Luminal Fluid Motion Inside an In Vitro Dissolution Model of the Human Ascending Colon Assessed Using Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dynamic Colon Model and Lumen Media
2.2. MR Protocol
2.3. MR Data Analysis
2.4. Statistics
3. Results and Discussion
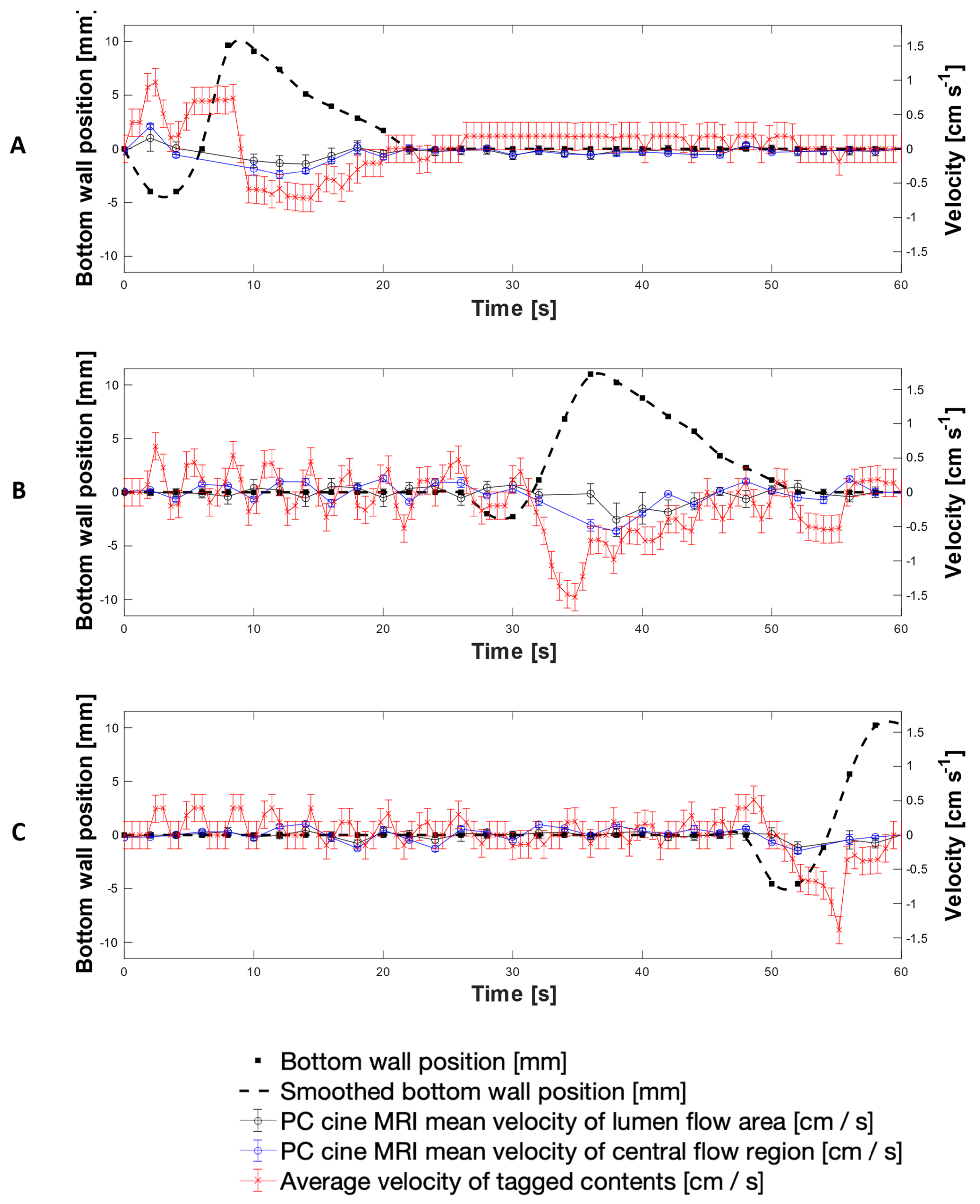
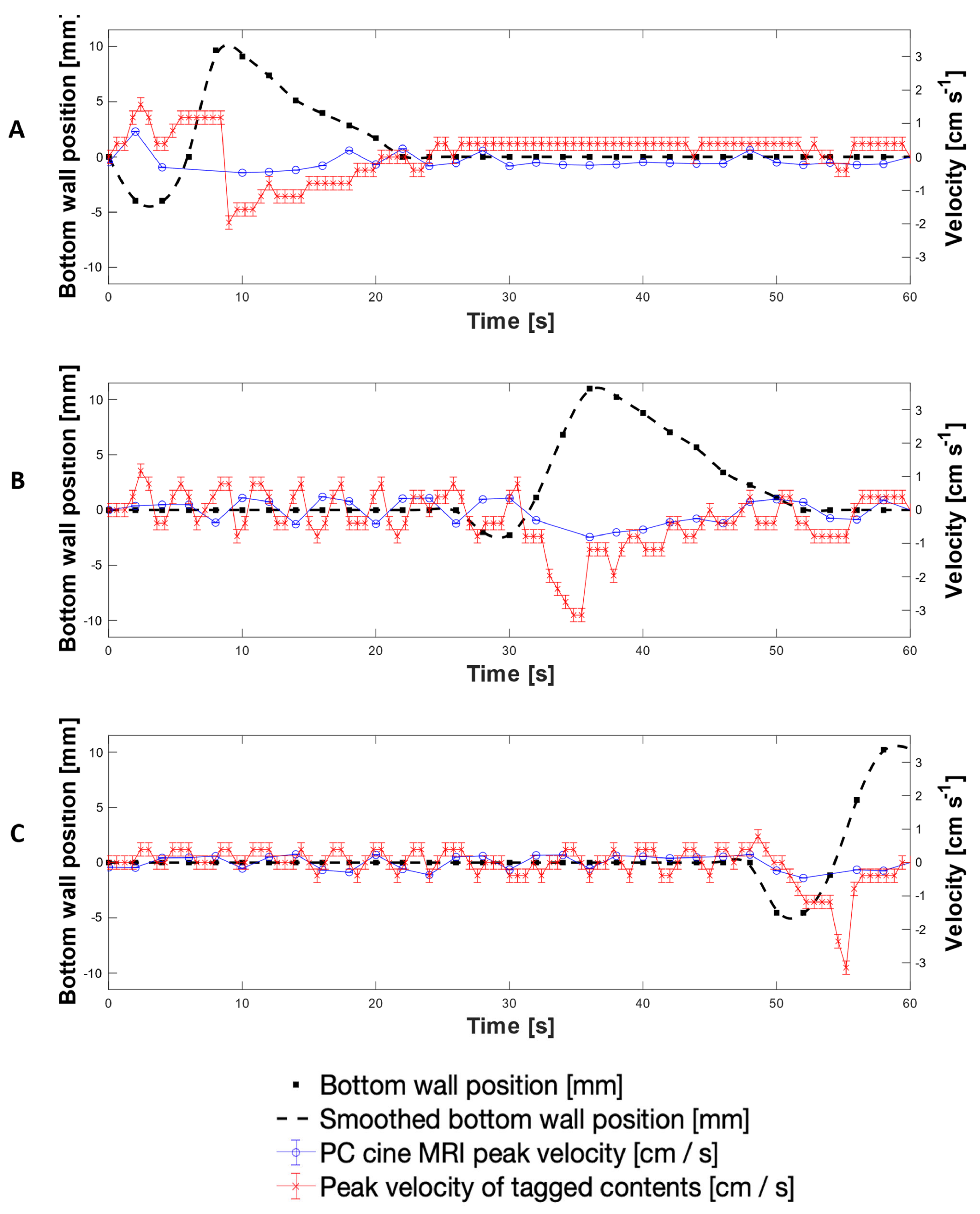
3.1. MR Tagging of Fluid Motion
3.2. Velocimetry of Fluid Motion In Vitro Using MR Tagging
3.3. Velocimetry of Fluid Motion In Vivo Using MR Tagging
3.4. PC Cine-MRI Velocimetry of the DCM Lumen Contents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Scrutininsation of Tagging versus Phase Contrast Cine-MRI Velocity Measurements
Appendix B
Exclusion of Phase Contrast Cine-MRI Datapoints
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Zaborenko, N.; Shi, Z.; Corredor, C.C.; Smith-Goettler, B.M.; Zhang, L.; Hermans, A.; Neu, C.M.; Alam, M.A.; Cohen, M.J.; Lu, X.; et al. First-Principles and Empirical Approaches to Predicting In Vitro Dissolution for Pharmaceutical Formulation and Process Development and for Product Release Testing. AAPS J. 2019, 21, 1–20. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Alomar, M.J. In Vitro Dissolution and in Silico Modeling Shortcuts in Bioequivalence Testing. Pharmaceutics 2020, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Schütt, M.; Stamatopoulos, K.; Batchelor, H.K.; Simmons, M.J.H.; Alexiadis, A. Modelling and Simulation of the Drug Release from a Solid Dosage Form in the Human Ascending Colon: The Influence of Different Motility Patterns and Fluid Viscosities. Pharmaceutics 2021, 13, 859. [Google Scholar] [CrossRef] [PubMed]
- Salehi, N.; Al-Gousous, J.; Mudie, D.M.; Amidon, G.L.; Ziff, R.M.; Amidon, G.E. Hierarchical Mass Transfer Analysis of Drug Particle Dissolution, Highlighting the Hydrodynamics, pH, Particle Size, and Buffer Effects for the Dissolution of Ionizable and Nonionizable Drugs in a Compendial Dissolution Vessel. Mol. Pharm. 2020, 17, 3870–3884. [Google Scholar] [CrossRef]
- Chen, J.-H.; Parsons, S.P.; Shokrollahi, M.; Wan, A.; Vincent, A.D.; Yuan, Y.; Pervez, M.; Chen, W.L.; Xue, M.; Zhang, K.K.; et al. Characterization of Simultaneous Pressure Waves as Biomarkers for Colonic Motility Assessed by High-Resolution Colonic Manometry. Front. Physiol. 2018, 9, 1248. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yu, Y.; Yang, Z.; Yu, W.-Z.; Chen, W.L.; Yu, H.; Kim, M.J.-M.; Huang, M.; Tan, S.; Luo, H.; et al. Intraluminal pressure patterns in the human colon assessed by high-resolution manometry. Sci. Rep. 2017, 7, 41436. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-W.; Yu, Y.-J.; Fei, F.; Zheng, M.-Y.; Zhang, S.-W. High-resolution colonic manometry and its clinical application in patients with colonic dysmotility: A review. World J. Clin. Cases. 2019, 7, 2675–2686. [Google Scholar] [CrossRef]
- Milkova, N.; Parsons, S.P.; Ratcliffe, E.; Huizinga, J.D.; Chen, J.-H. On the nature of high-amplitude propagating pressure waves in the human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G646–G660. [Google Scholar] [CrossRef] [Green Version]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Patton, V.; Lewis, H.; Arkwright, J.W.; Wattchow, D.A.; Lubowski, D.Z.; Costa, M.; Bampton, P.A. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterol. Motil. 2015, 27, 379–388. [Google Scholar] [CrossRef]
- Van der Paardt, M.P.; Sprengers, A.M.J.; Zijta, F.M.; Lamerichs, R.; Nederveen, A.J.; Stoker, J. Noninvasive automated motion assessment of intestinal motility by continuously tagged MR imaging. J. Magn. Reson. Imaging. 2014, 39, 9–16. [Google Scholar] [CrossRef]
- Sprengers, A.M.J.; van der Paardt, M.P.; Zijta, F.M.; Caan, M.W.A.; Lamerichs, R.M.; Nederveen, A.J.; Stoker, J. Use of continuously MR tagged imaging for automated motion assessment in the abdomen: A feasibility study. J. Magn. Reson. Imaging. 2012, 36, 492–497. [Google Scholar] [CrossRef]
- Jonge, C.S.; Sprengers, A.M.J.; Rijn, K.L.; Nederveen, A.J.; Stoker, J. Assessment of fasted and fed gastrointestinal contraction frequencies in healthy subjects using continuously tagged MRI. Neurogastroenterol. Motil. 2019, 32, e13747. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson-Smith, V.; Menys, A.; Bradley, C.; Corsetti, M.; Marciani, L.; Atkinson, D.; Coupland, C.; Taylor, S.A.; Gowland, P.; Spiller, R.; et al. The MRI colonic function test: Reproducibility of the Macrogol stimulus challenge. Neurogastroenterol. Motil. 2020, 32, e13942. [Google Scholar] [CrossRef]
- Axel, L.; Ann Shimakawa, B.S.E.E.; MacFall, J. A time-of-flight method of measuring flow velocity by magnetic resonance imaging. Magn. Reson. Imaging. 1986, 4, 199–205. [Google Scholar] [CrossRef]
- Moser, K.W.; Kutter, E.C.; Georgiadis, J.G.; Buckius, R.O.; Morris, H.D.; Torczynski, J.R. Velocity measurements of flow through a step stenosis using Magnetic Resonance Imaging. Exp. Fluids. 2000, 29, 438–447. [Google Scholar] [CrossRef]
- D’Avila, M.A.; Powell, R.L.; Phillips, R.J.; Shapley, N.C.; Walton, J.H.; Dungan, S.R. Magnetic resonance imaging (MRI): A technique to study flow an microstructure of concentrated emulsions. Braz. J. Chem. Eng. 2005, 22, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, S.E.; Paul, J.; Major, G.; Marciani, L.; Gowland, P.A.; Spiller, R.C.; Hoad, C.L. Assessment of motion of colonic contents in the human colon using MRI tagging. Neurogastroenterol. Motil. 2017, 29, e13091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatopoulos, K.; Batchelor, H.K.; Simmons, M.J.H. Dissolution profile of theophylline modified release tablets, using a biorelevant Dynamic Colon Model (DCM). Eur. J. Pharm. Biopharm. 2016, 108, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Stamatopoulos, K.; Karandikar, S.; Goldstein, M.; O’Farrell, C.; Marciani, L.; Sulaiman, S.; Hoad, C.L.; Simmons, M.J.H.; Batchelor, H.K. Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques. Pharmaceutics. 2020, 12, 659. [Google Scholar] [CrossRef]
- O’Farrell, C.; Stamatopoulos, K.; Simmons, M.; Batchelor, H. In vitro models to evaluate ingestible devices: Present status and current trends. Adv. Drug Deliv. Rev. 2021, 176, 113924. [Google Scholar] [CrossRef]
- Spratt, P.; Nicolella, C.; Pyle, D.L. An Engineering Model of the Human Colon. Food Bioprod. Process. 2005, 83, 147–157. [Google Scholar] [CrossRef]
- Grady, H.; Elder, D.; Webster, G.K.; Mao, Y.; Lin, Y.; Flanagan, T.; Mann, J.; Blanchard, A.; Cohen, M.J.; Lin, J.; et al. Industry’s View on Using Quality Control, Biorelevant, and Clinically Relevant Dissolution Tests for Pharmaceutical Development, Registration, and Commercialization. J. Pharm. Sci. 2018, 107, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Batchelor, H.K.; Alberini, F.; Ramsay, J.; Simmons, M.J.H. Understanding the impact of media viscosity on dissolution of a highly water soluble drug within a USP 2 mini vessel dissolution apparatus using an optical planar induced fluorescence (PLIF) method. Int. J. Pharm. 2015, 495, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Vertzoni, M.; Augustijns, P.; Grimm, M.; Koziolek, M.; Lemmens, G.; Parrott, N.; Pentafragka, C.; Reppas, C.; Rubbens, J.; Van Den Abeele, J.; et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019, 134, 153–175. [Google Scholar] [CrossRef]
- Tanaka, Y.; Goto, T.; Kataoka, M.; Sakuma, S.; Yamashita, S. Impact of Luminal Fluid Volume on the Drug Absorption After Oral Administration: Analysis Based on In Vivo Drug Concentration–Time Profile in the Gastrointestinal Tract. J. Pharm. Sci. 2015, 104, 3120–3127. [Google Scholar] [CrossRef]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Gibbins, I.; Patton, V.; Arkwright, J.W.; Lubowski, D.Z.; O’Grady, G.; Bampton, P.A.; Brookes, S.J.; et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. 2014, 26, 1443–1457. [Google Scholar] [CrossRef] [Green Version]
- Nayak, K.S.; Nielsen, J.-F.; Bernstein, M.A.; Markl, M.; Gatehouse, P.D.; Botnar, R.M.; Saloner, D.; Lorenz, C.; Wen, H.; Hu, B.S.; et al. Cardiovascular magnetic resonance phase contrast imaging. J. Cardiovasc. Magn. Reson. 2015, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Wymer, D.T.; Patel, K.P.; Burke, W.F.; Bhatia, V.K. Phase-Contrast MRI: Physics, Techniques, and Clinical Applications. RadioGraphics 2020, 40, 122–140. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mastikhin, I. Velocity Measurement of Fast Flows Inside Small Structures with Tagged MRI. Appl. Magn. Reson. 2020, 51, 431–448. [Google Scholar] [CrossRef]
- O’Brien, K.R.; Cowan, B.R.; Jain, M.; Stewart, R.A.H.; Kerr, A.J.; Young, A.A. MRI phase contrast velocity and flow errors in turbulent stenotic jets. J. Magn. Reson. Imaging. 2008, 28, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.P.; Ehrenpreis, E.D.; Putt, K.S.; Hannon, B. A dynamic model of colonic concentrations of delayed-release 5-aminosalicylic acid (Asacol). Aliment. Pharmacol. Ther. 2009, 29, 1193–1201. [Google Scholar] [CrossRef]
- Dinning, P.G.; Zarate, N.; Hunt, L.M.; Fuentealba, S.E.; Mohammed, S.D.; Szczesniak, M.M.; Lubowski, D.Z.; Preston, S.L.; Fairclough, P.D.; Lunniss, P.J.; et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol. Motil. 2010, 22, e340–e349. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Gaburri, M.; Imbimbo, B.P.; Rossi, L.; Farroni, F.; Pelli, M.A.; Morelli, A. Colonic mass movements in idiopathic chronic constipation. Gut 1988, 29, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Braun, R.J.; Parrott, E.L. Influence of Viscosity and Solubilization on Dissolution Rate. J. Pharm. Sci. 1972, 61, 175–178. [Google Scholar] [CrossRef]
- Schütt, M.; Stamatopoulos, K.; Simmons, M.J.H.; Batchelor, H.K.; Alexiadis, A. Modelling and simulation of the hydrodynamics and mixing profiles in the human proximal colon using Discrete Multiphysics. Comput. Biol. Med. 2020, 121, 103819. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Sulaiman, S.; Goumas, K.; Kersten, E.; Anlahr, J.; Muenster, U.; Reppas, C. Characteristics of Contents of Lower intestine in the 65–74 Years of Age Range Could Impact the Performance of Safe and Efficacious Modified Release Products. J. Pharm. Sci. 2021, 110, 251–258. [Google Scholar] [CrossRef]
- Perman, W.H.; Creswell, L.L.; Wyers, S.G.; Moulton, M.J.; Pasque, M.K. Hybrid dante and phase-contrast imaging technique for measurement of three- dimensional myocardial wall motion. J. Magn. Reson. Imaging. 1995, 5, 101–106. [Google Scholar] [CrossRef]
- Kuijer, J.P.A.; Marcus, T.; Götte, M.J.W.; van Rossum, A.C.; Heethaar, R.M. Simultaneous MRI tagging and through-plane velocity quantification: A three-dimensional myocardial motion tracking algorithm. J. Magn. Reson. Imaging. 1999, 9, 409–419. [Google Scholar] [CrossRef]
- Alexiadis, A.; Stamatopoulos, K.; Wen, W.; Batchelor, H.K.; Bakalis, S.; Barigou, M.; Simmons, M.J. Using discrete multi-physics for detailed exploration of hydrodynamics in an in vitro colon system. Comput. Biol. Med. 2017, 81, 188–198. [Google Scholar] [CrossRef]
- Wang, B.; Bredael, G.; Armenante, P.M. Computational hydrodynamic comparison of a mini vessel and a USP 2 dissolution testing system to predict the dynamic operating conditions for similarity of dissolution performance. Int. J. Pharm. 2018, 539, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kuwana, A.; Shibata, H.; Izutsu, K.-i.; Goda, Y. Particle Image Velocimetry Evaluation of Fluid Flow Profiles in USP 4 Flow-Through Dissolution Cells. Pharm. Res. 2015, 32, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, Y.; Li, B.; Chen, J. Analysis of the Velocity Distribution in Partially-Filled Circular Pipe Employing the Principle of Maximum Entropy. PLoS ONE 2016, 11, e0151578. [Google Scholar] [CrossRef]
- Sinnott, M.D.; Cleary, P.W.; Arkwright, J.W.; Dinning, P.G. Investigating the relationships between peristaltic contraction and fluid transport in the human colon using Smoothed Particle Hydrodynamics. Comput. Biol. Med. 2012, 42, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Espersen, M.; Gregersen, T.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol. Motil. 2018, 31, e13451. [Google Scholar] [CrossRef] [PubMed]
- Hiroz, P.; Schlageter, V.; Givel, J.C.; Kucera, P. Colonic movements in healthy subjects as monitored by a Magnet Tracking System. Neurogastroenterol. Motil. 2009, 21, e838–e857. [Google Scholar] [CrossRef] [Green Version]
- Haughton, V.M.; Korosec, F.R.; Medow, J.E.; Dolar, M.T.; Iskandar, B.J. Peak systolic and diastolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control participants. AJNR Am. J. Neuroradiol. 2003, 24, 169–176. [Google Scholar]
- Armonda, R.A.; Citrin, C.M.; Foley, K.T.; Ellenbogen, R.G. Quantitative Cine-mode Magnetic Resonance Imaging of Chiari I Malformations. Neurosurgery 1994, 35, 214–224. [Google Scholar] [CrossRef]
- Levy, L.M.; Di Chiro, G. MR phase imaging and cerebrospinal fluid flow in the head and spine. Neuroradiology 1990, 32, 399–406. [Google Scholar] [CrossRef]
- Enzmann, D.R.; Pelc, N.J. Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR Am. J. Neuroradiol. 1993, 14, 1301–1307; discussion 1309–1310. [Google Scholar]
- Enzmann, D.R.; Pelc, N.J. Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology 1991, 178, 467–474. [Google Scholar] [CrossRef]
- Macgowan, C.K.; Henkelman, R.M.; Wood, M.L. Pulse-wave velocity measured in one heartbeat using MR tagging. Magn. Reson. Med. 2002, 48, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.W.; Georgiadis, J.G.; Buckius, R.O. On the Use of Optical Flow Methods with Spin-Tagging Magnetic Resonance Imaging. Ann. Biomed. Eng. 2001, 29, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Pelc, N.J.; Herfkens, R.J.; Shimakawa, A.; Enzmann, D.R. Phase contrast cine magnetic resonance imaging. Magn. Reson. Q. 1991, 7, 229–254. [Google Scholar] [PubMed]
- Ståhlberg, F.; Søndergaard, L.; Thomsen, C.; Henriksen, O. Quantification of complex flow using MR phase imaging—A study of parameters influencing the phase/velocity relation. Magn. Reson. Imaging. 1992, 10, 13–23. [Google Scholar] [CrossRef]
- Eichenberger, A.C.; Jenni, R.; von Schulthess, G.K. Aortic valve pressure gradients in patients with aortic valve stenosis: Quantification with velocity-encoded cine MR imaging. Am. J. Roentgenol. 1993, 160, 971–977. [Google Scholar] [CrossRef]
- Pelc, N.J.; Drangova, M.; Pelc, L.R.; Zhu, Y.; Noll, D.C.; Bowman, B.S.; Herfkens, R.J. Tracking of cyclic motion with phase-contrast cine MR velocity data. J. Magn. Reson. Imaging. 1995, 5, 339–345. [Google Scholar] [CrossRef] [PubMed]
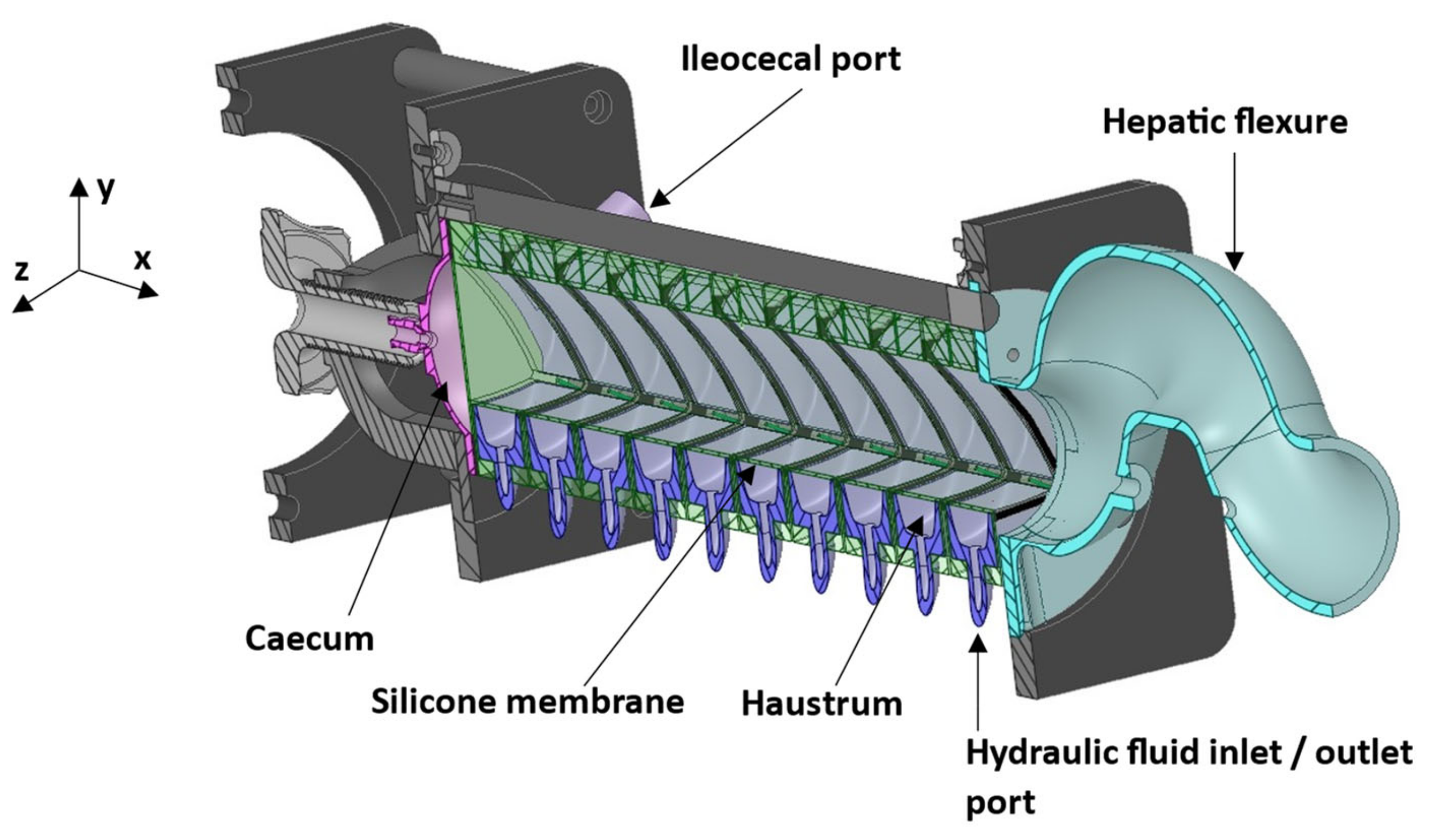
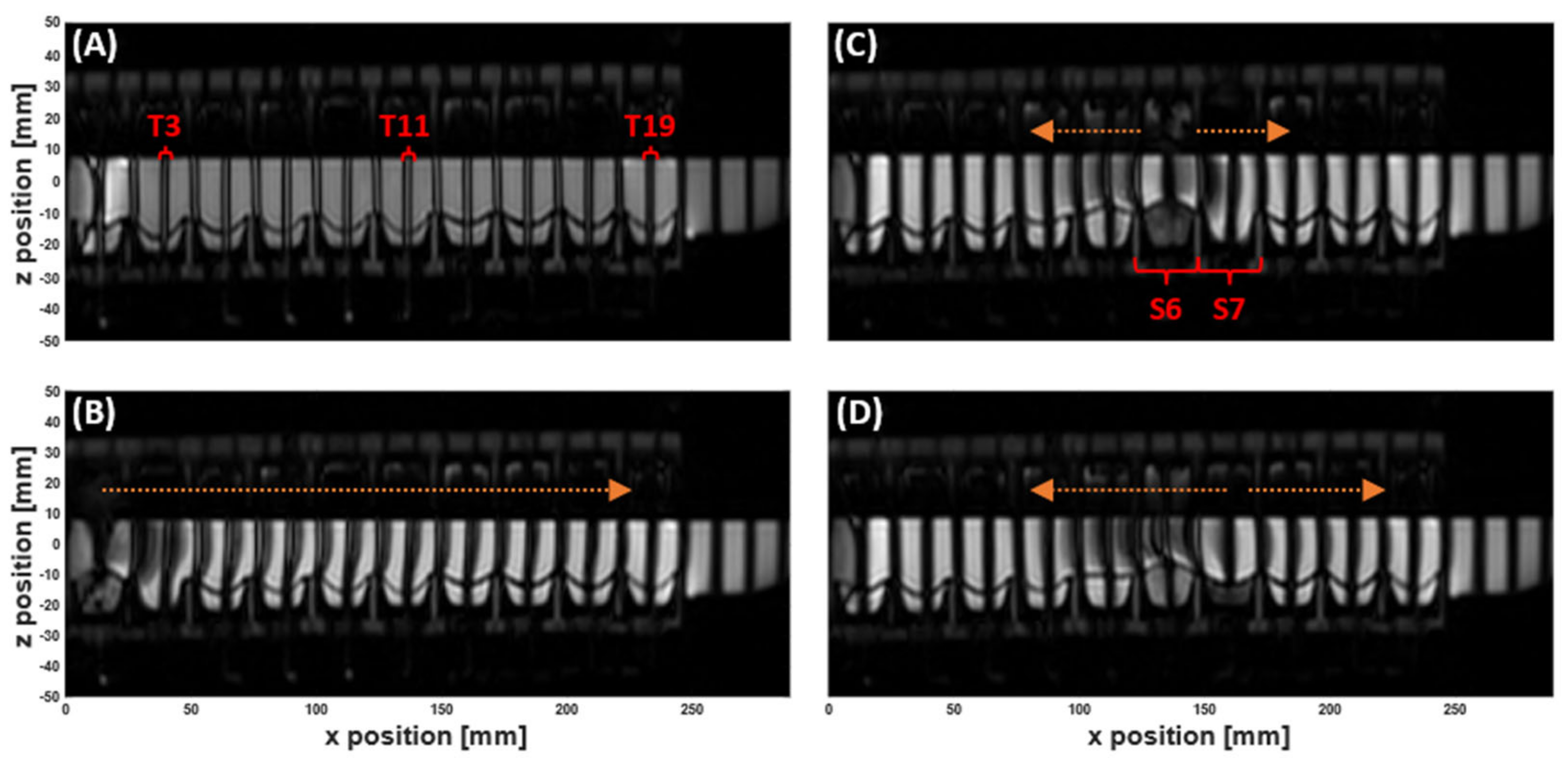
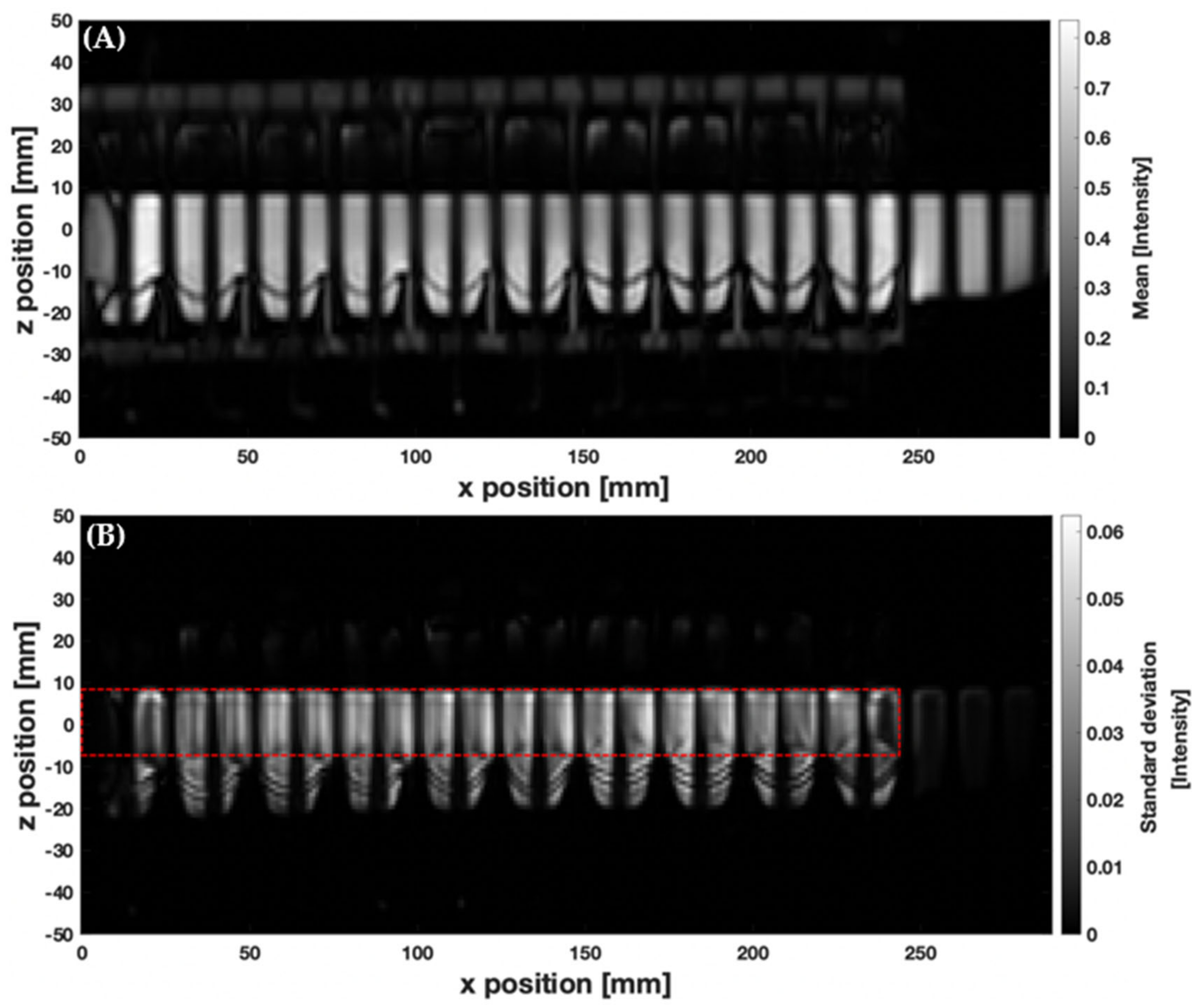



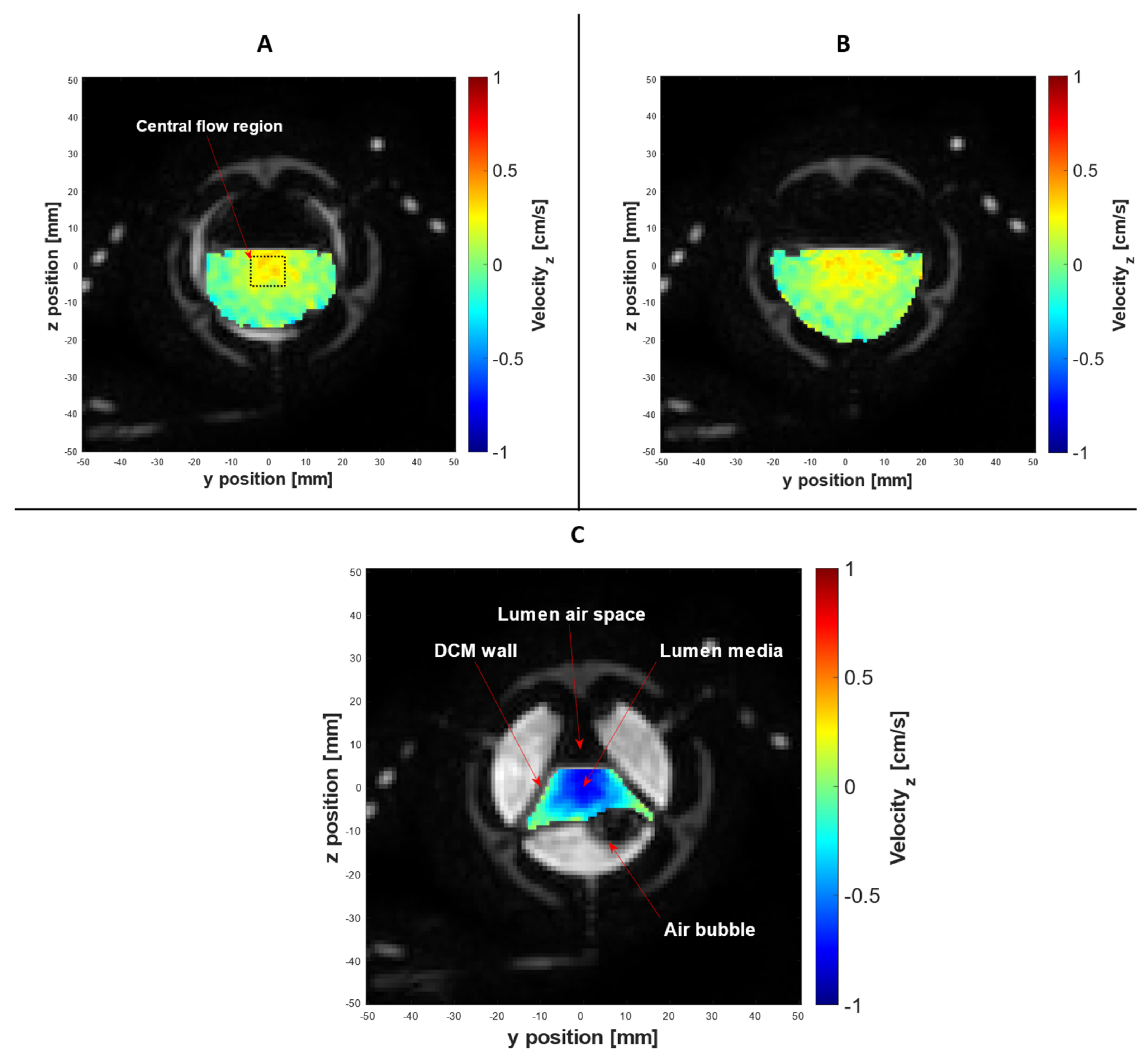
| Parameter | DCM PC cine-MRI | DCM Tagging | Human Colon Tagging [20] |
|---|---|---|---|
| Scan duration [s] | 60 | 60 | 20 |
| TR [ms] | 9.21 | 2.43 | 2.30 |
| TE [ms] | 7.60 | 1.22 | 1.15 |
| FA [°] | 10 | 45 | 45 |
| FOV [mm2] | 177 × 200 | 259 × 330 | 222–264 × 330 |
| Recon resolution [mm2] | 1.136 × 1.136 | 0.982 × 0.982 | 0.982 × 0.982 |
| Slice thickness [mm] | 8 | 15 | 15 |
| SENSE | 2.0 | 1.5 | 1.5 |
| No. dynamics | 30 | 100 | 33 |
| Temporal Resolution [s] | 2 | 0.6 | 0.6 |
| No. averages | 1 | 1 | 1 |
| Orientation | Transverse | Sagittal | Sagittal |
| Media | Volume | CoV (%) | |
|---|---|---|---|
| Slower CPPW (0.4 cm·s−1) | |||
| LOVIS | 150 mL | 32.16 | |
| 200 mL | 30.23 | ||
| HIVIS | 150 mL | 26.52 | |
| 200 mL | 25.68 | ||
| Faster CPPW (0.7 cm·s−1) | |||
| LOVIS | 150 mL | 37.29 | |
| 200 mL | 37.42 | ||
| HIVIS | 150 mL | 32.35 | |
| 200 mL | 31.98 | ||
| Post-hoc multiple comparisons of CoV using Tukey’s HSD test | |||
| Variable | Level difference | Mean difference | p-value |
| Motility pattern | Slower–Faster CPPW | 6.113 | p < 0.05 |
| Media viscosity | Media LOVIS-HIVIS | 5.142 | p < 0.05 |
| Media volume | 150–200 mL | −0.752 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Farrell, C.; Hoad, C.L.; Stamatopoulos, K.; Marciani, L.; Sulaiman, S.; Simmons, M.J.H.; Batchelor, H.K. Luminal Fluid Motion Inside an In Vitro Dissolution Model of the Human Ascending Colon Assessed Using Magnetic Resonance Imaging. Pharmaceutics 2021, 13, 1545. https://doi.org/10.3390/pharmaceutics13101545
O’Farrell C, Hoad CL, Stamatopoulos K, Marciani L, Sulaiman S, Simmons MJH, Batchelor HK. Luminal Fluid Motion Inside an In Vitro Dissolution Model of the Human Ascending Colon Assessed Using Magnetic Resonance Imaging. Pharmaceutics. 2021; 13(10):1545. https://doi.org/10.3390/pharmaceutics13101545
Chicago/Turabian StyleO’Farrell, Connor, Caroline L. Hoad, Konstantinos Stamatopoulos, Luca Marciani, Sarah Sulaiman, Mark J. H. Simmons, and Hannah K. Batchelor. 2021. "Luminal Fluid Motion Inside an In Vitro Dissolution Model of the Human Ascending Colon Assessed Using Magnetic Resonance Imaging" Pharmaceutics 13, no. 10: 1545. https://doi.org/10.3390/pharmaceutics13101545






