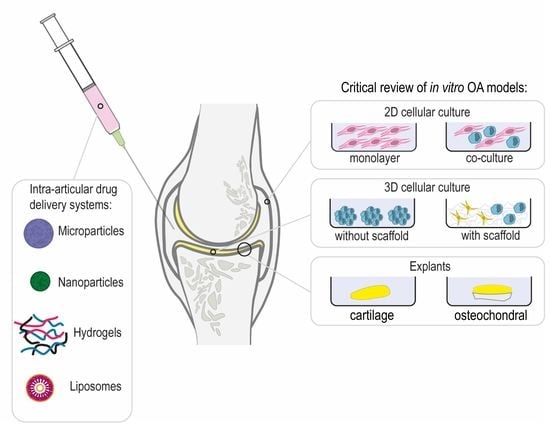
Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems
Abstract
1. Introduction
2. Osteoarthritis
3. Intra-Articular Drug Delivery Systems and Interactions with OA Joints
4. In Vitro Models of OA
4.1. 2D Cellular Models
4.1.1. Monolayer Culture
4.1.2. Co-Culture
4.2. 3D Cellular Models
4.2.1. 3D Cellular Models without Scaffold
4.2.2. 3D Cellular Models with Scaffold
4.3. Explants
4.4. Considerations on OA In Vitro Models for Development of IA DDSs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Koszowska, A.; Hawranek, R.; Nowak, J. Osteoarthritis -a multifactorial issue. Reumatologia 2014, 52, 319–325. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Rockel, J.S.; Kapoor, M. Understanding osteoarthritis pathogenesis: A multiomics system-based approach. Curr. Opin. Rheumatol. 2020, 32, 80–91. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- Gómez-Gaete, C.; Retamal, M.; Chávez, C.; Bustos, P.; Godoy, R.; Torres-Vergara, P. Development, characterization and in vitro evaluation of biodegradable rhein-loaded microparticles for treatment of osteoarthritis. Eur. J. Pharm. Sci. 2017, 96, 390–397. [Google Scholar] [CrossRef]
- Lin, J.B.; Poh, S.; Panitch, A. Controlled release of anti-inflammatory peptides from reducible thermosensitive nanoparticles suppresses cartilage inflammation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2095–2100. [Google Scholar] [CrossRef]
- Maudens, P.; Meyer, S.; Seemayer, C.A.; Jordan, O.; Allémann, E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale 2018, 10, 1845. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, D.; Wang, Z.; Wu, G.; Miao, L.; Huang, L. Intra-articular delivery of liposomal celecoxib–hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int. J. Pharm. 2013, 441, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Emery, C.A.; Whittaker, J.L.; Mahmoudian, A.; Lohmander, L.S.; Roos, E.M.; Bennell, K.L.; Toomey, C.M.; Reimer, R.A.; Thompson, D.; Ronsky, J.L.; et al. Establishing outcome measures in early knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 438–448. [Google Scholar] [CrossRef]
- Brakke, R.; Singh, J.; Sullivan, W. Physical Therapy in Persons With Osteoarthritis. PM R 2012, 4, S53–S58. [Google Scholar] [CrossRef]
- Allen, K.D.; Choong, P.F.; Davis, A.M.; Dowsey, M.M.; Dziedzic, K.S.; Emery, C.; Hunter, D.J.; Losina, E.; Page, A.E.; Roos, E.M.; et al. Osteoarthritis: Models for appropriate care across the disease continuum. Best Pract. Res. Clin. Rheumatol. 2016, 30, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Deshpande, B.R.; Collins, J.E.; Katz, J.N.; Losina, E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthr. Cartil. 2016, 24, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Naidoo, P. Viscosupplementation for knee osteoarthritis: A focus on hylan G-F 20. Orthop. Res. Rev. 2018, 10, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Leong, N.L.; Cole, B.J. The Use of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis. J. Knee Surg. 2019, 32, 37–45. [Google Scholar] [CrossRef]
- Geiger, B.C.; Grodzinsky, A.J.; Hammond, P. Designing drug delivery systems for articular joints. Chem. Eng. Prog. 2018, 114, 46–51. [Google Scholar]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Drug Delivery Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019. [Google Scholar] [CrossRef]
- Kang, M.L.; Ko, J.Y.; Kim, J.E.; Im, G. Il Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef]
- Maudens, P.; Jordan, O.; Allémann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef]
- Rai, M.F.; Pham, C.T. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018, 40, 67–73. [Google Scholar] [CrossRef]
- Brown, S.; Kumar, S.; Sharma, B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef]
- Mancipe Castro, L.M.; García, A.J.; Guldberg, R.E. Biomaterial strategies for improved intra-articular drug delivery. J. Biomed. Mater. Res. Part. A 2020. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Timur, U.T.; Woike, N.; Welting, T.J.M.; Draaisma, G.; Gijbels, M.; van Rhijn, L.W.; Mihov, G.; Thies, J.; Emans, P.J. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J. Control. Release 2016, 244, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gu, L.; Ren, L.; Chen, J.; Li, T.; Wang, X.; Yang, J.; Chen, C.; Sun, L. Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am. J. Transl. Res. 2019, 11, 6775–6789. [Google Scholar]
- Petit, A.; Redout, E.M.; van de Lest, C.H.; de Grauw, J.C.; Müller, B.; Meyboom, R.; van Midwoud, P.; Vermonden, T.; Hennink, W.E.; René van Weeren, P. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials 2015, 53, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-L.; Kim, J.-E.; Im, G.-I. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016, 39, 65–78. [Google Scholar] [CrossRef]
- Steven, R.; Goldring, M.B.G. Kelley’s Textbook of Rheumatology; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J., Eds.; Saunders: Broomall, PA, USA, 2013; pp. 1–19. ISBN 9781455737673. [Google Scholar]
- Marieb, E.N.; Wilhelm, P.B.; Mallatt, J. Human Anatomy; Person: London, UK, 2017; ISBN 9780134243818. [Google Scholar]
- Piluso, S.; Li, Y.; Abinzano, F.; Levato, R.; Teixeira, L.M.; Karperien, M.; Leijten, J.; Van Weeren, R.; Malda, J. Mimicking the Articular Joint with In Vitro Models. Trends Biotechnol. 2019, 37, 1063–1077. [Google Scholar] [CrossRef]
- Tellier, L.E.; Treviño, E.A.; Brimeyer, A.L.; Reece, D.S.; Willett, N.J.; Guldberg, R.E.; Temenoff, J.S. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis †. Biomater. Sci. 2018, 6, 1159. [Google Scholar] [CrossRef]
- Pradal, J.; Zuluaga, M.-F.; Maudens, P.; Waldburger, J.-M.; Seemayer, C.A.; Doelker, E.; Gabay, C.; Jordan, O.; Allémann, E. Intra-articular bioactivity of a p38 MAPK inhibitor and development of an extended-release system. Eur. J. Pharm. Biopharm. 2015, 93, 110–117. [Google Scholar] [CrossRef]
- Goldring, M.B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000, 43, 1916–1926. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Maudens, P.; Seemayer, C.A.; Thauvin, C.; Gabay, C.; Jordan, O.; Allémann, E. Nanocrystal-Polymer Particles: Extended Delivery Carriers for Osteoarthritis Treatment. Small 2018, 14, 1703108. [Google Scholar] [CrossRef] [PubMed]
- Aydin, O.; Korkusuz, F.; Korkusuz, P.; Tezcaner, A.; Bilgic, E.; Yaprakci, V.; Keskin, D. In vitro and in vivo evaluation of doxycycline-chondroitin sulfate/PCLmicrospheres for intraarticular treatment of osteoarthritis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.; Indulekha, S.; Vijayalakshmi, S.; Srivastava, R. Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles-a potential Intra-articular drug delivery system for the treatment of Osteoarthritis. J. Biomater. Sci. Polym. Ed. 2016, 27, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, H.; Kamel, A.O.; Sammour, O.A. Injectable long acting chitosan/tripolyphosphate microspheres for the intra-articular delivery of lornoxicam: Optimization and in vivo evaluation. Carbohydr. Polym. 2016, 149, 263–273. [Google Scholar] [CrossRef]
- Goto, N.; Okazaki, K.; Akasaki, Y.; Ishihara, K.; Murakami, K.; Koyano, K.; Ayukawa, Y.; Yasunami, N.; Masuzaki, T.; Nakashima, Y. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J. Orthop. Res. 2017, 35, 2465–2475. [Google Scholar] [CrossRef]
- Maudens, P.; Seemayer, C.A.; Pfefferlé, F.; Jordan, O.; Allémann, E. Nanocrystals of a potent p38 MAPK inhibitor embedded in microparticles: Therapeutic effects in inflammatory and mechanistic murine models of osteoarthritis. J. Control. Release 2018, 276, 102–112. [Google Scholar] [CrossRef]
- Kumar, A.; Bendele, A.M.; Blanks, R.C.; Bodick, N. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthr. Cartil. 2015, 23, 151–160. [Google Scholar] [CrossRef]
- Kraus, V.B.; Conaghan, P.G.; Aazami, H.A.; Mehra, P.; Kivitz, A.J.; Lufkin, J.; Hauben, J.; Johnson, J.R.; Bodick, N. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 34–42. [Google Scholar] [CrossRef]
- Getgood, A.; Dhollander, A.; Malone, A.; Price, J.; Helliwell, J. Pharmacokinetic Profile of Intra-articular Fluticasone Propionate Microparticles in Beagle Dog Knees. Cartilage 2019, 10, 139–147. [Google Scholar] [CrossRef]
- Salgado, C.; Guénée, L.; Černý, R.; Allémann, E.; Jordan, O. Nano wet milled celecoxib extended release microparticles for local management of chronic inflammation. Int. J. Pharm. 2020, 589. [Google Scholar] [CrossRef]
- Dhanabalan, K.M.; Gupta, V.K.; Agarwal, R. Rapamycin-PLGA microparticles prevent senescence, sustain cartilage matrix production under stress and exhibit prolonged retention in mouse joints. Biomater. Sci. 2020, 8, 4308–4321. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, A.G.; Quadir, M.A.; Hammond, P.T.; Grodzinsky, A.J. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr. Cartil. 2016, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Niazvand, F.; Khorsandi, L.; Abbaspour, M.; Orazizadeh, M.; Varaa, N.; Maghzi, M.; Ahmadi, K. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on mono-iodoacetate-induced osteoarthritis in rats. Vet. Res. Forum 2017, 8, 155–161. [Google Scholar]
- Bajpayee, A.G.; De La Vega, R.E.; Scheu, M.; Varady, N.H.; Yannatos, I.A.; Brown, L.A.; Krishnan, Y.; Fitzsimons, T.J.; Bhattacharya, P.; Frank, E.H.; et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of posttraumatic osteoarthritis. Eur. Cells Mater. 2017, 34, 341–364. [Google Scholar] [CrossRef]
- Mcmasters, J.; Poh, S.; Lin, J.B.; Panitch, A. Delivery of Anti-inflammatory Peptides from Hollow PEGylated Poly(NIPAM) Nanoparticles Reduces Inflammation in an Ex Vivo Osteoarthritis Model Graphical abstract HHS Public Access. J. Control. Release 2017, 258, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, Q.; Yan, X.; Ding, B.; Chen, D.; Cheng, L. Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage. Nanomedicine 2018, 13, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, J.; Yuan, L.; Chen, J.; Wang, Z.; Wang, Y.; Guo, C.; Mo, X.; Yan, Z. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018, 25, 1004–1012. [Google Scholar] [CrossRef]
- Liu, X.; Corciulo, C.; Arabagian, S.; Ulman, A.; Cronstein, B.N. Adenosine-Functionalized Biodegradable PLA-b-PEG Nanoparticles Ameliorate Osteoarthritis in Rats. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Mota, A.H.; Direito, R.; Carrasco, M.P.; Rijo, P.; Ascensão, L.; Viana, A.S.; Rocha, J.; Eduardo-Figueira, M.; Rodrigues, M.J.; Custódio, L.; et al. Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis. Int. J. Pharm. 2019, 559, 13–22. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef]
- Jin, T.; Wu, D.; Liu, X.M.; Xu, J.T.; Ma, B.J.; Ji, Y.; Jin, Y.Y.; Wu, S.Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, S.E.; Kim, H.J.; Park, K.; Song, G.G.; Choi, S.J. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int. J. Pharm. 2020, 581. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Abdelkhalek, A.A.; Elkasabgy, N.A. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int. J. Pharm. 2020, 578. [Google Scholar] [CrossRef] [PubMed]
- Deloney, M.; Smart, K.; Christiansen, B.A.; Panitch, A. Thermoresponsive, hollow, degradable core-shell nanoparticles for intra-articular delivery of anti-inflammatory peptide. J. Control. Release 2020, 323, 47–58. [Google Scholar] [CrossRef]
- Alarçin, E.; Demirbağ, Ç.; Karsli-Ceppioglu, S.; Kerimoğlu, O.; Bal-Ozturk, A. Development and characterization of oxaceprol-loaded poly-lactide-co-glycolide nanoparticles for the treatment of osteoarthritis. Drug Dev. Res. 2020, 81, 501–510. [Google Scholar] [CrossRef]
- She, P.; Bian, S.; Cheng, Y.; Dong, S.; Liu, J.; Liu, W.; Xiao, C. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. Int. J. Biol. Macromol. 2020, 158, 1082–1089. [Google Scholar] [CrossRef]
- Shan-Bin, G.; Yue, T.; Ling-Yan, J. Long-term sustained-released in situ gels of a water-insoluble drug amphotericin B for mycotic arthritis intra-articular administration: Preparation, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2015, 41, 573–582. [Google Scholar] [CrossRef]
- Reum Son, A.; Kim, D.Y.; Hun Park, S.; Yong Jang, J.; Kim, K.; Ju Kim, B.; Yun Yin, X.; Ho Kim, J.; Hyun Min, B.; Keun Han, D.; et al. Direct chemotherapeutic dual drug delivery through intra-articular injection for synergistic enhancement of rheumatoid arthritis treatment. Sci. Rep. 2015, 5, 14713. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, X.; Ma, P.; Tao, Y.; Wu, X.; Wu, X.; Chu, X.; Gui, S. Phytantriol-Based In Situ Liquid Crystals with Long-Term Release for Intra-articular Administration. AAPS PharmSciTech 2015, 16. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Gao, J.; Zhao, Y.; Zhao, Y.; Guo, L.; Chen, C.; Duan, Z.; Li, P.; Wei, L. Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: An experimental study in a rat model of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 411. [Google Scholar] [CrossRef]
- Kang, M.L.; Jeong, S.Y.; Im, G. Il Hyaluronic Acid Hydrogel Functionalized with Self-Assembled Micelles of Amphiphilic PEGylated Kartogenin for the Treatment of Osteoarthritis. Tissue Eng. Part A 2017, 23, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Cokelaere, S.M.; Plomp, S.G.M.; de Boef, E.; de Leeuw, M.; Bool, S.; van de Lest, C.H.A.; van Weeren, P.R.; Korthagen, N.M. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur. J. Pharm. Biopharm. 2018, 128, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A.; et al. Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsushita, T.; Nishida, K.; Takayama, K.; Nagai, K.; Araki, D.; Matsumoto, T.; Tabata, Y.; Kuroda, R. Attenuation of osteoarthritis progression in mice following intra-articular administration of simvastatin-conjugated gelatin hydrogel. J. Tissue Eng. Regen. Med. 2019, 13, 423–432. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; El-Ganainy, S.O. Thermoresponsive Hyalomer intra-articular hydrogels improve monoiodoacetate-induced osteoarthritis in rats. Int. J. Pharm. 2020, 573, 118859. [Google Scholar] [CrossRef]
- Kawanami, T.; LaBonte, L.R.; Amin, J.; Thibodeaux, S.J.; Lee, C.C.; Argintaru, O.A.; Adams, C.M. A novel diclofenac-hydrogel conjugate system for intraarticular sustained release: Development of 2-pyridylamino-substituted 1-phenylethanol (PAPE) and its derivatives as tunable traceless linkers. Int. J. Pharm. 2020, 585, 119519. [Google Scholar] [CrossRef]
- Tsubosaka, M.; Kihara, S.; Hayashi, S.; Nagata, J.; Kuwahara, T.; Fujita, M.; Kikuchi, K.; Takashima, Y.; Kamenaga, T.; Kuroda, Y.; et al. Gelatin hydrogels with eicosapentaenoic acid can prevent osteoarthritis progression in vivo in a mouse model. J. Orthop. Res. 2020, 38, 2157–2169. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Shou, X.; Ni, D.; Kong, T.; Zhao, Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale 2020, 12, 17093–17102. [Google Scholar] [CrossRef]
- Mok, S.W.; Fu, S.C.; Cheuk, Y.C.; Chu, I.M.; Chan, K.M.; Qin, L.; Yung, S.H.; Kevin Ho, K.W. Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model. Cartilage 2020, 11, 490–499. [Google Scholar] [CrossRef]
- Sarkar, A.; Carvalho, E.; D’Souza, A.A.; Banerjee, R. Liposome-encapsulated fish oil protein-tagged gold nanoparticles for intra-articular therapy in osteoarthritis. Nanomedicine 2019, 14, 871–887. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Sun, T.; Zhang, Q.; Wang, Y.; Zhang, M.; Zhang, H.; Zhao, X. Glucosamine sulphate-loaded distearoyl phosphocholine liposomes for osteoarthritis treatment: Combination of sustained drug release and improved lubrication. Biomater. Sci. 2019, 7, 2716–2728. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ming Kuo, S.; Tien, Y.-C.; Shen, P.-C.; Kuo, Y.-W.; Hsiang Huang, H. Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound. Int. J. Nanomed. 2020. [Google Scholar] [CrossRef] [PubMed]
- Verbost, P.M.; Van Der Valk, J.; Hendriksen, C.F.M. Effects of the introduction of in vitro assays on the use of experimental animals in pharmacological research. ATLA Altern. Lab. Anim. 2007, 35, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of Osteoarthritis: Relevance and New Insights. Calcif. Tissue Int. 2020, 1, 3. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Bastiaansen-Jenniskens, Y.M.; Koevoet, W.; Saris, D.B.F.; Dhert, W.J.A.; Creemers, L.B.; Van Osch, G.J.V.M. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage: Establishment and characterization of a long-term cartilage-synovium coculture. Arthritis Rheum. 2011, 63, 1918–1927. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Dionisio, K.L.; Jiang, J.; Lu, H.H. Osteoblast and Chondrocyte Interactions during Coculture on Scaffolds. IEEE Eng. Med. Biol. Mag. 2003, 22, 27–34. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef]
- Geurts, J.; Jurić, D.; Müller, M.; Schären, S.; Netzer, C. Novel Ex Vivo Human Osteochondral Explant Model of Knee and Spine Osteoarthritis Enables Assessment of Inflammatory and Drug Treatment Responses. Int. J. Mol. Sci. 2018, 19, 1314. [Google Scholar] [CrossRef]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway Protective Effects of MicroRNAs on Human Chondrocytes in an In Vitro Model of Osteoarthritis. Mol. Ther. Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef]
- Thysen, S.; Luyten, F.P.; Lories, R.J.U. Targets, models and challenges in osteoarthritis research. DMM Dis. Model. Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Riesle, J.; van Blitterswijk, C.A. Co-culture in cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2007, 1, 170–178. [Google Scholar] [CrossRef]
- Ziadlou, R.; Barbero, A.; Stoddart, M.J.; Wirth, M.; Li, Z.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Regulation of Inflammatory Response in Human Osteoarthritic Chondrocytes by Novel Herbal Small Molecules. Int. J. Mol. Sci. 2019, 20, 5745. [Google Scholar] [CrossRef]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Martin, I. Human articular chondrocytes culture. In Methods in Molecular Medicine; Hauser, H., Fussenegger, M., Eds.; Human Press: Totowa, NJ, USA, 2007; Volume 140, pp. 237–247. [Google Scholar]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, H.; Li, J.; Feng, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Bioactive scaffolds for regeneration of cartilage and subchondral bone interface. Theranostics 2018, 8, 1940–1955. [Google Scholar] [CrossRef] [PubMed]
- Bicho, D.; Pina, S.; Oliveira, J.M.; Reis, R.L. In vitro mimetic models for the bone-cartilage interface regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1059, pp. 373–394. [Google Scholar]
- Pradal, J.; Maudens, P.; Gabay, C.; Seemayer, C.A.; Jordan, O.; Allémann, E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int. J. Pharm. 2016, 498, 119–129. [Google Scholar] [CrossRef]
- Smith, D.; Herman, C.; Razdan, S.; Abedin, M.R.; Van Stoecker, W.; Barua, S. Microparticles for Suspension Culture of Mammalian Cells. ACS Appl. Bio Mater. 2019. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Wu, M.; Fu, A. Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater. Sci. 2019, 7, 3143–3157. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Z.; Li, E.N.; Li, X.; Del Duke, C.J.; Shen, H.; Hao, T.; O’Donnell, B.; Bunnell, B.A.; Goodman, S.B.; et al. Osteochondral Tissue Chip Derived From iPSCs: Modeling OA Pathologies and Testing Drugs. Front. Bioeng. Biotechnol. 2019, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Mainardi, A.; Votta, E.; Vallmajo-Martin, Q.; Ehrbar, M.; Martin, I.; Barbero, A.; Rasponi, M. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat. Biomed. Eng. 2019, 3, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Rosser, J.; Bachmann, B.; Jordan, C.; Ribitsch, I.; Haltmayer, E.; Gueltekin, S.; Junttila, S.; Galik, B.; Gyenesei, A.; Haddadi, B.; et al. Microfluidic nutrient gradient–based three-dimensional chondrocyte culture-on-a-chip as an in vitro equine arthritis model. Mater. Today Bio 2019, 4, 100023. [Google Scholar] [CrossRef]

| Formulation | Drug | Carrier | Type of Study | Main Target Tissue | In Vitro Model | In Vivo Model | Authors; Year; References |
|---|---|---|---|---|---|---|---|
| Microparticles | Doxycycline | PCL | Pre-clinical studies | Cartilage | 3D rabbit chondrocyte agarose model | Rabbits | Aydin et al. 2015 [44] |
| Celecoxib | PEA | Pre-clinical studies | Synovium | Differentiated HI-60 cells and lysates | Human synovium and synovial fluid (ex vivo); rat ACLT model | Janssen et al. 2016 [32] | |
| Etoricoxib | PCL | Pre-clinical studies | Synovium, cartilage | Not reported | Rats | Arunkumar et al. 2016 [45] | |
| Lornoxicam | Chitosan/TPP | Pre-clinical studies | Synovium, cartilage | Not reported | Rat MIA model | Abd-Allah et al. 2016 [46] | |
| Fluvastatin | PLGA | Pre-clinical studies | Cartilage | Human primary chondrocytes | Rabbit ACLT model | Goto et al. 2017 [47] | |
| Rhein (cassic acid) | PLGA | Pre-clinical studies | Synovium | THP-1 macrophages | Not reported | Gomez-Gaete et al. 2017 [8] | |
| Kartogenin | PLA | Pre-clinical studies | Cartilage | Human synoviocytes | Mice DMM model | Maudens et al. 2018 [43] | |
| PH-797804, Dexamethasone | PLA | Pre-clinical studies | Synovium | Human synoviocytes | Mice AIA model | Maudens et al. 2018 [48] | |
| Triamcinolone acetonide (Zilretta™) | PLGA | Phase II/III clinical trials in OA patients 1 | Synovium, cartilage | Not reported | Rat knee model 2 | Kumar et al. 2015 2; Kraus et al. 2018 1 [49,50] | |
| TSG-6 (tumor necrosis factor-alpha stimulated gene-6) | Heparin | Pre-clinical studies | Cartilage | Not reported | Rat MMT model | Tellier et al. 2018 [39] | |
| Fluticasone propionate | PVA | Pre-clinical studies | Synovium | Not reported | Beagle dogs | Getgood et al. 2019 [51] | |
| Celecoxib | PLA | Pre-clinical studies | Synovium | Human synoviocytes | Not reported | Salgado et al. 2020 [52] | |
| Rapamycin | PLGA | Pre-clinical studies | Cartilage | Human immortal chondrocytes | Mice | Dhanabalan et al. 2020 [53] | |
| Nanoparticles | VX-745 (p38 MAPK inhibitor) | PLA and PLGA | Pre-clinical studies | Synovium | Human synoviocytes | Mice AIA model | Pradal et al. 2015 [40] |
| Dexamethasone | Avidin/PEG | Pre-clinical studies | Synovium, cartilage | Bovine knee cartilage explants | Not reported | Bajpayee et al. 2016 [54] | |
| KAFAK (anti-inflammatory mitogen-activated protein kinase-activated protein kinase 2 (MK2)-inhibiting cell-penetrating peptide) | pNiPAM-PEG | Pre-clinical studies | Synovium, cartilage | Bovine knee cartilage explants | Not reported | Lin et al. 2016 [9] | |
| Kartogenin; Diclofenac | Chitosan/Pluronic F127 | Pre-clinical studies | Synovium, cartilage | Human BMSCs (bone marrow mesenchymal stem cells); Human primary chondrocytes | Rats | Kang et al. 2016 [35] | |
| Curcumin | PLGA | Pre-clinical studies | Synovium, cartilage | Not reported | Rats | Niazvand et al. 2017 [55] | |
| Dexamethasone | Avidin | Pre-clinical studies | Synovium | Not reported | Rabbit ACLT model | Bajpayee et al. 2017 [56] | |
| KAFAK | pNiPAM-PEG | Pre-clinical studies | Synovium, cartilage | RAW 264.7 macrophages; Bovine knee cartilage explants | Not reported | McMasters et al. 2017 [57] | |
| CAP (chondrocyte affinity peptide) | PEG-PAMAM | Pre-clinical studies | Cartilage | Human primary chondrocytes | Rats | Hu et al. 2018 [58] | |
| Kartogenin | Polyurethane | Pre-clinical studies | Cartilage | Rat primary chondrocytes | Rat ACLT model | Fan et al. 2018 [59] | |
| Adenosine | PEG-b-PLA | Pre-clinical studies | Synovium, cartilage | RAW 264.7 macrophages | Rat ACLT model | Liu et al. 2019 [60] | |
| Etoricoxib | PLGA-PEG-PLGA | Pre-clinical studies | Synovium, cartilage | Human primary chondrocytes | Rat ACLT model | Liu et al. 2019 [33] | |
| Hyaluronic acid | PLGA | Pre-clinical studies | Cartilage | RAW 264.7 macrophages | Brine shrimp; Rats | Mota et al. 2019 [61] | |
| Hyaluronic acid and near-infrared dye | PLGA | Pre-clinical studies | Cartilage | Human primary chondrocytes | Mice DMM model | Zerrillo et al. 2019 [62] | |
| Celastrol | Mesoporous silica | Pre-clinical studies | Cartilage | Rat primary chondrocytes | Rat MIA model | Jin et al. 2020 [63] | |
| Diacerein | PLGA | Pre-clinical studies | Synovium, cartilage | Rat synoviocytes | Rat MIA model | Jung et al. 2020 [64] | |
| Etoricoxib | PLA/Chitosan | Pre-clinical studies | Synovium | MC3T3-E1 cells (mouse osteoblast precursor) | Not reported | Salama et al. 2020 [65] | |
| MK2i (anti-inflammatory MK2-inhibiting peptide) | Linked and non-linked NIPAm | Pre-clinical studies | Synovium, cartilage | Bovine primary chondrocytes | Rats | Deloney et al. 2020 [66] | |
| Oxaceprol | PLGA | Pre-clinical studies | Synovium | Human primary LCLs (lymphoblastoid cell lines) | Not reported | Alarçin et al. 2020 [67] | |
| Triamcinolone acetonide | Dextran sulfate conjugated | Pre-clinical studies | Synovium | RAW 264.7 macrophages; L929 cells (mouse fibroblast) | Mice MIA model | She et al. 2020 [68] | |
| Hydrogels | Amphotericin B | Hyaluronic acid/glyceryl monooleate | Pre-clinical studies | Synovium, cartilage | Not reported | Rabbits | Shan-Bin et al. 2015 [69] |
| Celecoxib | PCLA-PEG-PCLA | Pre-clinical studies | Synovium | Not reported | Horse | Petit et al. 2015 [34] | |
| Methotrexate/dexamethasone/near-infrared dye | Hyaluronic acid + PLGA microcapsules | Pre-clinical studies | Synovium | RAW 264.7 macrophages | Rat RA model | Son et al. 2015 [70] | |
| Sinomenine hydrochloride | Phytantriol | Formulation studies | Not reported | Not reported | Not reported | Chen et al. 2015 [71] | |
| Dexamethasone | Hyaluronic acid | Pre-clinical studies | Synovium, cartilage | Human primary chondrocytes | Rat ACLT model | Zhang et al. 2016 [72] | |
| PEGylated Kartogenin | Hyaluronic acid | Pre-clinical studies | Cartilage | Human BMSCs; human primary chondrocytes | Rat ACLT model | Kang et al. 2017 [73] | |
| Celecoxib | PCLA-PEG-PCLA | Pre-clinical studies | Synovium | Not reported | Equine synovitis model | Cokeleare et al. 2018 [74] | |
| Dexamethasone | Hyaluronic acid/pNiPAM | Pre-clinical studies | Synovium | Human synoviocytes | Mice DMM model | Maudens et al. 2018 [10] | |
| Triamcinolone hexacetonide (Cingal®) | Hyaluronic acid | Phase II/III clinical trials in OA patients | Synovium, cartilage | Not reported | Not reported | Hangody et al. 2018 [75] | |
| Simvastatin | Gelatin | Pre-clinical studies | Cartilage | Mouse primary chondrocytes | Mice | Tanaka et al. 2019 [76] | |
| Dexamethasone | Agarose gel + PLGA microspheres | Pre-clinical studies | Synovium, cartilage | 3D canine articular chondrocyte construct | Canine osteochondral autograft model | Stefani et al. 2020 [77] | |
| Diclofenac | Hyalomer (HA and poloxamer 407) | Pre-clinical studies | Synovium, cartilage | Not reported | Rat MIA model | Hanafy et al. 2020 [78] | |
| Diclofenac | Linked PAPE (2-Pyridylamino substituted 1-phenylethanol) | Formulation studies | Not reported | Not reported | Not reported | Kawanami et al. 2020 [79] | |
| Eicosapentanoic acid | Gelatin | Pre-clinical studies | Synovium | Human primary chondrocytes | Mouse DMM model | Tsubosaka et al. 2020 [80] | |
| Hyaluronic acid/diclofenac sodium | Silica colloidal crystal beads- pNiPAM | Pre-clinical studies | Synovium, cartilage | Human primary chondrocytes | Rat DMM model | Yang et al. 2020 [81] | |
| Liposomes | Quercetin | mPEG-PA (Methoxy-poly(ethylene glycol)-l-poly(alanine)) | Pre-clinical studies | Synovium, cartilage | Human primary chondrocytes | Rat ACLT model | Mok et al. 2020 [82] |
| Fish oil protein encapsulated in gold nanoparticles | DPPC | Pre-clinical studies | Synovium | Not reported | Rats | Sarkar et al. 2019 [83] | |
| Glucosamine sulphate | Distearoyl phosphocholine | Pre-clinical studies | Cartilage | Mouse primary chondrocytes | Not reported | Ji et al. 2019 [84] | |
| Rapamycin | DSPC combined with low-intensity pulsed ultrasound | Pre-clinical studies | Cartilage | Human primary chondrocytes | Guinea pigs | Chen et al. 2020 [85] |
| In Vitro OA Model | Advantages | Disadvantages | Models Applied in IA DDS Development (as per Table 1) | Outcome Evaluation (as per Table 1) | |
|---|---|---|---|---|---|
| 2D cellular culture | Monolayer | High throughput, low cost. Homogenous cell exposition to nutrients. Allows for differences in cellular phenotype studies [12] | Furthest from natural in vivo tissue conditions. High variability (different passages). Better suited for synoviocytes than chondrocytes. 2D substrate induces de-differentiation and changes in morphology [12] |
| RAW 264.7 macrophages [33,57,60,68,70]:
|
| Co-culture | Important in studies of cell-to-cell interactions and studies of influence of different cellular phenotypes together [12] | Expensive and difficult to maintain. Lacks in three-dimensional characteristics of cartilage growth [87] | (examples not included in Table 1)
| ||
| 3D cellular culture | Without Scaffold | High similarity with in vivo tissue conditions as it maintains structure from ECM growth. Cellular phenotype is preserved. Important in studies of intercellular and cell to ECM relationship and loading capacity assays [88,91] | Expensive and difficult to maintain. Restricted throughput (hard to propagate without compromising cell quality). Nature of scaffold plays role in cellular growth [92] |
|
|
| With Scaffold |
| ||||
| Explants | Easy to obtain and inexpensive. Allows for studies of intercellular and cell to ECM relationship because it maintains tissue as a whole [93] | High variability and limited amounts of replicates from source. Cell death at edge of extracted tissues [12] |
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, C.; Jordan, O.; Allémann, E. Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics 2021, 13, 60. https://doi.org/10.3390/pharmaceutics13010060
Salgado C, Jordan O, Allémann E. Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics. 2021; 13(1):60. https://doi.org/10.3390/pharmaceutics13010060
Chicago/Turabian StyleSalgado, Carlota, Olivier Jordan, and Eric Allémann. 2021. "Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems" Pharmaceutics 13, no. 1: 60. https://doi.org/10.3390/pharmaceutics13010060
APA StyleSalgado, C., Jordan, O., & Allémann, E. (2021). Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics, 13(1), 60. https://doi.org/10.3390/pharmaceutics13010060