Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer
Abstract
:1. Introduction
2. Single-Walled Carbon Nanohorns (SWCNH)
2.1. Definition and Structure of SWCNH
2.2. Synthesis
2.3. Properties of SWCNH
2.4. Non-Covalent and Covalent Functionalization of SWCNH
3. Use of SWCNH in Biomedicine: Drug Delivery and Gene Therapy
3.1. Biocompatibility and Toxicity of SWCNH
3.2. Biomedical Applications of SWCNH
4. SWCNH in Cancer Therapy
4.1. SWCNH as Anticancer Nanoparticles
4.2. SWCNH as Delivery Systems for Chemotherapeutic Drugs
4.2.1. Chemotherapeutic Binding to SWCNH for Cancer Therapy
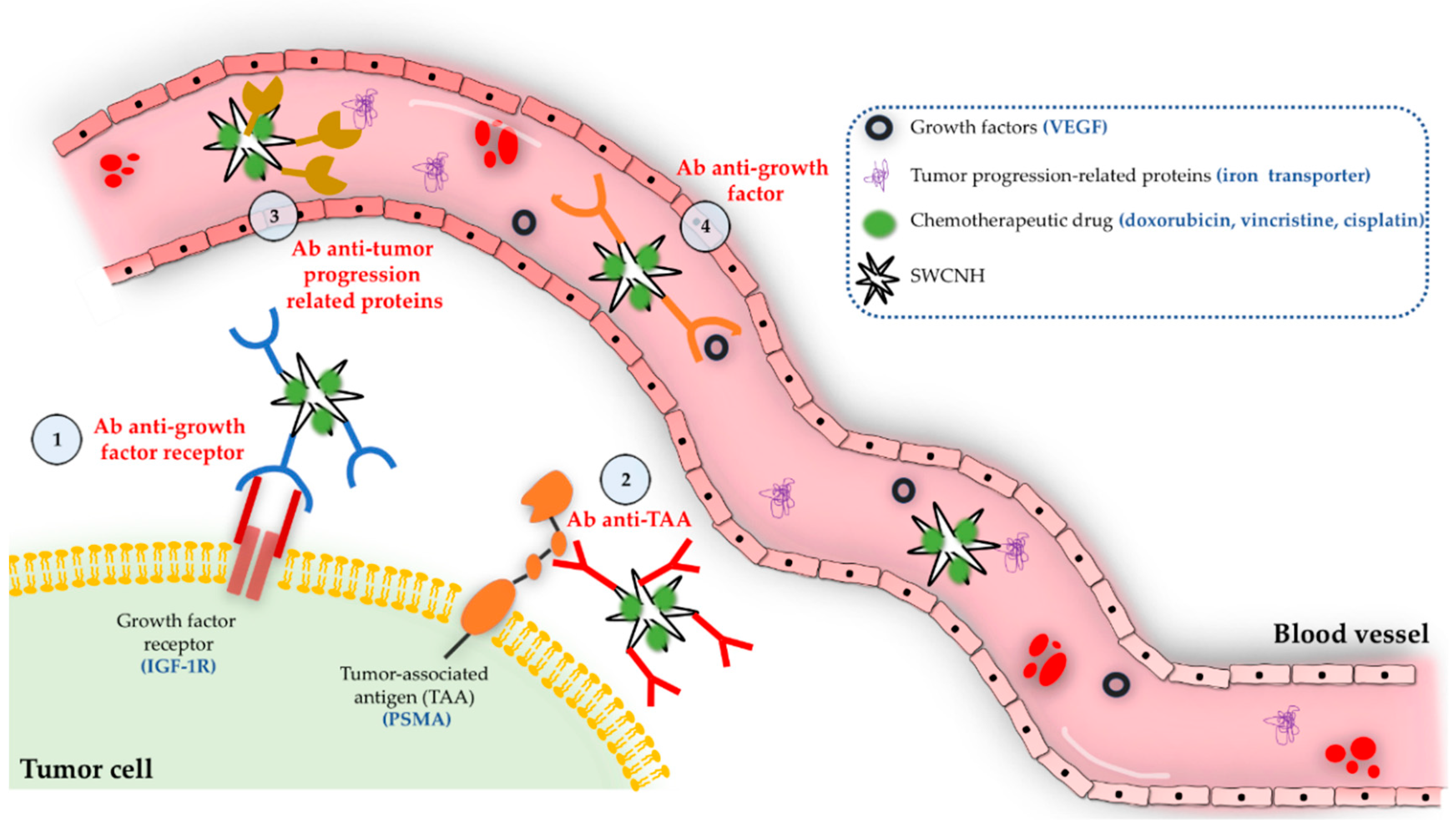
4.2.2. Targeted Chemotherapy Using SWCNH
4.3. SWCNH in Photothermal and Photodynamic Cancer Therapy
4.4. SWCNH in Cancer Gene Therapy and Immunosensing
5. Discussion and Conclusions
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Review Douglas. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Shen, S.; Liu, M.; Li, T.; Lin, S.; Mo, R. Recent progress in nanomedicine-based combination cancer therapy using a site-specific co-delivery strategy. Biomater. Sci. 2017, 5, 1367–1381. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Song, W.; Huang, L. Drug delivery systems targeting tumor-associated fibroblasts for cancer immunotherapy. Cancer Lett. 2019, 448, 31–39. [Google Scholar] [CrossRef]
- Singh, Y.; Pawar, V.K.; Meher, J.G.; Raval, K.; Kumar, A.; Shrivastava, R.; Bhadauria, S.; Chourasia, M.K. Targeting tumor associated macrophages (TAMs) via nanocarriers. J. Control. Release 2017, 254, 92–106. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon Nanomaterials for Targeted Cancer Therapy Drugs: A Critical Review. Chem. Rec. 2019, 19, 502–522. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 165–170. [Google Scholar] [CrossRef]
- Serban, B.-C.; Bumbac, M.; Buiu, O.; Cobianu, C.; Brezeanu, M.; Nicolescu, C. Carbon nanohorns and their nanocomposites: Synthesis, properties and applications. A concise review. Ann. Acad. Rom. Sci. Sci. Technol. Inf. 2018, 11, 5–18. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef] [Green Version]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D’Emanuele, A. Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Deliv. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Luquero, A.; Parra-Robert, M.; Mora, A.; Ribera, J.; Edelman, E.R.; Jiménez, W. Graphene-Dendrimer Nanostars for Targeted Macrophage Overexpression of Metalloproteinase 9 and Hepatic Fibrosis Precision Therapy. Nano Lett. 2018, 18, 5839–5845. [Google Scholar] [CrossRef] [PubMed]
- Yuge, R.; Yudasaka, M.; Toyama, K.; Yamaguchi, T.; Iijima, S.; Manako, T. Buffer gas optimization in CO2 laser ablation for structure control of single-wall carbon nanohorn aggregates. Carbon N. Y. 2012, 50, 1925–1933. [Google Scholar] [CrossRef]
- Azami, T.; Kasuya, D.; Yuge, R.; Yudasaka, M.; Iijima, S.; Yoshitake, T.; Kubo, Y. Large-scale production of single-wall carbon nanohorns with high purity. J. Phys. Chem. C 2008, 112, 1330–1334. [Google Scholar] [CrossRef]
- Joseph Berkmans, A.; Jagannatham, M.; Rohit Reddy, D.; Haridoss, P. Synthesis of thin bundled single walled carbon nanotubes and nanohorn hybrids by arc discharge technique in open air atmosphere. Diam. Relat. Mater. 2015, 55, 12–15. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Bandow, S.; Iijima, S. Synthesis of carbon nanohorn particles by simple pulsed arc discharge ignited between pre-heated carbon rods. Chem. Phys. Lett. 2004, 389, 181–185. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon N. Y. 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Wang, H.; Chhowalla, M.; Sano, N.; Jia, S.; Amaratunga, G.A.J. Large-scale synthesis of single-walled carbon nanohorns by submerged arc. Nanotechnology 2004, 15, 546–550. [Google Scholar] [CrossRef]
- Pasura, C.; Barison, S.; Battiston, S.; Schiavon, M. Synthesis and characterization of single wall carbon nanohorns produced by direct vaporization of graphite. Nanotechnol. 2010 Adv. Mater. CNTs Part. Films Compos. 2010, 1, 289–291. [Google Scholar]
- Murata, K.; Kaneko, K.; Steele, W.A.; Kokai, F.; Takahashi, K.; Kasuya, D.; Yudasaka, M.; Iijima, S. Porosity Evaluation of Intrinsic Intraparticle Nanopores of Single Wall Carbon Nanohorn. Nano Lett. 2001, 1, 197–199. [Google Scholar] [CrossRef]
- Utsuini, S.; Miyawaki, J.; Tanaka, H.; Hattori, Y.; Itoi, T.; Ichikuni, N.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Opening mechanism of internal nanoporosity of single-wall carbon nanohorn. J. Phys. Chem. B 2005, 109, 14319–14324. [Google Scholar] [CrossRef]
- Sano, N.; Taniguchi, K.; Tamon, H. Hydrogen storage in porous single-walled carbon nanohorns dispersed with Pd-Ni alloy nanoparticles. J. Phys. Chem. C 2014, 118, 3402–3408. [Google Scholar] [CrossRef]
- Pagona, G.; Tagmatarchis, N.; Fan, J.; Yudasaka, M.; Iijima, S. Cone-end functionalization of carbon nanohorns. Chem. Mater. 2006, 18, 3918–3920. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, S.; Wang, C.; Li, J.; Xu, G. Single-walled carbon nanohorns for energy applications. Nanomaterials 2015, 5, 1732–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaj, S.; Thien-Nga, L.; Gaal, R.; Forró, L.; Takahashi, K.; Kokai, F.; Yudasaka, M.; Iijima, S.; Iijima, S.; Iijima, S. Electronic properties of carbon nanohorns studied by ESR. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 62, 17115–17119. [Google Scholar] [CrossRef]
- Yang, C.M.; Kim, Y.J.; Endo, M.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Nanowindow-regulated specific capacitance of supercapacitor electrodes of single-wall carbon nanohorns. J. Am. Chem. Soc. 2007, 129, 20–21. [Google Scholar] [CrossRef]
- Unni, S.M.; Illathvalappil, R.; Bhange, S.N.; Puthenpediakkal, H.; Kurungot, S. Carbon Nanohorn-Derived Graphene Nanotubes as a Platinum-Free Fuel Cell Cathode. ACS Appl. Mater. Interfaces 2015, 7, 24256–24264. [Google Scholar] [CrossRef]
- Yoshitake, T.; Shimakawa, Y.; Kuroshima, S.; Kimura, H.; Ichihashi, T.; Kubo, Y.; Kasuya, D.; Takahashi, K.; Kokai, F.; Yudasaka, M.; et al. Preparation of fine platinum catalyst supported on single-wall carbon nanohorns for fuel cell application. Phys. B Condens. Matter 2002, 323, 124–126. [Google Scholar] [CrossRef]
- Pagona, G.; Sandanayaka, A.S.D.; Araki, Y.; Fan, J.; Tagmatarchis, N.; Yudasaka, M.; Iijima, S.; Ito, O. Electronic interplay on illuminated aqueous carbon nanohorn-porphyrin ensembles. J. Phys. Chem. B 2006, 110, 20729–20732. [Google Scholar] [CrossRef]
- Pagona, G.; Fan, J.; Maignè, A.; Yudasaka, M.; Iijima, S.; Tagmatarchis, N. Aqueous carbon nanohorn-pyrene-porphyrin nanoensembles: Controlling charge-transfer interactions. Diam. Relat. Mater. 2007, 16, 1150–1153. [Google Scholar] [CrossRef]
- Yang, M.; Wada, M.; Zhang, M.; Kostarelos, K.; Yuge, R.; Iijima, S.; Masuda, M.; Yudasaka, M. A high poly(ethylene glycol) density on graphene nanomaterials reduces the detachment of lipid-poly(ethylene glycol) and macrophage uptake. Acta Biomater. 2013, 9, 4744–4753. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 2018, 8, 1966–1984. [Google Scholar] [CrossRef] [PubMed]
- Mountrichas, G.; Ichihashi, T.; Pispas, S.; Yudasaka, M.; Iijima, S.; Tagmatarehis, N. Solubilization of carbon nanohorns by block polyelectrolyte wrapping and templated formation of gold nanoparticles. J. Phys. Chem. C 2009, 113, 5444–5449. [Google Scholar] [CrossRef]
- Kizhisseri, D.R.; Mahesh, S.; Joseph, K. Cardanol-derived azobenzene-induced phototunable conductance switching of single-walled carbon nanohorns. ACS Sustain. Chem. Eng. 2020, 8, 2698–2706. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Ajima, K.; Miyawaki, J.; Iijima, S. Light-assisted oxidation of single-wall carbon nanohorns for abundant creation of oxygenated groups that enable Chemical modifications with proteins to enhance biocompatibility. ACS Nano 2007, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Han, S.; Zhang, L.; Parveen, S.; Xu, G. A novel fluorescent aptasensor based on single-walled carbon nanohorns. Nanoscale 2011, 3, 4589–4592. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ichihashi, T.; Kasuya, D.; Kataura, H.; Iijima, S. Structure changes of single-wall carbon nanotubes and single-wall carbon nanohorns caused by heat treatment. Carbon N. Y. 2003, 41, 1273–1280. [Google Scholar] [CrossRef]
- Bekyarova, E.; Hashimoto, A.; Yudasaka, M.; Hattori, Y.; Murata, K.; Kanoh, H.; Kasuya, D.; Iijima, S.; Kaneko, K. Palladium nanoclusters deposited on single-walled carbon nanohorns. J. Phys. Chem. B 2005, 109, 3711–3714. [Google Scholar] [CrossRef]
- Isaac, K.M.; Sabaraya, I.V.; Ghousifam, N.; Das, D.; Pekkanen, A.M.; Romanovicz, D.K.; Long, T.E.; Saleh, N.B.; Rylander, M.N. Functionalization of single-walled carbon nanohorns for simultaneous fluorescence imaging and cisplatin delivery in vitro. Carbon N. Y. 2018, 138, 309–318. [Google Scholar] [CrossRef]
- Yuge, R.; Ichihashi, T.; Shimakawa, Y.; Kubo, Y.; Yudasaka, M.; Iijima, S. Preferential deposition of Pt nanoparticles inside single-walled carbon nanohorns. Adv. Mater. 2004, 16, 1420–1423. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, G.; Chen, X.; Luo, F.; Jiang, D.; Huang, S.; Li, Y.; Pu, X. A novel strategy of procalcitonin detection based on multi-nanomaterials of single-walled carbon nanohorns-hollow Pt nanospheres/PAMAM as signal tags. RSC Adv. 2014, 4, 13934–13940. [Google Scholar] [CrossRef]
- Xu, J.; Yudasaka, M.; Kouraba, S.; Sekido, M.; Yamamoto, Y.; Iijima, S. Single wall carbon nanohorn as a drug carrier for controlled release. Chem. Phys. Lett. 2008, 461, 189–192. [Google Scholar] [CrossRef]
- Jiang, B.P.; Hu, L.F.; Shen, X.C.; Ji, S.C.; Shi, Z.; Liu, C.J.; Zhang, L.; Liang, H. One-step preparation of a water-soluble carbon nanohorn/phthalocyanine hybrid for dual-modality photothermal and photodynamic therapy. ACS Appl. Mater. Interfaces 2014, 6, 18008–18017. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ajima, K.; Miyawaki, J.; Yudasaka, M.; Iijima, S.; Shiba, K. Drug-loaded carbon nanohorns: Adsorption and release of dexamethasone in vitro. Mol. Pharm. 2004, 1, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Tahara, Y.; Ikehara, Y.; Murakami, T.; Tsuchida, K.; Iijima, S.; Waga, I.; Yudasaka, M. Single-walled carbon nanohorns as drug carriers: Adsorption of prednisolone and anti-inflammatory effects on arthritis. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Yudasaka, M.; Azami, T.; Kubo, Y.; Iijima, S. Toxicity of single-walled carbon nanohorns. ACS Nano 2008, 2, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.M.; Voy, B.H.; Glass, D.F.; Mahurin, S.M.; Zhao, B.; Hu, H.; Saxton, A.M.; Donnell, R.L.; Cheng, M.D. Assessing the pulmonary toxicity of single-walled carbon nanohorns. Nanotoxicology 2007, 1, 157–166. [Google Scholar] [CrossRef]
- Tahara, Y.; Miyawaki, J.; Zhang, M.; Yang, M.; Waga, I.; Iijima, S.; Irie, H.; Yudasaka, M. Histological assessments for toxicity and functionalization-dependent biodistribution of carbon nanohorns. Nanotechnology 2011, 22, 265106. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Z.; Li, S.; Zhang, Y.; He, B.; Peng, D.; Tian, J.; Zhao, M.; Wang, X.; Zhang, Q. The interactions of single-wall carbon nanohorns with polar epithelium. Int. J. Nanomed. 2017, 12, 4177–4194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yang, M.; Bussy, C.; Iijima, S.; Kostarelos, K.; Yudasaka, M. Biodegradation of carbon nanohorns in macrophage cells. Nanoscale 2015, 7, 2834–2840. [Google Scholar] [CrossRef]
- He, B.; Shi, Y.; Liang, Y.; Yang, A.; Fan, Z.; Yuan, L.; Zou, X.; Chang, X.; Zhang, H.; Wang, X.; et al. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Boyer, P.D.; Holt, B.D.; Islam, M.F.; Dahl, K.N. Decoding membrane- versus receptor-mediated delivery of single-walled carbon nanotubes into macrophages using modifications of nanotube surface coatings and cell activity. Soft Matter 2013, 9, 758–764. [Google Scholar] [CrossRef]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.H. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells /631/80 /704/172 /82/29 /14/19 /14/34 /123 article. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, X.E.; You, J.; Xu, G.; Wang, H. Carboxylic-group-functionalized single-walled carbon nanohorns as peroxidase mimetics and their application to glucose detection. Analyst 2015, 140, 6398–6403. [Google Scholar] [CrossRef] [PubMed]
- Miyako, E.; Nagata, H.; Hirano, K.; Makita, Y.; Nakayama, K.I.; Hirotsu, T. Near-infrared laser-triggered carbon nanohorns for selective elimination of microbes. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Miyako, E.; Nagata, H.; Hirano, K.; Sakamoto, K.; Makita, Y.; Nakayama, K.I.; Hirotsu, T. Photoinduced antiviral carbon nanohorns. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Niu, W.; Li, H.; Xu, G. Amperometric glucose biosensor based on single-walled carbon nanohorns. Biosens. Bioelectron. 2008, 23, 1887–1890. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Niu, W.; Xu, G. Simultaneous electrochemical determination of uric acid, dopamine, and ascorbic acid at single-walled carbon nanohorn modified glassy carbon electrode. Biosens. Bioelectron. 2009, 25, 940–943. [Google Scholar] [CrossRef]
- Kasai, T.; Matsumura, S.; Iizuka, T.; Shiba, K.; Kanamori, T.; Yudasaka, M.; Iijima, S.; Yokoyama, A. Carbon nanohorns accelerate bone regeneration in rat calvarial bone defect. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Depan, D.; Shah, J.S. Structure-process-functional property relationship of nanostructured carbon mediated cellular response for soft-tissue reconstruction and replacement. Acta Biomater. 2012, 8, 1908–1917. [Google Scholar] [CrossRef]
- Guerra, J.; Herrero, M.A.; Vázquez, E. Carbon nanohorns as alternative gene delivery vectors. RSC Adv. 2014, 4, 27315–27321. [Google Scholar] [CrossRef]
- Guerra, J.; Herrero, M.A.; Carrión, B.; Pérez-Martínez, F.C.; Lucío, M.; Rubio, N.; Meneghetti, M.; Prato, M.; Ceña, V.; Vázquez, E. Carbon nanohorns functionalized with polyamidoamine dendrimers as efficient biocarrier materials for gene therapy. Carbon N. Y. 2012, 50, 2832–2844. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.; et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Martínez, F.C.; Carrión, B.; Lucío, M.I.; Rubio, N.; Herrero, M.A.; Vázquez, E.; Ceña, V. Enhanced docetaxel-mediated cytotoxicity in human prostate cancer cells through knockdown of cofilin-1 by carbon nanohorn delivered siRNA. Biomaterials 2012, 33, 8152–8159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, X.; Yang, W.; He, J.; He, K.; Xia, Z.; Zhang, J.; Xiang, G. Single-walled carbon nanohorn aggregates promotes mitochondrial dysfunction-induced apoptosis in hepatoblastoma cells by targeting SIRT3. Int. J. Oncol. 2018, 53, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Q.; Bo, J.; Huang, R.; Zhang, M.; Xia, Z.; Ju, L.; Xiang, G. Single-Walled Carbon Nanohorn (SWNH) aggregates inhibited proliferation of human liver cell lines and promoted apoptosis, especially for hepatoma cell lines. Int. J. Nanomed. 2014, 9, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; He, J.; Li, B.; Zhang, J.; He, K.; Duan, X.; Huang, R.; Xiang, G. SWNHs (Single-Wall Carbon Nanohorns) Supervises Endoplasmic Reticulum (ER) Stress in Hepatocellular Carcinoma. J. Nanosci. Nanotechnol. 2018, 18, 6740–6745. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Y.; Zhao, M. Single-walled carbon nanohorns inhibit proliferation of conjunctival melanoma cell lines CRMM-1 and involved in energy metabolism. J. Nanosci. Nanotechnol. 2015, 15, 1821–1830. [Google Scholar] [CrossRef]
- Ajima, K.; Murakami, T.; Mizoguchi, Y.; Tsuchida, K.; Ichihashi, T.; Iijima, S.; Yudasaka, M. Enhancement of In Vivo Anticancer Inside Single-Wall Carbon Nanohorns. ACS Nano 2008, 2, 2057–2064. [Google Scholar] [CrossRef]
- Lucío, M.I.; Opri, R.; Pinto, M.; Scarsi, A.; Fierro, J.L.G.; Meneghetti, M.; Fracasso, G.; Prato, M.; Vázquez, E.; Herrero, M.A. Targeted killing of prostate cancer cells using antibody-drug conjugated carbon nanohorns. J. Mater. Chem. B 2017, 5, 8821–8832. [Google Scholar] [CrossRef]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigne, A.; Shiba, K.; Iijima, S. Carbon Nanohorns as Anticancer Drug Carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef]
- Matsumura, S.; Ajima, K.; Yudasaka, M.; Iijima, S.; Shiba, K. Dispersion of cisplatin-loaded carbon nanohorns with a conjugate comprised of an artificial peptide aptamer and polyethylene glycol. Mol. Pharm. 2007, 4, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shu, C.; Guo, J.; Pang, L.; Su, L.; Fu, D.; Zhong, W. Targeted cancer therapy based on single-wall carbon nanohorns with doxorubicin in vitro and in vivo. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Jiang, F.; Liu, Z.; Shu, C.; Wan, L.J. In vitro and in vivo photothermally enhanced chemotherapy by single-walled carbon nanohorns as a drug delivery system. J. Mater. Chem. B 2014, 2, 4726–4732. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Fan, J.; Yudasaka, M.; Iijima, S.; Shiba, K. Solubilization of Single-Wall Carbon Nanohorns Using a PEG-Doxorubicin Conjugate. Mol. Pharm. 2006, 3, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sawada, H.; Tamura, G.; Yudasaka, M.; Iijima, S.; Tsuchida, K. Water-dispersed single-wall carbon nanohorns as drug carriers for local cancer chemotherapy. Nanomed 2008, 3, 453–463. [Google Scholar] [CrossRef]
- Wang, R.; Cui, H.; Wang, J.; Li, N.; Zhao, Q.; Zhou, Y.; Lv, Z.; Zhong, W. Enhancing the antitumor effect of methotrexate: In intro and in vivo by a novel targeted single-walled carbon nanohorn-based drug delivery system. RSC Adv. 2016, 6, 47272–47280. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Q.; Shu, C.; Ma, X.; Li, R.; Shen, H.; Zhong, W. Targeted killing of cancer cells in vivo and in vitro with IGF-IR antibody-directed carbon nanohorns based drug delivery. Int. J. Pharm. 2015, 478, 644–654. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2015, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2016, 22, 270–281. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yu, E. Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Bone 2008, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Qi, S.; Perrino, S.; Hashimoto, M.; Brodt, P. Targeting the IGF-Axis for Cancer Therapy: Development and Validation of an IGF-Trap as a Potential Drug. Cells 2020, 9, 1098. [Google Scholar] [CrossRef]
- Maris, C.; D’Haene, N.; Trépant, A.L.; Le Mercier, M.; Sauvage, S.; Allard, J.; Rorive, S.; Demetter, P.; Decaestecker, C.; Salmon, I. IGF-IR: A new prognostic biomarker for human glioblastoma. Br. J. Cancer 2015, 113, 729–737. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Haberkorn, U.; Kopka, K. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Fan, K.; Yan, X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release 2019, 311–312, 288–300. [Google Scholar] [CrossRef]
- Gomes, I.P.; Duarte, J.A.; Maia, A.L.C.; Rubello, D.; Townsend, D.M.; de Barros, A.L.B.; Leite, E.A. Thermosensitive nanosystems associated with hyperthermia for cancer treatment. Pharmaceuticals 2019, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Whitney, J.R.; Sarkar, S.; Zhang, J.; Do, T.; Young, T.; Manson, M.K.; Campbell, T.A.; Puretzky, A.A.; Rouleau, C.M.; More, K.L.; et al. Single walled carbon nanohorns as photothermal cancer agents. Lasers Surg. Med. 2011, 43, 43–51. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, S.; Dai, H.; Lin, Y. An ultrasensitive ratiometric electrochemiluminescence immunosensor combining photothermal amplification for ovarian cancer marker detection. Biosens. Bioelectron. 2019, 146, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Zhang, F.; Yin, Y.; Mei, L.; Song, F.; Tao, M.; Yue, W.; Zhong, W. Overcoming multidrug resistance by a combination of chemotherapy and photothermal therapy mediated by carbon nanohorns. J. Mater. Chem. B 2016, 4, 6043–6051. [Google Scholar] [CrossRef]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic therapy based on graphene and MXene in cancer theranostics. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Department photodynamic therapy of cancer: An update Patrizia. CA Cancer J. Clin. 2012, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Dong, P.; Lin, Z.; Guo, X.; Jiang, B.P.; Ji, S.; Liang, H.; Shen, X.C. Near-Infrared Light Responsive Imaging-Guided Photothermal and Photodynamic Synergistic Therapy Nanoplatform Based on Carbon Nanohorns for Efficient Cancer Treatment. Chem. A Eur. J. 2018, 24, 12827–12837. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, C.; Song, L.; Cui, H.; Gao, G.; Liu, P.; Sheng, Z.; Cai, L. Indocyanine green-loaded polydopamine-iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, B.P.; Liang, J.; Wen, C.; Shen, X.C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon N. Y. 2019, 143, 814–827. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1–37. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of nanomaterials for immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Han, J.; Zhuo, Y.; Yang, Z.; Chai, Y.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanohorns as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef]
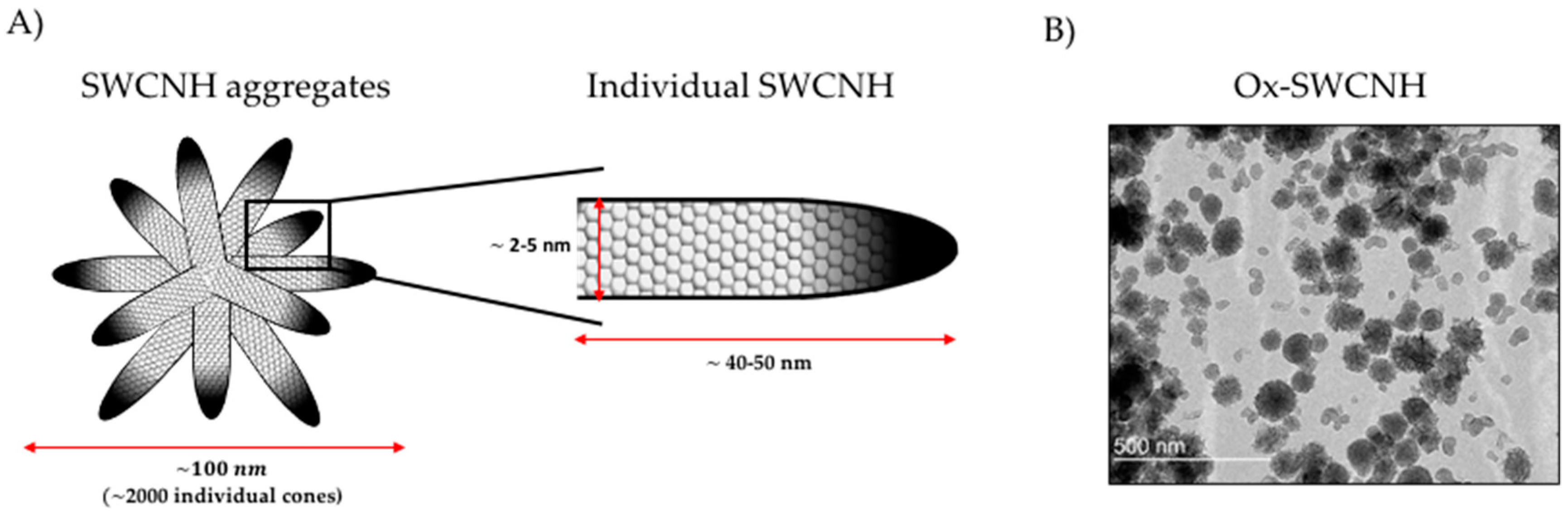
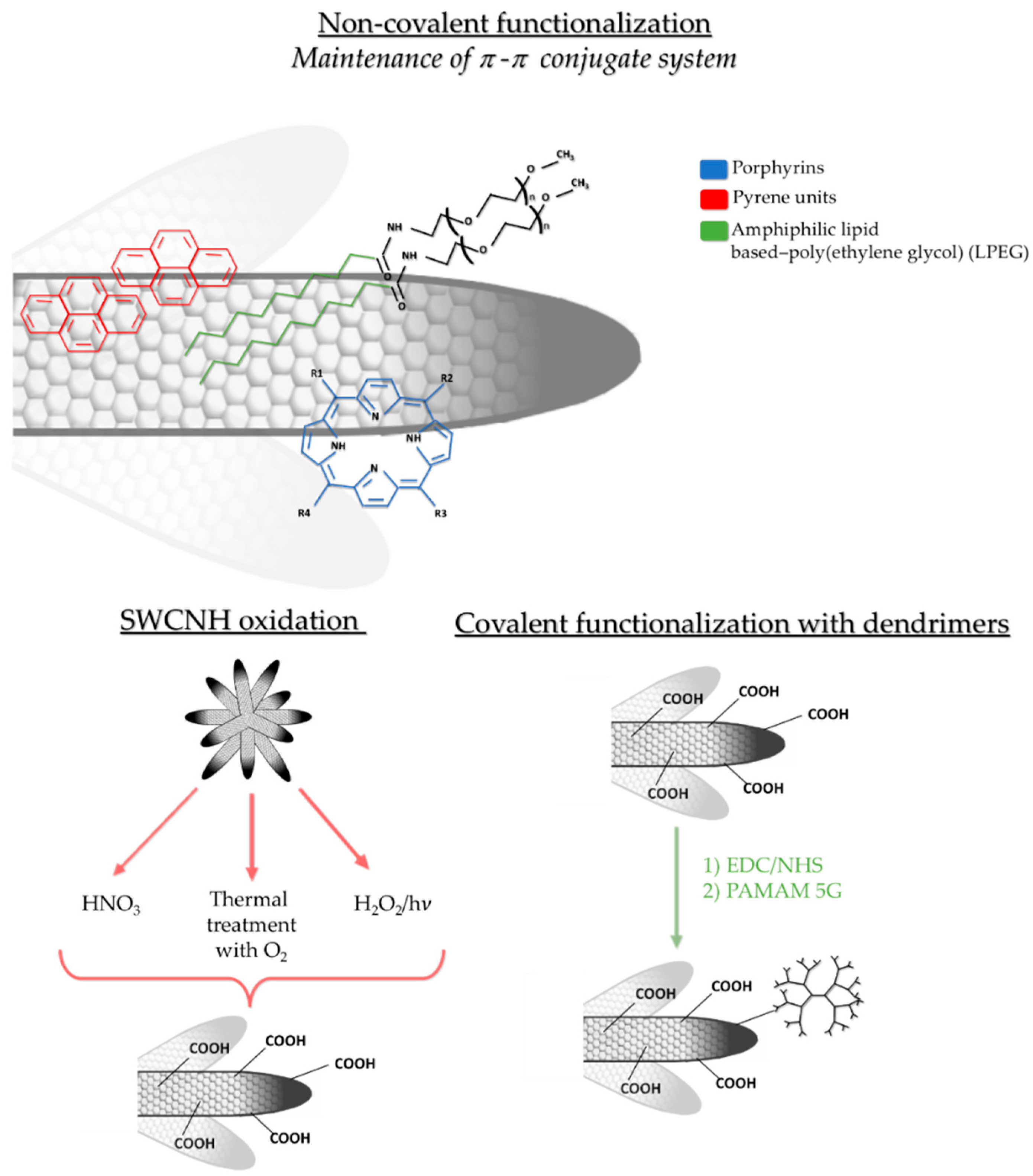
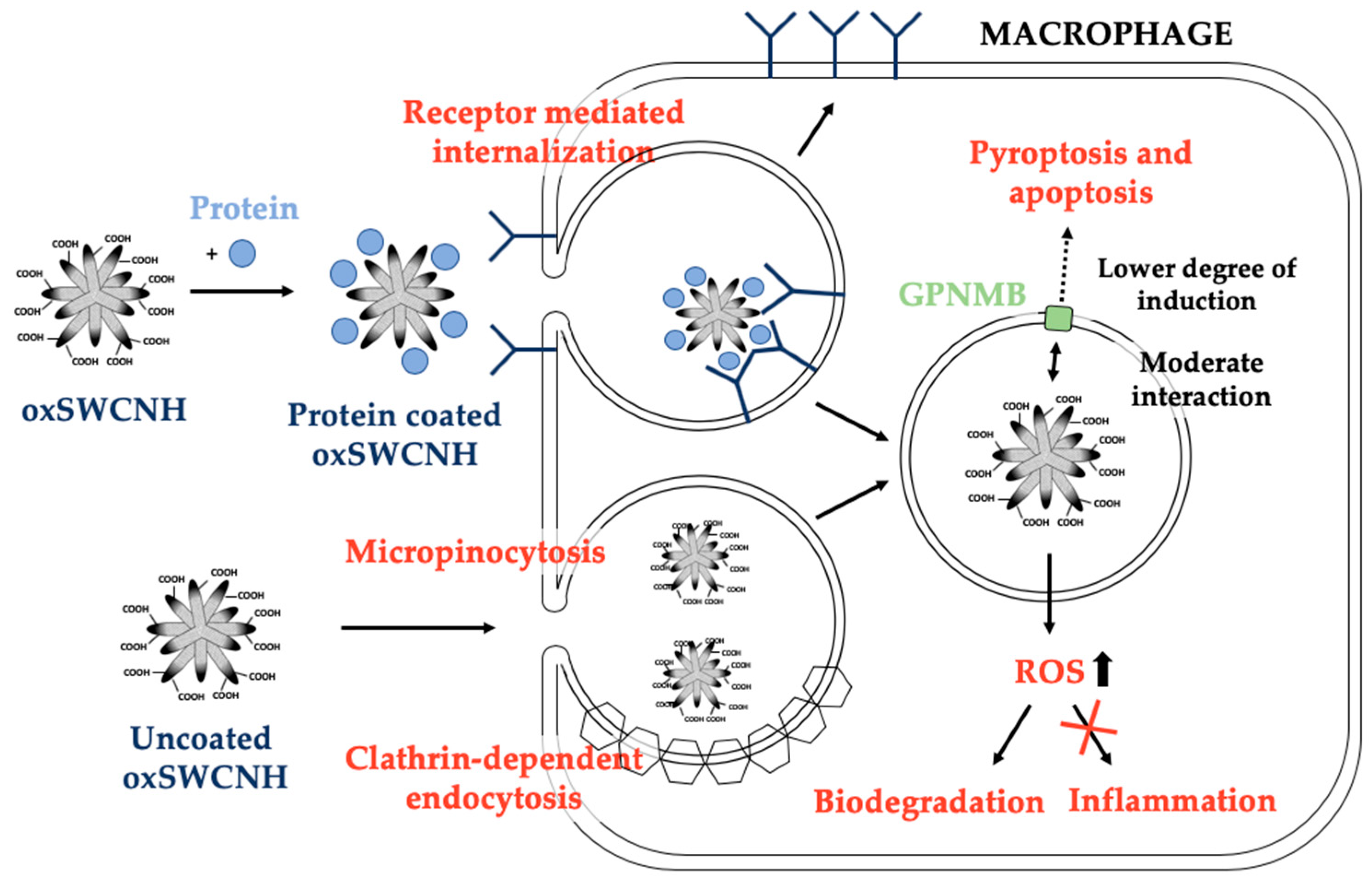
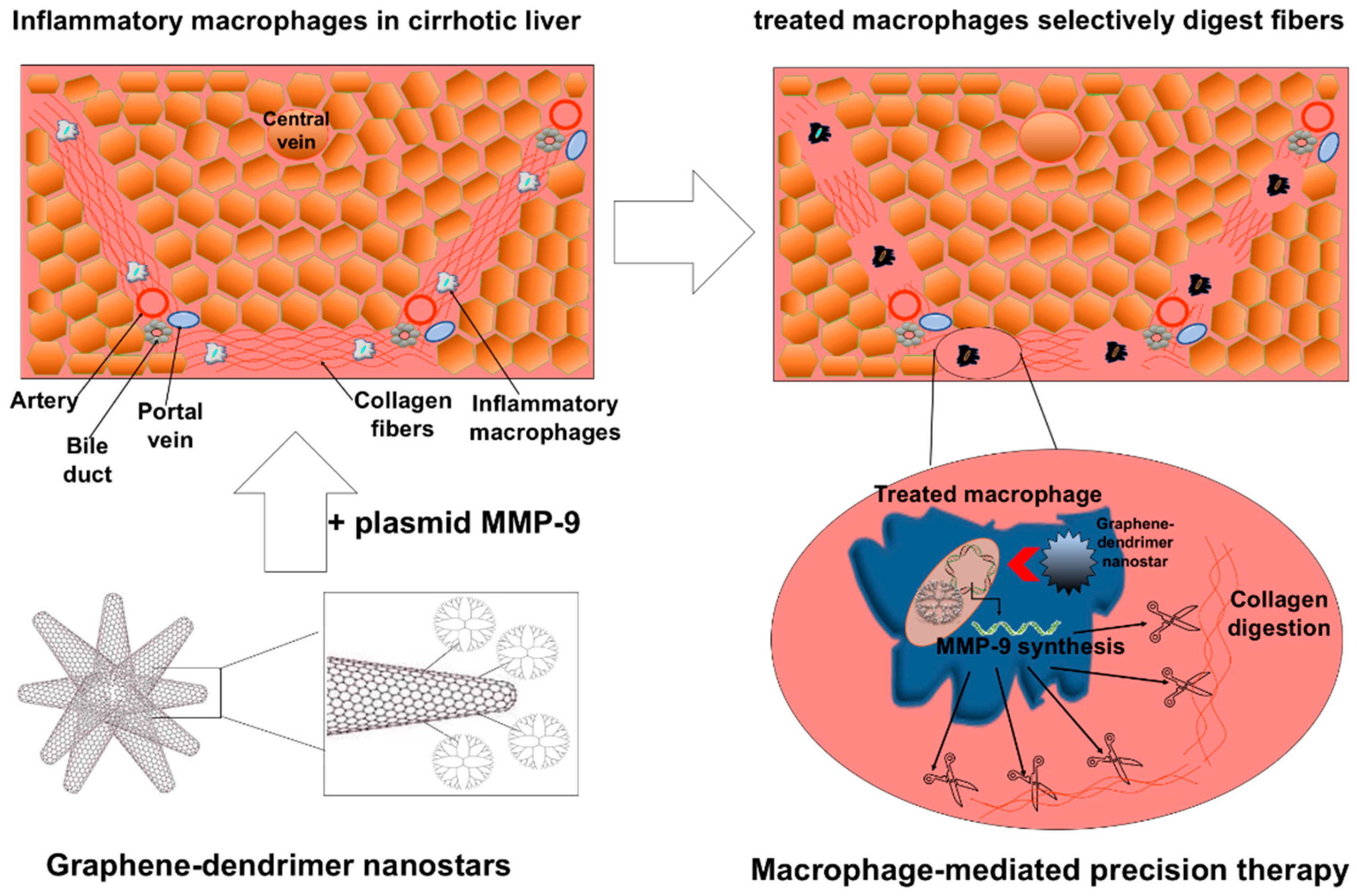

| Administration Route | Dosage | Mice Monitorization | Evaluated Parameters | References |
|---|---|---|---|---|
| Eye contact | 0.02 g/eye | 1 h after injection | No irritation response | [55] |
| Skin contact | 0.015 g/site | 1 h after injection | No erythema or edema formation | [55] |
| Oral administration | 2000 mg/kg of body weight. | For 14 days | No body weight changes | [55] |
| Intratracheal instillation | 2.25 mg/animal | 90-day test period | No clinical symptoms of distress and no changes in a whole-lung microarray analysis | [56] |
| Intravenous administration | 6 mg/kg of body weight. | 26 weeks test period | Normal gross appearance and no severe abnormalities in the tissues were found on histological observations | [57] |
| Chemotherapy | Functionalization and Union to SWCNH | Target | References |
|---|---|---|---|
| Cisplatin (CDDP) | Incorporation into SWCNH cone | AY-27 rat bladder carcinoma cells | [48] |
| Oxidized drug attachment to amine functionalized SWCNH | PC-3 PSMA+ prostate cancer cells | [79] | |
| Drug incorporated with nanoprecipitation [80] | NCI-H460 human lung cancer cells | [78,80,81] | |
| Balb/c nu/nu mice bearing NCI-H460 human lung cancer cells | [78] | ||
| Dual cisplatin (CDDP) and doxorubicin (DOX) | Poly(maleic anhydride-alt-1-octadecene) (C18PMH) and methoxypolyethyleneglycol-b-poly-d, l-lactide (mPEG-PLA) | Balb/c mice bearing 4T1 breast cancer cell line | [41] |
| Doxorubicin (DOX) | Hydrophobic π-π stacking interactions | MCF-7 human breast adenocarcinoma cells | [82] |
| 4T1 breast cancer cells and Balb/c mice bearing 4T1 cells | [83] | ||
| Polyethylene glycol (PEG) | NCI-H460 human non-small lung cancer cells | [84,85] | |
| Balb/c nu/nu mice bearing NCI-H460 human non-small lung cancer cells | [85] | ||
| Methotrexate | 1,2-Disteatoyl-sn-glycero-3- phosphoethanolamine–N-poly(ethylene glycol)-amine (DSPE–PEG-NH2) | Human lung adenocarcinoma (A549) and breast adenocarcinoma (MAD-MB-231) cell line. ICR mice bearing H22 hepatocellular carcinoma cell line. | [86] |
| Vincristine | Physical adsorption | MCF-7 human breast adenocarcinoma cells and ICR mice bearing H22 hepatocellular carcinoma cell line. | [87] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Lanceta, A.; Medrano-Bosch, M.; Melgar-Lesmes, P. Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer. Pharmaceutics 2020, 12, 850. https://doi.org/10.3390/pharmaceutics12090850
Moreno-Lanceta A, Medrano-Bosch M, Melgar-Lesmes P. Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer. Pharmaceutics. 2020; 12(9):850. https://doi.org/10.3390/pharmaceutics12090850
Chicago/Turabian StyleMoreno-Lanceta, Alazne, Mireia Medrano-Bosch, and Pedro Melgar-Lesmes. 2020. "Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer" Pharmaceutics 12, no. 9: 850. https://doi.org/10.3390/pharmaceutics12090850
APA StyleMoreno-Lanceta, A., Medrano-Bosch, M., & Melgar-Lesmes, P. (2020). Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer. Pharmaceutics, 12(9), 850. https://doi.org/10.3390/pharmaceutics12090850






