Fisher) | 210 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | PR capsular devices | [83] | |
| 145–150 | 20–25 | 10–20 | 1.75 | Haake™ MiniCTW (Thermo Fisher) | 210 30 | --- | 90 | 0, 10, 20, 30 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | SR intra-gastric floating tablets | [13] | |
| 135 | 35 | --- | 1.75 | Filabot Original EX2 (Filabot) | 185 55 | --- | 80 | 0 | 0.5 | 0.10 | MF2200-D (Mutoh Industries) | SR floating tablets | [66] | |
| 165 | 50 | 40–50 | --- | Process 11(Thermo Fisher) | 190 60 | 50 | 50 | 50, 75, 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | SR floating tablets | [71] | |
| 130 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 160 0 | 3 | 25 | 100 | --- | 0.10 | Replicator 2 (MakerBot Ind.) | CR tablets | [35] | |
| Hydroxypropyl methylcellulose(HPMC) | 160 | 70 | 70 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | CR disks | [25] |
| 190 | 50 | 84 | --- | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | CR tablets | [86] | |
| 180 | 50 | --- | 2.00 | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | CR tablets | [4] | |
| 160 | 50 | --- | 2.00 | Haake™ MiniLab II (Thermo Fisher) | 200 50 | 50 | 50 | 20–100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | CR tablets | [48] | |
| 110 | 50 | --- | --- | Haake™ MiniLab II (Thermo Fisher) | 180 RT | 30 | --- | 20 | 0.4 | 0.30 | CraftBot Plus (Craftunique, Budapest, Hungary) | CR tablets | [57] | |
| 160 | 70 | 70 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.3 0.4 | 0.10 | Replicator 2 (MakerBot Ind.) | PR capsular devices | [34] | |
| 150–170 | 200 | --- | 1.55 | Haake™ MiniCTW (Thermo Fisher) | 210 RT | 45 | 150 | 60–100 | 0.4 | 0.10 | Replicator 2 (MakerBot Ind.) | IM tablets | [61] | |
| Hydroxypropylmethylcellulose acetate succinate (HPMCAS) | 180 | 100 | 100 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | MR disks | [25] |
| 200 | 50 | 46–66 | --- | (Thermo Fisher) | 200 50 | 50 | 50 | 100 | 0.4 | 0.10 | Prusa i3 (Prusa Research) | MR tablets | [86] | |
| 180 | 100 | 100 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.3 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | PR capsular devices | [34] | |
| 80–110 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 180–190 | 90 | 150 | 20,100 | --- | 0.10 | Replicator 2 (MakerBot Ind.) | MR tablets | [49] | |
| 105 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 175 0 | 3 | 25 | 100 | --- | 0.10 | Replicator 2 (MakerBot Ind.) | CR tablets | [35] | |
| 40–120 | 50 | --- | --- | Eurolab 16 (Thermo Fisher) | 165 | 25 | ---- | 7 | --- | 0.15 | HD2xR (Airwolf, Pigeon Forge, TN, USA) | MR Starmix® structures | [65] | |
| ACRYLATES | ||||||||||||||
| Poly(butyl methacrylate-co-(2-demethylaminoeethyl) methacrylate-co-methyl methacrylate) 1:2:1 (Eudragit E PO) | 100–130 | --- | 8 | --- | Haake™ MiniLab II (Thermo Fisher) | 150 | --- | --- | 100 | 0.4 | 0.2 | Replicator 2 (MakerBot Ind.) | ER disks | [42] |
| 90–100 | --- | 80 | --- | Haake™ MiniCTW (Thermo Fisher) | 135 | 90 | 150 | 100 | 0.4 | 0.2 | MakerWare (Makerbot Ind.) | IR tablets | [29] | |
| 100, 120 | 100 | --- | 1.75 | Haake™ MiniCTW (Thermo Fisher) | 230 | --- | --- | --- | --- | --- | Replicator 2 (MakerBot Ind.) | --- | [44] | |
| 90–100 | --- | 0.8 | --- | Haake™ MiniCTW (Thermo Fisher) | 135 | 90 | 150 | 100 | 0.4 | 0.2 | MakerWare (Makerbot Ind.) | CR tablets | [74] | |
| 130 | --- | 0.6 | 1.50 | Haake™ MiniCTW (Thermo Fisher) | 140 | 90 | 150 | 100 | --- | 0.2 | Replicator 2 (Makerbot Ind.) | IR; ER capsules | [26] | |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonio ethyl methacrylate chloride) 1:2:0.2 (Eudragit RL) | 160 | 20 | --- | 1.6–1.7 | Pharmalab HME 16 (Thermo Fisher) | 140–180 | 20–40 | 46.65–74.55 | 20–40 | 0.4 | 0.20 | XXL Pro (Prodim Int., Helmond, The Netherlands) | SR tablets | [72] |
| 160 | 20 | --- | 1.75 | Pharmalab HME 16 (Thermo Fisher) | 180 | 15 | --- | 10, 15, 20, 25, 30, 40 | 0.4 | --- | XXL Pro (Prodim Int.) | SR tablets | [18] | |
| 110 | --- | --- | 1.50 | Noztec Pro Hot Melt (Noztec) | 170 | 90 | 150 | 100 | --- | 0.2 | Replicator 2 (MakerBot Ind.) | CR tablets | [5] | |
| 140 | 35 | --- | 1.75 | Filabot Original® (Filabot) | 170 | 70 | 90 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | SR tablets | [59] | |
| 130 | --- | 0.6 | 1.50 | --- | 170 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR and ER tablets | [26] | |
| 120 | 95 | 60 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 160 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | CR disks | [25] | |
| Poly(methacylic acid-co-methyl methacrylate) 1:1 (Eudragit L) | 160 | 80 | 120 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 160 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | CR disks | [25] |
| Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonioethyl methacrylate chloride) 1:2:0.1 (Eudragit RS) | 130 | --- | 0.6 | 1.50 | --- | 150 | 90 | 150 | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | IR and ER tablets | [26] |
| 55 | 0.94 | --- | 3.00 | --- | 155 | 144 | 40 | 100 | 0.5 | 0.10 | Multirap M420 (Multec GmbH). | IR tablets | [69] | |
| OTHER POLYMERS | ||||||||||||||
| Polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer (PCL: PVA: PEG) | 120 | 80 | 80 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 200 | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | CR disks | [25] |
| 120 | 100 | 80 | 1.75 | Haake™ MiniLab II (Thermo Fisher) | 150 | --- | --- | 100 | --- | 0.20 | Replicator 2 (MakerBot Ind.) | CR disks | [42] | |
| 100, 110, 120 | 100 | --- | 1.75 | Haake™ MiniCTW (Thermo Fisher) | 230 | --- | --- | --- | --- | --- | Replicator 2 (MakerBot Ind.) | --- | [44] | |
| Polyvinyl alcohol/polyethylene glycol graft copolymer (PVA/PEG) | 160 | 100 | 80 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 180 --- | --- | --- | 100 | 0.4 | 0.30 | Replicator 2 (MakerBot Ind.) | IR disks | [25] |
| 160 | 100 | 80 | 1.80 | Haake™ MiniLab II (Thermo Fisher) | 180 --- | --- | --- | 100 | 0.3 0.4 | 0.10 | Replicator 2 (MakerBot Ind.) | PR Capsular devices | [34] | |
| 145 | 15 | --- | 1.75 | Noztec Pro Hot Melt (Noztec) | 155 40 | 3 | 25 | 100 | 0.5 | 0.10 | Replicator 2 (MakerBot Ind.) | CR tablets | [35] | |
| 175 | 1 | --- | 1.75 | Noztek, (Noztek) | 198–204 | 15 | 25 | 75, 70, 100 | - | - | --- | IR films | [90] | |
| 150–170 | 200 | --- | 1.55 | (Thermo Fisher) | 210 RT | 45 | 150 | 60–100 | 0.4 | 0.10 | Replicator 2 (MakerBot Ind.) | IR tablets | [61] | |
| Polyethylene oxide (PEO) | 60 | --- | --- | 1.60 | Noztek Pro (Noztek) | 165 RT | 70 | 60 | 40 | 0.4 | 0.10 | Wanhao Duplicator 4 (Wanhao) | IR films | [93] |
| 70 | 35 | --- | 1.50 | Haake™ MiniCTW (Thermo Fisher) | 110 40 | 50 | 150 | 100 | 0.4 | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | [73] | |
| 80 | 35 | --- | 1.50 | Haake™ MiniCTW (Thermo Fisher) | 145 40 | 50 | 150 | 100 | 0.4 | 0.20 | Replicator 2 (MakerBot Ind.) | IR tablets | ||
| Poly(e-caprolactone) | 65 | --- | --- | 2.00 | Noztec Pro Hot Melt (Noztec) | 95 | 90 | 150 | 100 | --- | 0.2 | Replicator 2 (MakerBot Ind.) | CR tablets | [5] |
| 100 | 30 | --- | 1.75 | Haake™ MiniCTW (Thermo Fisher) | 100 | 45 | 150 | 10, 30 | --- | 0.10 | Replicator 2 (MakerBot Ind.) | CR implants | [94] | |
| 47 | 0.94 | --- | 3.00 | --- | 53 | 144 | 40 | 100 | 0.5 | 0.10 | Multirap M420 (Multec GmbH). | IR tablets | [69] | |
| Ethylene vinyl acetate (EVA) | 110 | 10 | --- | 1.5, 2.5 | Haake™ MiniCTW (Thermo Fisher) | 165 | 10 | 150 | 100 | --- | 0.1 | Replicator 2 (MakerBot Ind.) | CR implants | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. https://doi.org/10.3390/pharmaceutics12090795
Pereira GG, Figueiredo S, Fernandes AI, Pinto JF. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics. 2020; 12(9):795. https://doi.org/10.3390/pharmaceutics12090795
Chicago/Turabian StylePereira, Gabriela G., Sara Figueiredo, Ana Isabel Fernandes, and João F. Pinto. 2020. "Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics" Pharmaceutics 12, no. 9: 795. https://doi.org/10.3390/pharmaceutics12090795
APA StylePereira, G. G., Figueiredo, S., Fernandes, A. I., & Pinto, J. F. (2020). Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics, 12(9), 795. https://doi.org/10.3390/pharmaceutics12090795