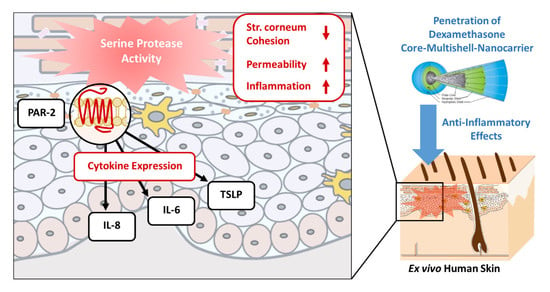
Serine Protease-Mediated Cutaneous Inflammation: Characterization of an Ex Vivo Skin Model for the Assessment of Dexamethasone-Loaded Core Multishell-Nanocarriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carrier Synthesis and Characterization
2.2. Serine Protease Treatment and Drug Application on Ex Vivo Human Skin
2.3. Protein Extraction
2.4. Analysis of Protein Extracts
2.5. Gene Expression Analysis
2.6. Histology and Immunohistochemistry
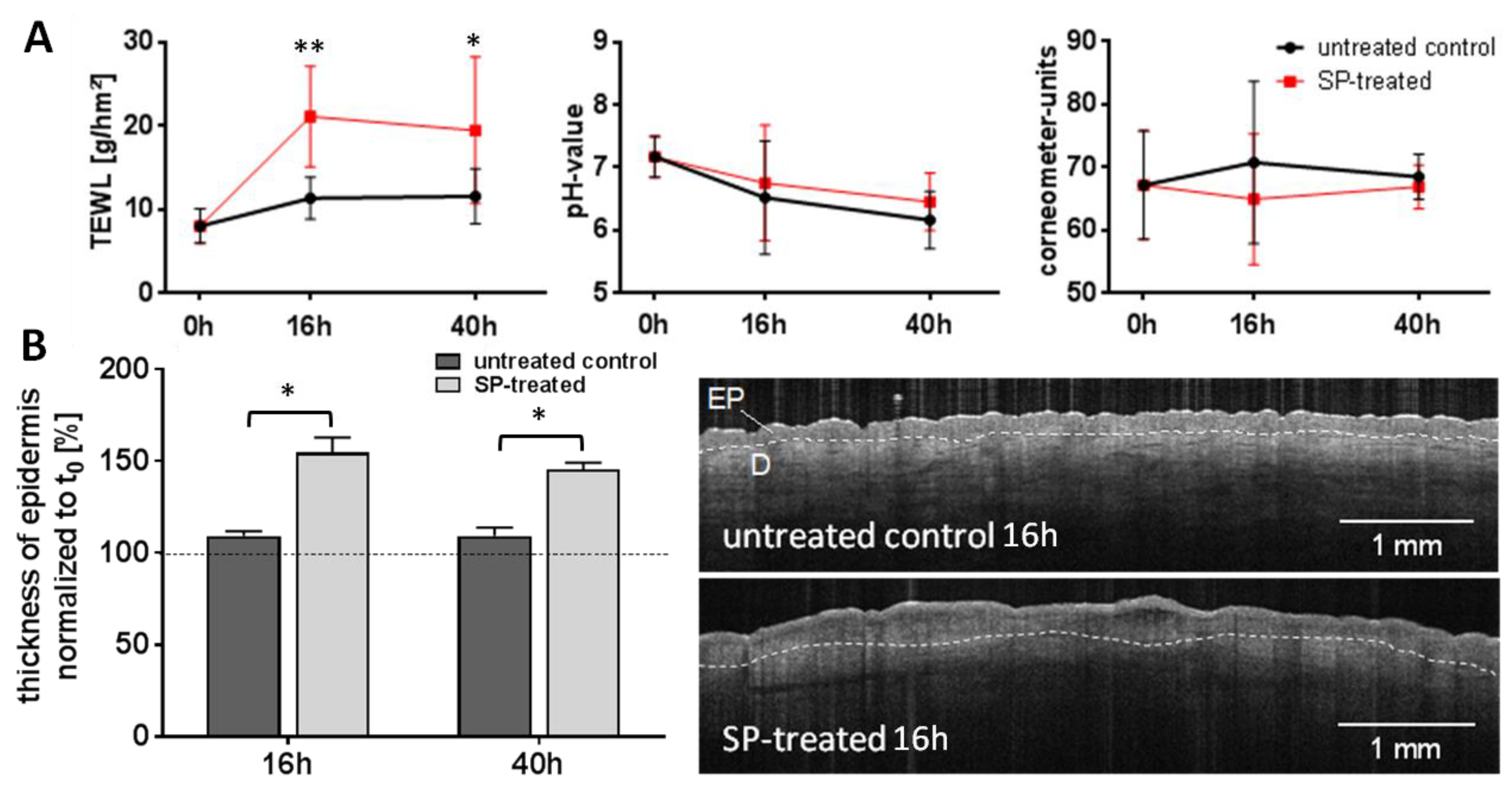
2.7. Non-Invasive Skin Assessments
2.8. Statistical Analysis
3. Results
3.1. Expression of Inflammatory Mediators in Response to Serine Protease Treatment
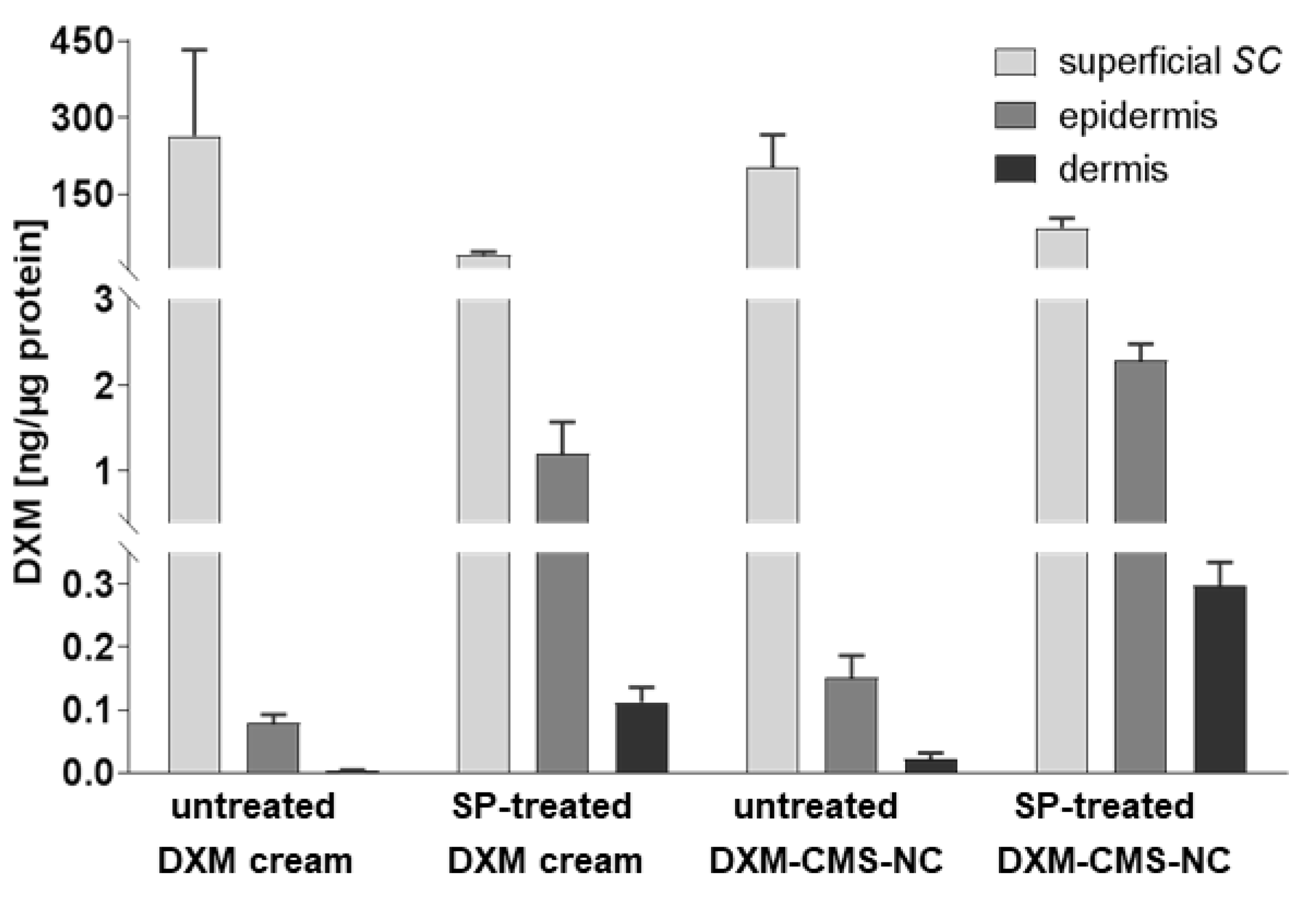
3.2. Impact of Serine Protease-Treatment on Dexamethasone Penetration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Semlin, L.; Schafer-Korting, M.; Borelli, C.; Korting, H.C. In vitro models for human skin disease. Drug Discov. Today 2011, 16, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell Biotech. 2017, 3, 21–39. [Google Scholar] [CrossRef] [Green Version]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berroth, A.; Kühnl, J.; Kurschat, N.; Schwarz, A.; Stäb, F.; Schwarz, T.; Wenck, H.; Fölster-Holst, R.; Neufang, G. Role of fibroblasts in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 1547–1554. [Google Scholar] [CrossRef]
- Eckl, K.M.; Alef, T.; Torres, S.; Hennies, H.C. Full-thickness human skin models for congenital ichthyosis and related keratinization disorders. J. Investig. Dermatol. 2011, 131, 1938–1942. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.; Weindl, G. Models of oral and vaginal candidiasis based on in vitro reconstituted human epithelia for the study of host-pathogen interactions. Methods Mol. Biol. 2009, 470, 327–345. [Google Scholar]
- Zoschke, C.; Ulrich, M.; Sochorová, M.; Wolff, C.; Vávrová, K.; Ma, N.; Ulrich, C.; Brandner, J.M.; Schäfer-Korting, M. The barrier function of organotypic non-melanoma skin cancer models. J. Control. Release 2016, 233, 10–18. [Google Scholar] [CrossRef]
- Honzke, S.; Wallmeyer, L.; Ostrowski, A.; Radbruch, M.; Mundhenk, L.; Schafer-Korting, M.; Hedtrich, S. Influence of Th2 Cytokines on the Cornified Envelope, Tight Junction Proteins, and ss-Defensins in Filaggrin-Deficient Skin Equivalents. J. Investig. Dermatol. 2016, 136, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Vávrová, K.; Henkes, D.; Strüver, K.; Sochorová, M.; Školová, B.; Witting, M.Y.; Friess, W.; Schreml, S.; Meier, R.J.; Schäfer-Korting, M.; et al. Filaggrin Deficiency Leads to Impaired Lipid Profile and Altered Acidification Pathways in a 3D Skin Construct. J. Investig. Dermatol. 2014, 134, 746–753. [Google Scholar] [CrossRef] [Green Version]
- De Wever, B.; Kurdykowski, S.; Descargues, P. Human Skin Models for Research Applications in Pharmacology and Toxicology: Introducing NativeSkin®, the “Missing Link” Bridging Cell Culture and/or Reconstructed Skin Models and Human Clinical Testing. Appl. Toxicol. 2015, 1, 26–32. [Google Scholar] [CrossRef]
- Chiang, A.; Tudela, E.; Maibach, H.I. Percutaneous absorption in diseased skin: An overview. J. Appl. Toxicol. 2012, 32, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.G. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydr Polym. 2020, 243, 116477. [Google Scholar] [CrossRef]
- Voegeli, R.; Rawlings, A.V.; Breternitz, M.; Doppler, S.; Schreier, T.; Fluhr, J.W. Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br. J. Dermatol. 2009, 161, 70–77. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U. Reddish, scaly, and itchy: How proteases and their inhibitors contribute to inflammatory skin diseases. Arch. Immunol. Ther. Exp. (Warsz.) 2009, 57, 345–354. [Google Scholar] [CrossRef]
- Two, A.M.; Hata, T.R.; Nakatsuji, T.; Coda, A.B.; Kotol, P.F.; Wu, W.; Shafiq, F.; Huang, E.Y.; Gallo, R.L. Reduction in serine protease activity correlates with improved rosacea severity in a small, randomized pilot study of a topical serine protease inhibitor. J. Investig. Dermatol. 2014, 134, 1143–1145. [Google Scholar] [CrossRef] [Green Version]
- De Veer, S.J.; Furio, L.; Harris, J.M.; Hovnanian, A. Proteases: Common culprits in human skin disorders. Trends Mol. Med. 2014, 20, 166–178. [Google Scholar] [CrossRef]
- De Veer, S.J.; Furio, L.; Harris, J.M.; Hovnanian, A. Proteases and proteomics: Cutting to the core of human skin pathologies. Proteom. Clin. Appl. 2014, 8, 389–402. [Google Scholar] [CrossRef]
- Voegeli, R.; Doppler, S.; Joller, P.; Breternitz, M.; Fluhr, J.W.; Rawlings, A.V. Increased mass levels of certain serine proteases in the stratum corneum in acute eczematous atopic skin. Int. J. Cosmet. Sci. 2011, 33, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Hachem, J.P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J. Investig. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Sun, R.; Eder, A.R.; Wakefield, J.S.; Man, M.-Q. Treating atopic dermatitis at the source: Corrective barrier repair therapy based upon new pathogenic insights. Expert Rev. Dermatol. 2013, 8, 27–36. [Google Scholar] [CrossRef]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattenholl, A.; Steinhoff, M. Proteinase-activated receptor-2 in the skin: Receptor expression, activation and function during health and disease. Drug News Perspect. 2008, 21, 369–381. [Google Scholar] [CrossRef]
- Rattenholl, A.; Steinhoff, M. Role of proteinase-activated receptors in cutaneous biology and disease. Drug Dev. Res. 2003, 59, 408–416. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.R.; Shpacovitch, V.; Rattenholl, A.; Moormann, C.; Vergnolle, N.; Luger, T.A.; Hollenberg, M.D. Proteinase-Activated Receptors: Transducers of Proteinase-Mediated Signaling in Inflammation and Immune Response. Endocr. Rev. 2005, 26, 1–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakita, H.; Furukawa, F.; Takigawa, M. Thrombin and trypsin induce granulocyte-macrophage colony-stimulating factor and interleukin-6 gene expression in cultured normal human keratinocytes. Proc. Assoc. Am. Physicians 1997, 109, 190–207. [Google Scholar] [PubMed]
- Lourbakos, A.; Potempa, J.; Travis, J.; D’Andrea, M.R.; Andrade-Gordon, P.; Santulli, R.; Mackie, E.J.; Pike, R.N. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect. Immun. 2001, 69, 5121–5130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpacovitch, V.M.; Brzoska, T.; Buddenkotte, J.; Luger, T.A.; Steinhoff, M.; Stroh, C.; Sommerhoff, C.P.; Ansel, J.C.; Schulze-Osthoff, K.; Bunnett, N.W. Agonists of Proteinase-Activated Receptor 2 Induce Cytokine Release and Activation of Nuclear Transcription Factor κB in Human Dermal Microvascular Endothelial Cells. J. Investig. Dermatol. 2002, 118, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.; Corvera, C.U.; Thoma, M.S.; Kong, W.; McAlpine, B.E.; Caughey, G.H.; Ansel, J.C.; Bunnett, N.W. Proteinase-activated receptor-2 in human skin: Tissue distribution and activation of keratinocytes by mast cell tryptase. Exp. Dermatol. 1999, 8, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kapas, S.; Cruchley, A.T.; Macey, M.G.; Harriott, P.; Chinni, C.; Stone, S.R.; Howells, G.L. Immunolocalization of protease-activated receptor-2 in skin: Receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology 1998, 94, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Nilsson, G.; Harvima, I.T. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp. Dermatol. 2010, 19, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takai, T.; Fujimura, T.; Matsuoka, H.; Ogawa, T.; Murayama, K.; Ishii, A.; Ikeda, S.; Okumura, K.; Ogawa, H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy 2009, 64, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.; Fu, Y.; He, S. Induction of interleukin-8 secretion and activation of ERK1/2, p38 MAPK signaling pathways by thrombin in dermal fibroblasts. Int. J. Biochem. Cell Biol. 2006, 38, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef]
- Hovnanian, A. Netherton syndrome: Skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013, 351, 289–300. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahrens, K.; Park, H.M.; Marsella, R. First report in a dog model of atopic dermatitis: Expression patterns of protease-activated receptor-2 and thymic stromal lymphopoietin. Vet. Dermatol. 2015, 26, 180-e37. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Nishikawa, A. P152 Synergistic activation of proteinase-activated receptor 2 and toll-like receptor 4 induces production of thymic stromal lymphopoietin production by human keratinocytes. Cytokine 2012, 59, 568. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U.; Rogalski, C.; Seifert, S.; Schmeling, G.; Wingertszahn, J.; Proksch, E.; Wiedow, O. Trypsin induces epidermal proliferation and inflammation in murine skin. Exp. Dermatol. 2004, 13, 234–241. [Google Scholar] [CrossRef]
- Li, Q.; Ke, F.; Zhang, W.; Shen, X.; Xu, Q.; Wang, H.; Yu, X.-Z.; Leng, Q.; Wang, H. Plasmin Plays an Essential Role in Amplification of Psoriasiform Skin Inflammation in Mice. PLoS ONE 2011, 6, e16483. [Google Scholar] [CrossRef]
- Frombach, J.; Unbehauen, M.; Kurniasih, I.N.; Schumacher, F.; Volz, P.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Kleuser, B.; Haag, R.; et al. Core-multishell nanocarriers enhance drug penetration and reach keratinocytes and antigen-presenting cells in intact human skin. J. Control. Release 2019, 299, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Honzke, S.; Neumann, F.; Keilitz, J.; Chen, W.; Ma, N.; Hedtrich, S.; Haag, R. Development of biodegradable hyperbranched core-multishell nanocarriers for efficient topical drug delivery. J. Control. Release 2016, 242, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Alnasif, N.; Zoschke, C.; Fleige, E.; Brodwolf, R.; Boreham, A.; Ruhl, E.; Eckl, K.M.; Merk, H.F.; Hennies, H.C.; Alexiev, U.; et al. Penetration of normal, damaged and diseased skin—An in vitro study on dendritic core-multishell nanotransporters. J. Control. Release 2014, 185, 45–50. [Google Scholar] [CrossRef]
- Unbehauen, M.L.; Fleige, E.; Paulus, F.; Schemmer, B.; Mecking, S.; Moré, S.D.; Haag, R. Biodegradable Core-Multishell Nanocarriers: Influence of Inner Shell Structure on the Encapsulation Behavior of Dexamethasone and Tacrolimus. Polymers 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadir, M.A.; Radowski, M.R.; Kratz, F.; Licha, K.; Hauff, P.; Haag, R. Dendritic multishell architectures for drug and dye transport. J. Control. Release 2008, 132, 289–294. [Google Scholar] [CrossRef]
- Kuchler, S.; Abdel-Mottaleb, M.; Lamprecht, A.; Radowski, M.R.; Haag, R.; Schafer-Korting, M. Influence of nanocarrier type and size on skin delivery of hydrophilic agents. Int. J. Pharm. 2009, 377, 169–172. [Google Scholar] [CrossRef]
- Walker, K.A.; Stumbé, J.F.; Haag, R. Polyester-Based, Biodegradable Core-Multishell Nanocarriers for the Transport of Hydrophobic Drugs. Polymers 2016, 8, 192. [Google Scholar] [CrossRef]
- Honzke, S.; Gerecke, C.; Elpelt, A.; Zhang, N.; Unbehauen, M.; Kral, V.; Fleige, E.; Paulus, F.; Haag, R.; Schafer-Korting, M.; et al. Tailored dendritic core-multishell nanocarriers for efficient dermal drug delivery: A systematic top-down approach from synthesis to preclinical testing. J. Control. Release 2016, 242, 50–63. [Google Scholar] [CrossRef]
- Yamamoto, K.; Klossek, A.; Flesch, R.; Ohigashi, T.; Fleige, E.; Rancan, F.; Frombach, J.; Vogt, A.; Blume-Peytavi, U.; Schrade, P.; et al. Core-multishell nanocarriers: Transport and release of dexamethasone probed by soft X-ray spectromicroscopy. J. Control. Release 2016, 242, 64–70. [Google Scholar] [CrossRef]
- Radbruch, M.; Pischon, H.; Ostrowski, A.; Volz, P.; Brodwolf, R.; Neumann, F.; Unbehauen, M.; Kleuser, B.; Haag, R.; Ma, N.; et al. Dendritic Core-Multishell Nanocarriers in Murine Models of Healthy and Atopic Skin. Nanoscale Res. Lett. 2017, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischon, H.; Radbruch, M.; Ostrowski, A.; Volz, P.; Gerecke, C.; Unbehauen, M.; Honzke, S.; Hedtrich, S.; Fluhr, J.W.; Haag, R.; et al. Stratum corneum targeting by dendritic core-multishell-nanocarriers in a mouse model of psoriasis. Nanomedicine 2017, 13, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Rabe, C.; Fleige, E.; Vogt, K.; Szekely, N.; Lindner, P.; Burchard, W.; Haag, R.; Ballauff, M. The multi-domain nanoparticle structure of a universal core-multishell nanocarrier. Polymer 2014, 55, 6735–6742. [Google Scholar] [CrossRef]
- Frombach, J.; Lohan, S.B.; Lemm, D.; Gruner, P.; Hasler, J.; Ahlberg, S.; Blume-Peytavi, U.; Unbehauen, M.; Haag, R.; Meinke, M.C.; et al. Protease-mediated Inflammation: An In Vitro Human Keratinocyte-based Screening Tool for Anti-inflammatory Drug Nanocarrier Systems. Z. Phys. Chem. 2018, 232, 919–933. [Google Scholar] [CrossRef]
- Vogt, A.; Hadam, S.; Deckert, I.; Schmidt, J.; Stroux, A.; Afraz, Z.; Rancan, F.; Lademann, J.; Combadiere, B.; Blume-Peytavi, U. Hair follicle targeting, penetration enhancement and Langerhans cell activation make cyanoacrylate skin surface stripping a promising delivery technique for transcutaneous immunization with large molecules and particle-based vaccines. Exp. Dermatol. 2015, 24, 73–75. [Google Scholar] [CrossRef]
- Döge, N.; Avetisyan, A.; Hadam, S.; Pfannes, E.K.B.; Rancan, F.; Blume-Peytavi, U.; Vogt, A. Assessment of skin barrier function and biochemical changes of ex vivo human skin in response to physical and chemical barrier disruption. Eur. J. Pharm. Biopharm. 2017, 116, 138–148. [Google Scholar] [CrossRef]
- Weiss, B.; Wolk, K.; Grunberg, B.H.; Volk, H.D.; Sterry, W.; Asadullah, K.; Sabat, R. Cloning of murine IL-22 receptor alpha 2 and comparison with its human counterpart. Genes Immun. 2004, 5, 330–336. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]
- Stefansson, K.; Brattsand, M.; Roosterman, D.; Kempkes, C.; Bocheva, G.; Steinhoff, M.; Egelrud, T. Activation of Proteinase-Activated Receptor-2 by Human Kallikrein-Related Peptidases. J. Investig. Dermatol. 2008, 128, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Lee, K.-P.; Park, S.-B. Preparation and characterization of poly(ester)-silver and nylon-silver nanocomposites. Stud. Surf. Sci. Catal. 2003, 146, 93–96. [Google Scholar]
- Laudanska, H.; Reduta, T.; Szmitkowska, D. Evaluation of skin barrier function in allergic contact dermatitis and atopic dermatitis using method of the continuous TEWL measurement. Rocz. Akad. Med. Bialymst. 2003, 48, 123–127. [Google Scholar]
- Addor, F.A.S.; Takaoka, R.; Rivitti, E.A.; Aoki, V. Atopic dermatitis: Correlation between non-damaged skin barrier function and disease activity. Int. J. Dermatol. 2012, 51, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early-Onset Atopic Dermatitis. JAMA Dermatol 2019, 155, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Castells-Rodellas, A.; Castell, J.V.; Ramirez-Bosca, A.; Nicolas, J.F.; Valcuende-Cavero, F.; Thivolet, J. Interleukin-6 in normal skin and psoriasis. Acta Derm. Venereol. 1992, 72, 165–168. [Google Scholar] [PubMed]
- Ackermann, L.; Harvima, I.T. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-alpha and their degranulation is associated with expression of ICAM-1 in the epidermis. Arch. Dermatol. Res. 1998, 290, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Prens, E.P.; Benne, K.; van Damme, J.; Bakkus, M.; Brakel, K.; Benner, R.; van Joost, T. Interleukin-1 and interleukin-6 in psoriasis. J. Investig. Dermatol. 1990, 95, 121S–124S. [Google Scholar] [CrossRef] [Green Version]
- Kemeny, L.; Ruzicka, T.; Dobozy, A.; Michel, G. Role of interleukin-8 receptor in skin. Int. Arch. Allergy Immunol. 1994, 104, 317–322. [Google Scholar] [CrossRef]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [Green Version]
- Bou-Dargham, M.J.; Khamis, Z.I.; Cognetta, A.B.; Sang, Q.-X.A. The Role of Interleukin-1 in Inflammatory and Malignant Human Skin Diseases and the Rationale for Targeting Interleukin-1 Alpha. Med. Res. Rev. 2017, 37, 180–216. [Google Scholar] [CrossRef]
- Ye, L.; Mauro, T.M.; Dang, E.; Wang, G.; Hu, L.Z.; Yu, C.; Jeong, S.; Feingold, K.; Elias, P.M.; Lv, C.Z.; et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: A pilot clinical study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Doge, N.; Honzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schafer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin-assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frombach, J.; Rancan, F.; Kübrich, K.; Schumacher, F.; Unbehauen, M.; Blume-Peytavi, U.; Haag, R.; Kleuser, B.; Sabat, R.; Wolk, K.; et al. Serine Protease-Mediated Cutaneous Inflammation: Characterization of an Ex Vivo Skin Model for the Assessment of Dexamethasone-Loaded Core Multishell-Nanocarriers. Pharmaceutics 2020, 12, 862. https://doi.org/10.3390/pharmaceutics12090862
Frombach J, Rancan F, Kübrich K, Schumacher F, Unbehauen M, Blume-Peytavi U, Haag R, Kleuser B, Sabat R, Wolk K, et al. Serine Protease-Mediated Cutaneous Inflammation: Characterization of an Ex Vivo Skin Model for the Assessment of Dexamethasone-Loaded Core Multishell-Nanocarriers. Pharmaceutics. 2020; 12(9):862. https://doi.org/10.3390/pharmaceutics12090862
Chicago/Turabian StyleFrombach, Janna, Fiorenza Rancan, Katharina Kübrich, Fabian Schumacher, Michael Unbehauen, Ulrike Blume-Peytavi, Rainer Haag, Burkhard Kleuser, Robert Sabat, Kerstin Wolk, and et al. 2020. "Serine Protease-Mediated Cutaneous Inflammation: Characterization of an Ex Vivo Skin Model for the Assessment of Dexamethasone-Loaded Core Multishell-Nanocarriers" Pharmaceutics 12, no. 9: 862. https://doi.org/10.3390/pharmaceutics12090862
APA StyleFrombach, J., Rancan, F., Kübrich, K., Schumacher, F., Unbehauen, M., Blume-Peytavi, U., Haag, R., Kleuser, B., Sabat, R., Wolk, K., & Vogt, A. (2020). Serine Protease-Mediated Cutaneous Inflammation: Characterization of an Ex Vivo Skin Model for the Assessment of Dexamethasone-Loaded Core Multishell-Nanocarriers. Pharmaceutics, 12(9), 862. https://doi.org/10.3390/pharmaceutics12090862