A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
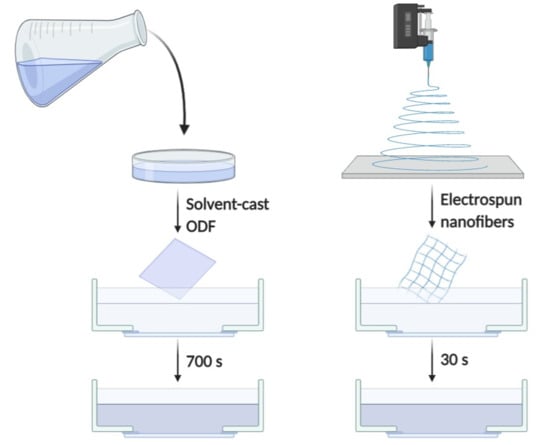
2.2.1. Preparation of Electrospun Nanofibers
2.2.2. Preparation of Solvent-Cast ODFs
2.2.3. Morphological Characteristics
2.2.4. Thermal Analysis and Solid-State Characterization
Thermogravimetric Analysis
Fourier Transform Infrared
X-ray Diffraction
2.2.5. Ultraviolet Assay for Drug Determination
2.2.6. Drug Loading (DL) and Encapsulation Efficiency (EE%)
2.2.7. Disintegration Test
2.2.8. Drug Release Study
2.2.9. E-Tongue and Bitterness Threshold Determination
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characteristics
3.2. Thermogravimetric Analysis
3.3. Fourier Transform Infrared
3.4. X-ray Diffraction
3.5. Ultraviolet Assay for Drug Determination
3.6. Drug Loading (DL) and Encapsulation Efficiency (EE%)
3.7. Disintegration Test
3.8. Drug Release Study
3.9. E-Tongue and Bitterness Threshold
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, D.-H.; Jeong, S.-H. Multi-layered matrix tablets with various tablet designs and release profiles. J. Korean Pharm. Sci. 2011, 41, 263–272. [Google Scholar] [CrossRef]
- Douroumis, D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin. Drug Deliv. 2011, 8, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Nagar, P.; Chauhan, I.; Yasir, M. Insights into polymers: Film formers in mouth dissolving films. Drug Invent. Today 2011, 3, 280–289. [Google Scholar]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, K.B.; Tan, Y.T.; Peh, K.K. Characterization of oral disintegrating film containing donepezil for alzheimer disease. AAPS PharmSciTech 2012, 13, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Barghi, L.; Jahangiri, A. Fast-dissolving nanofibers: As an emerging platform in pediatric and geriatric drug delivery. J. Adv. Chem. Pharm. Mater. (JACPM) 2018, 1, 26–28. [Google Scholar]
- Choudhary, D.R.; Patel, V.A.; Chhalotiya, U.K.; Patel, H.V.; Kundawala, A.J. Development and characterization of pharmacokinetic parameters of fast-dissolving films containing levocetirizine. Sci. Pharm. 2012, 80, 779–787. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry Orally Disintegrating Tablets-CDER Data Standards Manual. Available online: https://www.fda.gov/media/70877/download (accessed on 5 January 2021).
- Tho, I. Orally disintegrating tablets—Advantages and drawbacks. Tidsskr. Nor. Laegeforen. Tidsskr. Prakt. Med. Raekke 2012, 132, 424–425. [Google Scholar] [CrossRef] [Green Version]
- Al-Khattawi, A.; Mohammed, A.R. Challenges and emerging solutions in the development of compressed orally disintegrating tablets. Expert Opin. Drug Dis. 2014, 9, 1109–1120. [Google Scholar] [CrossRef]
- Amelian, A.; Wasilewska, K.; Wesoły, M.; Ciosek-Skibińska, P.; Winnicka, K. Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles. Saudi Pharm. J. 2017, 25, 1144–1150. [Google Scholar] [CrossRef]
- Lazar, R.B. Formulation and Evaluation of Orally Disintegrating Tablet of Taste Masked Drug. Ph.D. Thesis, Edayathangudy GS Pillay College of Pharmacy, Nagapattinam, India, 2014. [Google Scholar]
- Hesari, Z.; Shafiee, A.; Hooshfar, S.; Mobarra, N.; Mortazavi, S.A. Formulation and taste masking of ranitidine orally disintegrating tablet. Iran. J. Pharm. Res. IJPR 2016, 15, 677–686. [Google Scholar] [PubMed]
- Electronic Medicines Compendium, Prednisolone 10 mg/mL Oral Solution. 2017. Available online: https://www.medicines.org.uk/emc/product/3370/smpc#gref (accessed on 18 November 2020).
- Bíró, T.; Horvát, G.; Budai-Szűcs, M.; Csányi, E.; Urbán, E.; Facskó, A.; Szabó-Révész, P.; Csóka, I.; Aigner, Z. Development of prednisolone-containing eye drop formulations by cyclodextrin complexation and antimicrobial, mucoadhesive biopolymer. Drug Des. Dev. Ther. 2018, 12, 2529–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Kanjwal, M.A.; Lin, L.; Chronakis, I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces 2013, 103, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Deliverynull; UCL Press: London, UK, 2018. [Google Scholar]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Electrospinning optimization of eudragit e po with and without chlorpheniramine maleate using a design of experiment approach. Mol. Pharm. 2019, 16, 2557–2568. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Drug-loaded electrospun mats of poly (vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology 2006, 17, 2317. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (pva). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.C.; Wolffsohn, J.S.; Nick, J.; Winterton, L.; Lally, J. Clinical performance of daily disposable soft contact lenses using sustained release technology. Contact Lens Anterior Eye 2006, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chang, Y.S.; Oka, M. A two-year in vivo study of polyvinyl alcohol-hydrogel (pva-h) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Cedarstaff, T.H.; Tomlinson, A. A comparative study of tear evaporation rates and water content of soft contact lenses. Am. J. Optom. Physiol. Opt. 1983, 60, 167–174. [Google Scholar] [CrossRef]
- Sorbara, L.; Simpson, T.; Vaccari, S.; Jones, L.; Fonn, D. Tear turnover rate is reduced in patients with symptomatic dry eye. Contact Lens Anterior Eye 2004, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Nyúl, K.; Wagner, I.; Molnár, K.; Marosi, G. Electrospun water soluble polymer mat for ultrafast release of donepezil hcl. Express Polym. Lett. 2010, 4, 763–772. [Google Scholar] [CrossRef]
- Thitiwongsawet, P.; Supaphol, P. Carbendazim-loaded electrospun poly(vinyl alcohol) fiber mats and release characteristics of carbendazim therefrom. Polym. Adv. Technol. 2011, 22, 1366–1374. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Williams, G.R.; Craig, D.Q.M.; Orlu, M.; Tuleu, C. Human mouthfeel panel investigating the acceptability of electrospun and solvent cast orodispersible films. Int. J. Pharm. 2020, 585, 119532. [Google Scholar] [CrossRef]
- Aljebab, F.; Alanazi, M.; Choonara, I.; Conroy, S. Observational study on the palatability and tolerability of oral prednisolone and oral dexamethasone in children in saudi arabia and the uk. Arch. Dis. Child. 2018, 103, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.Y.; Keeney, M.P. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int. J. Pharm. 2006, 310, 118–124. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the electrospinning of poly(vinyl alcohol) nanofiber mats: A revisit. J. Appl. Polym. Sci. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New crosslinked cast films based on poly (vinyl alcohol): Preparation and physico-chemical properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Pinďáková, L.; Kašpárková, V.; Kejlová, K.; Dvořáková, M.; Krsek, D.; Jírová, D.; Kašparová, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bhusnure, O.G.; Gholve, S.; Manoj, B.; Todkar, V.; Giram, P.S. Analytical method development and validation of prednisolone sodium phosphate by qbd approach. IOSR J. Pharm. Biol. Sci. 2015, 10, 64–75. [Google Scholar]
- Tawfik, E.A.; Craig, D.Q.M.; Barker, S.A. Dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm. 2020, 581, 119296. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, J.; Keeley, A.; Keating, A.V.; Mohamed-Ahmed, A.H.A.; Sheng, Y.; Winzenburg, G.; Turner, R.; Desset-Brèthes, S.; Orlu, M.; Tuleu, C. Rats can predict aversiveness of active pharmaceutical ingredients. Eur. J. Pharm. Biopharm. 2018, 133, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous pva produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Sukyte, J.; Adomaviciute, E.; Milasius, R.; Bendoraitiene, J.; Danilovas, P.P. Formation of poly (vinyl alcohol)/cationic starch blend nanofibres via the electrospinning technique: The influence of different factors. Fibres Text. East. Eur. 2012, 3, 16–20. [Google Scholar]
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Antibacterial activity and inhibition of adherence of streptococcus mutans by propolis electrospun fibers. AAPS PharmSciTech 2015, 16, 182–191. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- ElShaer, A.; Mustafa, S.; Kasar, M.; Thapa, S.; Ghatora, B.; Alany, R.G. Nanoparticle-laden contact lens for controlled ocular delivery of prednisolone: Formulation optimization using statistical experimental design. Pharmaceutics 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.L.; Dasari, A.; Kong, L.B. Chinese ink-facilitated fabrication of carbon nanotube/polyvinyl alcohol composite sheets with a high nanotube loading. Compos. Part A Appl. Sci. Manuf. 2014, 61, 209–215. [Google Scholar] [CrossRef]
- De Gennes, P.-G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827. [Google Scholar] [CrossRef]
- Zuo, B.; Hu, Y.; Lu, X.; Zhang, S.; Fan, H.; Wang, X. Surface properties of poly(vinyl alcohol) films dominated by spontaneous adsorption of ethanol and governed by hydrogen bonding. J. Phys. Chem. C 2013, 117, 3396–3406. [Google Scholar] [CrossRef]
- Yazgan, G.; Dmitriev, R.I.; Tyagi, V.; Jenkins, J.; Rotaru, G.M.; Rottmar, M.; Rossi, R.M.; Toncelli, C.; Papkovsky, D.B.; Maniura-Weber, K.; et al. Steering surface topographies of electrospun fibers: Understanding the mechanisms. Sci. Rep. 2017, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- González, E.; Shepherd, L.M.; Saunders, L.; Frey, M.W. Surface functional poly(lactic acid) electrospun nanofibers for biosensor applications. Materials 2016, 9, 47. [Google Scholar] [CrossRef]
- Yuan, Y.; Shu, J.; Kolman, K.; Kiersnowski, A.; Bubeck, C.; Zhang, J.; Hansen, M.R. Multiple chain packing and phase composition in regioregular poly(3-butylthiophene) films. Macromolecules 2016, 49, 9493–9506. [Google Scholar] [CrossRef]
- Palanisamy, M.; Khanam, J. Solid dispersion of prednisolone: Solid state characterization and improvement of dissolution profile. Drug Dev. Ind. Pharm. 2011, 37, 373–386. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, 21. [Google Scholar] [CrossRef]
- El-Setouhy, D.A.; Abd El-Malak, N.S. Formulation of a novel tianeptine sodium orodispersible film. AAPS PharmSciTech 2010, 11, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- ElMeshad, A.N.; El Hagrasy, A.S. Characterization and optimization of orodispersible mosapride film formulations. AAPS PharmSciTech 2011, 12, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharm. Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ma, J.; Jiang, L. Manipulating and dispensing micro/nanoliter droplets by superhydrophobic needle nozzles. ACS Nano 2013, 7, 10371–10379. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.A.; Ranganath, A.S.; Baji, A.; Wong, H.C.; Raut, H.K.; Sahay, R.; Ramakrishna, S. Electrospun differential wetting membranes for efficient oil–water separation. Macromol. Mater. Eng. 2016, 301, 812–817. [Google Scholar] [CrossRef]
- Scheubel, E.; Lindenberg, M.; Beyssac, E.; Cardot, J.M. Small volume dissolution testing as a powerful method during pharmaceutical development. Pharmaceutics 2010, 2, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Krstić, M.; Radojević, M.; Stojanović, D.; Radojević, V.; Uskoković, P.; Ibrić, S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef]
- Adinarayana, N.; Raj, S.B.; Rajasekhar, M.; Reddy, K.B.; Mohanambal, E. Formulation and evaluation of prednisolone sodium phosphate orally disintegrating tablets. Int. J. Res. Pharm. Sci. 2011, 2, 192–199. [Google Scholar]
- Ekweremadu, C.S.; Abdelhakim, H.E.; Craig, D.Q.M.; Barker, S.A. Development and evaluation of feline tailored amlodipine besylate mini-tablets using l-lysine as a candidate flavouring agent. Pharmaceutics 2020, 12, 917. [Google Scholar] [CrossRef] [PubMed]










| Formulation | DL (µg/mg) | EE% |
|---|---|---|
| Nanofibers | 146.2 ± 1.8 | 87.5 ± 1.1 |
| Solvent-cast ODFs | 167.2 ± 2.4 | 100.1 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfik, E.A.; Scarpa, M.; Abdelhakim, H.E.; Bukhary, H.A.; Craig, D.Q.M.; Barker, S.A.; Orlu, M. A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets. Pharmaceutics 2021, 13, 120. https://doi.org/10.3390/pharmaceutics13010120
Tawfik EA, Scarpa M, Abdelhakim HE, Bukhary HA, Craig DQM, Barker SA, Orlu M. A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets. Pharmaceutics. 2021; 13(1):120. https://doi.org/10.3390/pharmaceutics13010120
Chicago/Turabian StyleTawfik, Essam A., Mariagiovanna Scarpa, Hend E. Abdelhakim, Haitham A. Bukhary, Duncan Q. M. Craig, Susan A. Barker, and Mine Orlu. 2021. "A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets" Pharmaceutics 13, no. 1: 120. https://doi.org/10.3390/pharmaceutics13010120
APA StyleTawfik, E. A., Scarpa, M., Abdelhakim, H. E., Bukhary, H. A., Craig, D. Q. M., Barker, S. A., & Orlu, M. (2021). A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets. Pharmaceutics, 13(1), 120. https://doi.org/10.3390/pharmaceutics13010120