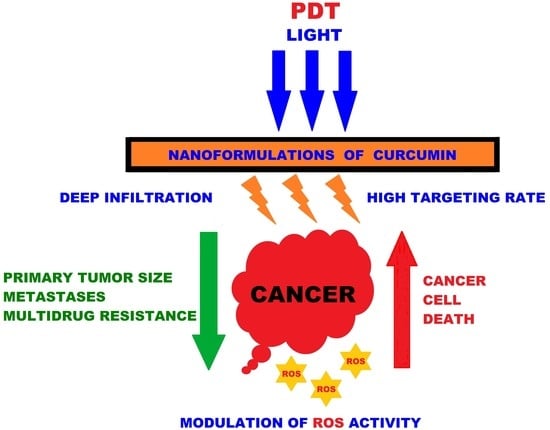
Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer
Abstract
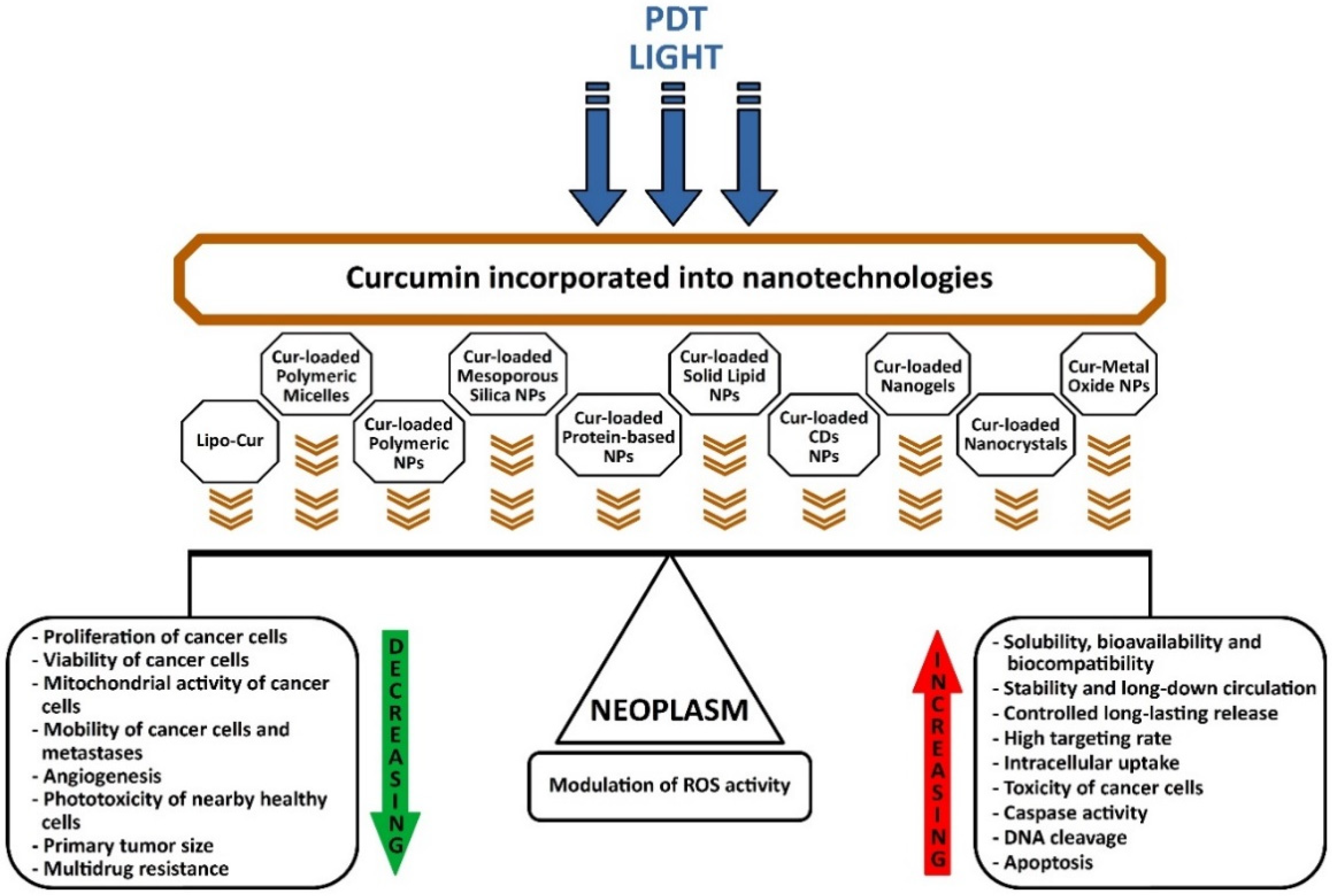
:1. Introduction
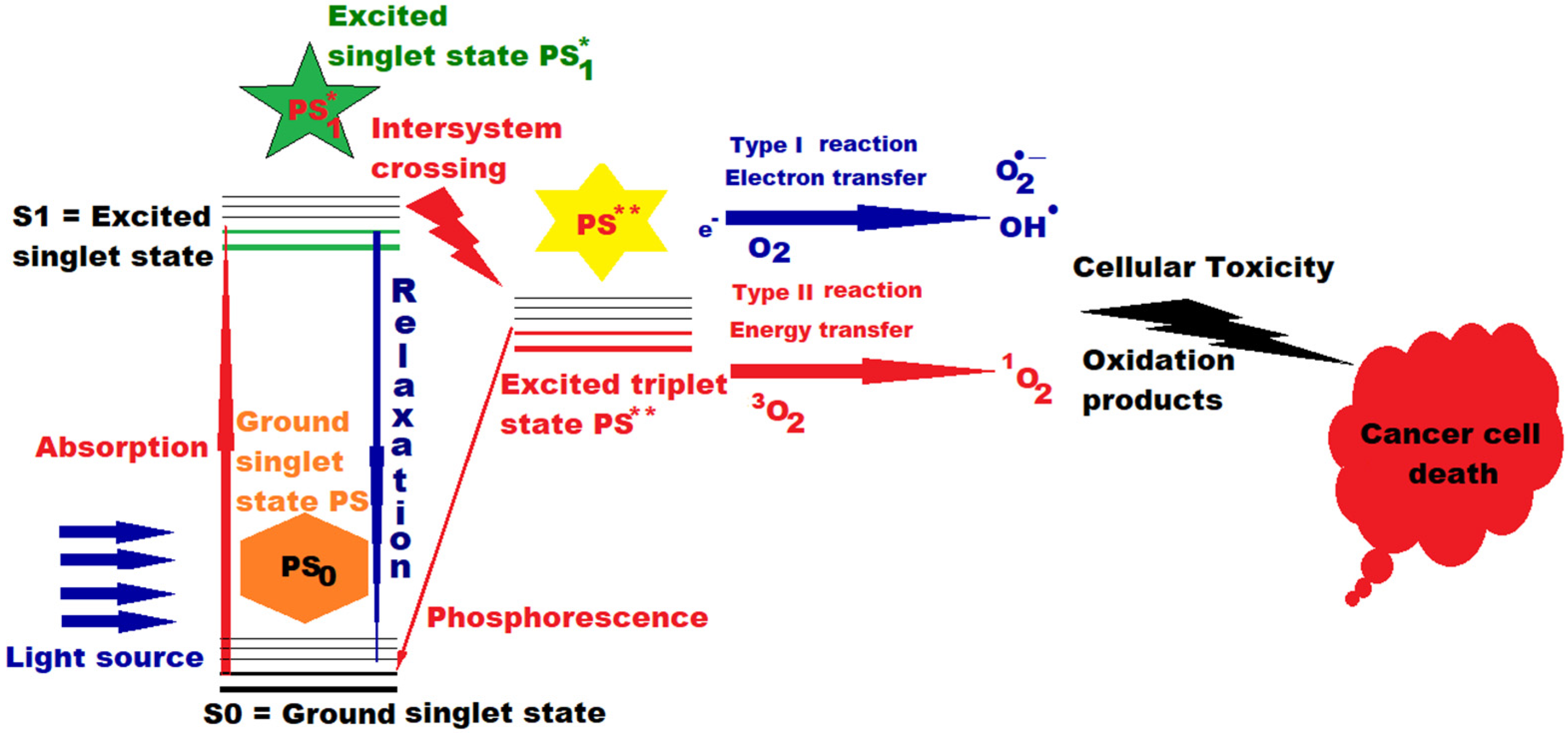
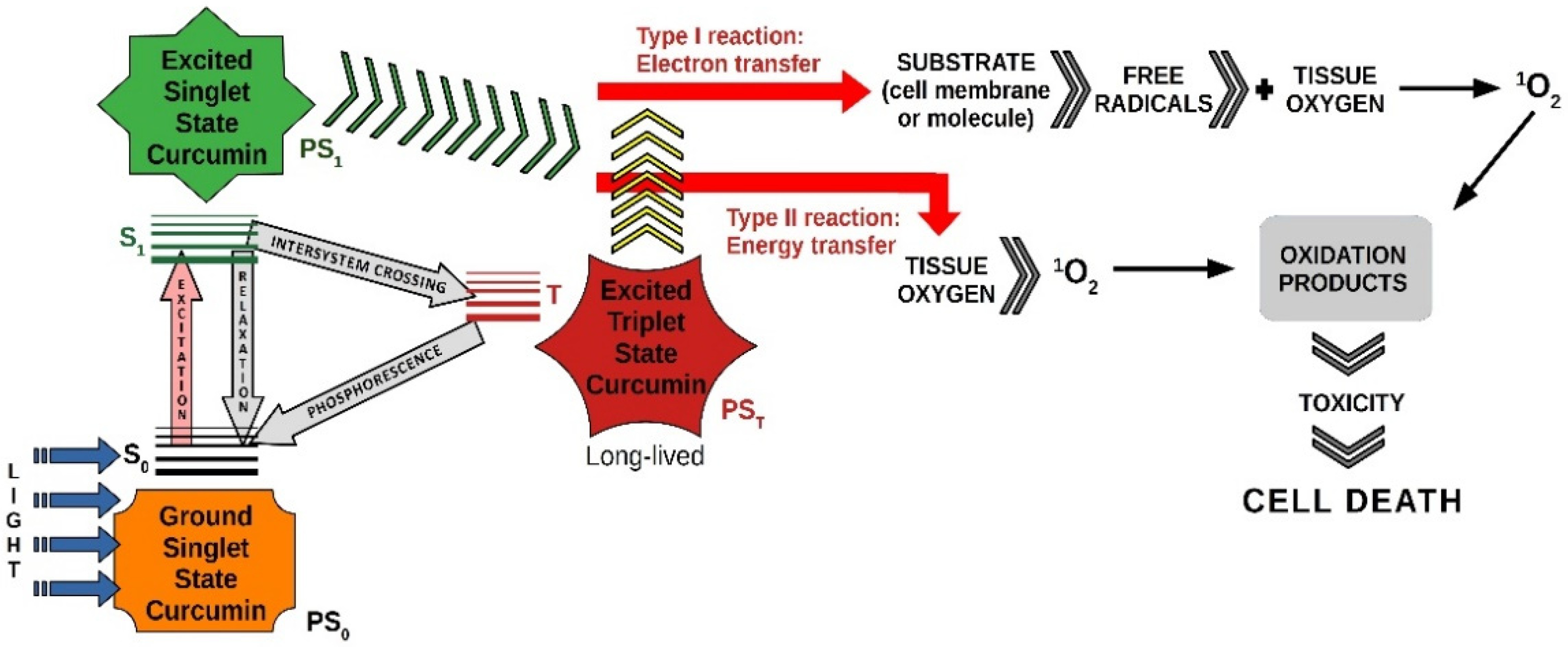
2. Photosensitizers and Photodynamic Therapy
- -
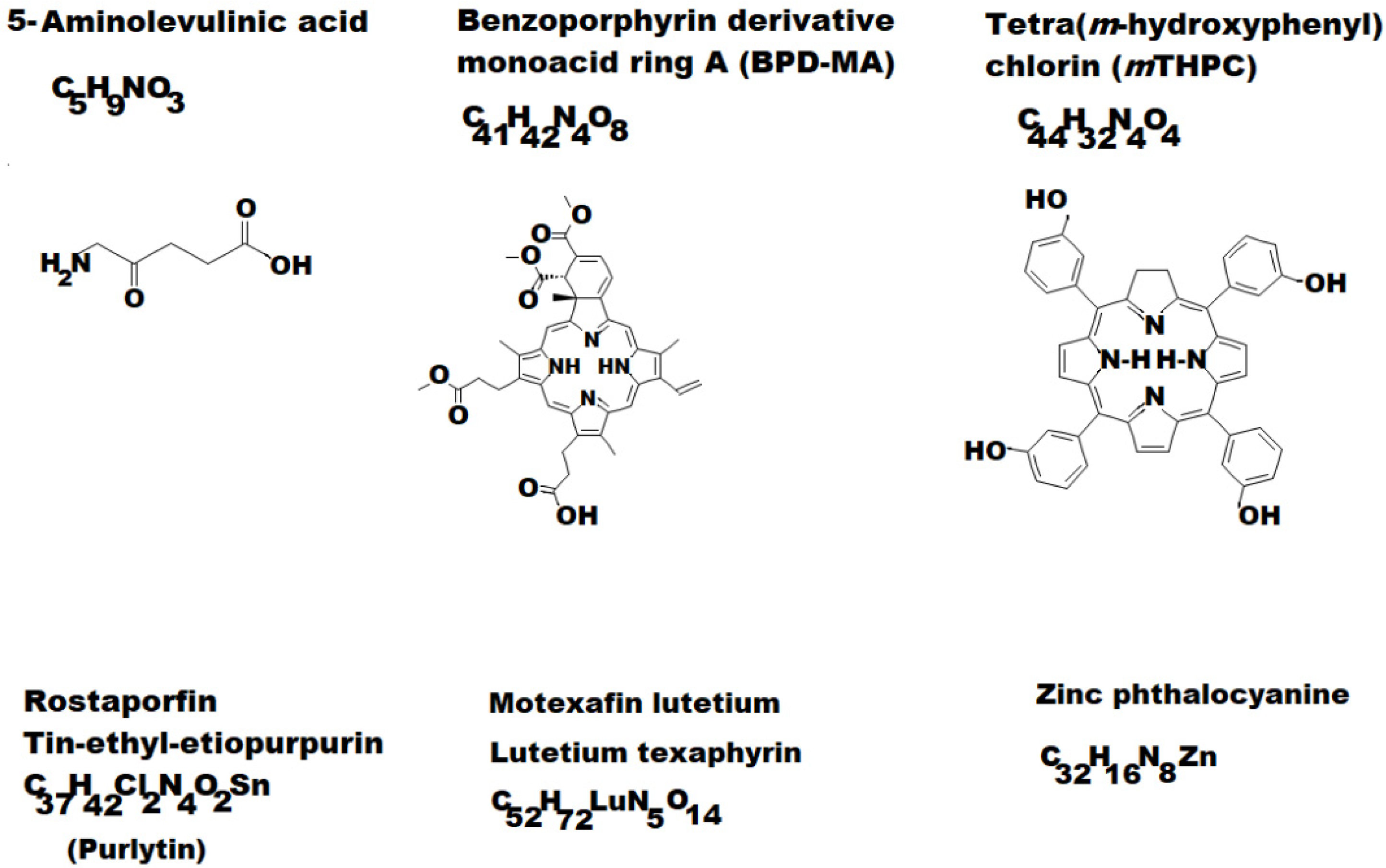
- the first generation (HpD and Photofrin) had poor absorption in the red visible range, limited applications and an unpleasant side effect, the residual sensitivity of the skin;
- -
- the second generation (see some examples depicted in Figure 2) allowed a much more accelerated development of PDT (5-Aminolaevulinic acid (ALA); Benzoporphyrin derivative monoacid ring A (BPD-MA) or Verteporfin; Chlorins sold as Purlytin; Tetra(m-hydroxyphenyl)chlorin (mTHPC) or Foscan; Lutetium texaphyrin with tradename Lutex or Lutrin; 9-Acetoxy-2,7,12,17-tetrakis-(β-methoxyethyl)-porphycene or ATMPn; Zinc phthalocyanine (CGP55847); Naphthalocyanines (NCs) and Porphyrin-type Chromophores (PC) with modified marginal operation by different functional groups, such as nitrophenyl, aminophenyl, hydroxyphenyl, pyridiniumyl derivatives etc. [7,27,28,29].
2.1. Curcumin-Loaded Liposomes (Lipo-Cur)
2.2. Cur-Loaded Polymeric Micelles
2.3. Cur-Loaded Polymeric NPs
2.4. Cur-Loaded Mesoporous Silica NPs
2.5. Cur-Loaded Protein-Based NPs
2.6. Cur-Loaded Solid Lipid NPs
2.7. Cur-Loaded CDs NPs
2.8. Cur-Loaded Nanogels
2.9. Cur-Loaded Nanocrystals
2.10. Cur-Metal Oxide NPs
3. Curcumin and Latest Cancer Applications
4. Effects of Curcumin and PDT in Various Forms of Cancer
4.1. Breast Cancer
- type 1 breast cancer with estrogen receptor (ER+) positive hormone or progesterone receptor (PR+) positive; this type responds to hormonal treatment.
- type 2 breast cancer with a positive test for the human epidermal growth factor receptor 2 (HER2), a protein which fosters the development of cancer cells, and may respond to HER2-targeted treatments.
- type 3 breast cancer is the one known as triple negative breast cancer (TNBC), because here we do not find ER, PR or HER2. This type of cancer is the most difficult to treat with pharmacological means that have already become classic. Various drugs [Sacituzumab govitecan (Trodelvy)] and immunotherapeutic products, [Pembrolizumab (Keytruda), PARP inhibitors], are being tested in combination with conventional chemotherapy for this type of breast cancer [76].
4.2. Gynecologic Cancers
4.3. Skin Cancer
4.4. Gastrointestinal Cancers
4.5. Lung Cancer
4.6. Other Cancers
5. Final Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-Aminolevulinic acid | (5-ALA) |
| Activated partial thromboplastin time tests | (aPTT) |
| Adenosine triphosphate | (ATP) |
| Basal cell carcinoma | (BCC) |
| Baseline or ground state | (S0) |
| B-cell lymphoma 2 | (Bcl-2) |
| Bcl-2–associated X | (Bax) |
| Blue light-emitting diode | (BLED) |
| Blue-light-emitting diode- photodynamic therapy | (BLED-PDT). |
| Cervical intraepithelial neoplasia | (CIN) |
| Chitosan | (CHT) |
| Cholesteryl hemisuccinate | (CHEMS) |
| Chondroitin sulfate | (CS) |
| Circulating tumor cells | (CTCs) |
| Colorectal cancer | (CRC) |
| Confocal laser scanning microscopy | (CLSM) |
| Continuous wave | (CW) |
| Curcumin | (Cur) |
| Curcumin conjugated silver nanoparticles | (CUR-AgNPs) |
| Curcumin loaded PLGA nanoparticles | (CUR-PLGA NPs) |
| Curcumin nanocrystals | (Cur-NCs) |
| Curcumin nanoparticles | (CUR-NPs) |
| Curcumin-loaded liposomes | (Lipo-Cur) |
| Curcumin-nanoemulsion | (CNE) |
| Curcumin-loaded solid lipid nanoparticles | (Cur-SLNs) |
| Cutaneous squamous cell carcinoma | (cSCC) |
| Cyclodextrins | (CDs) |
| Demethoxycurcumin | (DMC) |
| Dendrosomal Nano Curcumin | (DNC) |
| Deoxyadenosine triphosphate | (dATP) |
| Dichlorodihydrofluorescein diacetate | (DCFH-DA) |
| Dioleoyl phosphatidylethanolamine | (DOPE) |
| Dissolved in dimethyl sulfoxide | (DMSO) |
| Distearoyl phosphoethanolamine | (DSPE) |
| EGFRvIII overexpressed human glioblastoma cell line | (DKMG/EGFRvIII cells) |
| Epidermal growth factor | (EGF) |
| Epidermal growth factor receptor | (EGFR) |
| European Medicines Agency | (EMA) |
| Ferric chloride hexahydrate | (FeCl3·6H2O) |
| Ferrous sulfate heptahydrate | (FeSO4·7H2O) |
| Fluorescein isothiocyanate | (FITC) |
| Fluorescence microscopy imaging system | (FMI) |
| Food and Drug Administration | (FDA) |
| Fourier-transform infrared spectroscopy | (FTIR) |
| Glyceraldehydes 3-phosphate dehydrogenase | (GAPDH) |
| Immunohistochemistry | (IHC) |
| Indocyanine Green photosensitizer | (ICG) |
| Laser irradiation in near-infrared | (NIR) |
| Light emitting diode | (LED) |
| Liposome nanocarriers curcumin | (LIP-CUR) |
| Liposomes | (LPs) |
| Mesoporous silica nanoparticles | (MSNs) |
| Monoclonal antibody | (MAb) |
| Monoclonal antibody against EGFRvIII | (A-EGFRvIII-f) |
| Mouse embryonic fibroblasts | (MEFs) |
| Multidrug resistance | (MDR) |
| Multidrug resistance protein 1 | (MDR1) |
| Myeloid cell leukemia 1 | (Mcl-1) |
| Nanocomposite | (NC) |
| Nanoparticles | (NPs) |
| Nanoparticle | (NP) |
| Nanostructured lipid carriers | (NLCs) |
| Neutral comet assay | (NCA) |
| Notch receptor blocker human | (DAPT) |
| Nuclear Factor-Kappa-B | (NF-κB) |
| Papillomavirus | (HPV) |
| Polyethylene glycol | (PEG) |
| PEGylated lipid nanocarriers | (PLN) |
| P-glycoprotein | (P-gp) |
| Phosphate buffer saline | (PBS) |
| Photodynamic inactivation | (PDI) |
| Photodynamic therapy | (PDT) |
| Photosensitizer | (PS) |
| Photothermal therapy | (PTT) |
| Poly (lactic-co-glycolic acid) nanoparticles | (PLGA NPs) |
| Poly (ethylene glycol) | (PEG) |
| Poly (lactic acid) | (PLA) |
| Poly (lactic-co-glycolic acid) | (PLGA) |
| Poly (ε-caprolactone) | (PCL) |
| Poly (ethylene glycol) polymer | (PEG) |
| Polyoxyethylene(40)stearate | (Myrj52) |
| Polyvinylpyrrolidone | (PVP) |
| Pressure ulcers | (PU) |
| Reactive oxygen species | (ROS) |
| Scanning electron microscopy | (SEM) |
| Silver nanoparticles | (AgNPs) |
| Excited Singlet state | (S1) |
| Solid lipid nanoparticles | (SLNs) |
| Squamous cell carcinoma | (SCC) |
| Tetraethyl orthosilicate | (TEOS) |
| The half maximal inhibitory concentration | (IC50) |
| Transforming growth factor beta | (TGF-β) |
| Tripolyphosphate | (TPP). |
| Tumor necrosis factor alpha | (TNF-α) |
| Tumor Nodes Metastasized | (TNM) |
| Ultraviolet A | (UVA) |
| Ultraviolet radiation B | (UVB) |
| Vascular endothelial growth factor | (VEGF) |
| Viability measurements | (MTT) |
| Zinc oxide nanoparticles | (ZnONPCS) |
References
- Raab, O. Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z. Biol. 1900, 39, 524–526. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Grzybowski, A.; Sak, J.; Pawlikowski, J. A brief report on the history of phototherapy. Clin. Dermatol. 2016, 34, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Von Tappeiner, H.; Jodlbauer, A. Die Sensiblilisierende Wirkung Fluoreszierender Substanzen. Gesamte Untersuchungen über die Photodynamische Erscheinung; FCW Vogel: Leipzig, Germany, 1907. [Google Scholar]
- Pröhl, M.; Schubert, U.S.; Weigand, W.; Gottschaldt, M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord. Chem. Rev. 2016, 307, 32–41. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.P.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 3, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figge, F.H.J.; Weiland, G.S.; Nanganiello, L.O.J. Cancer detection and therapy. Affinity of neoplastic, embryonic, and traumatized tissues for porphyrins and metalloporphyrins. Proc. Soc. Exp. Biol. Med. 1948, 68, 640–641. [Google Scholar] [CrossRef]
- Rasmussen-Taxdal, D.S.; Ward, G.E.; Figge, F.H.J. Fluorescence of human lymphatic and cancer tissues following high doses of intravenous hematoporphyrin. Cancer 1955, 8, 78–81. [Google Scholar] [CrossRef]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. Hematoporphyrin derivative: A new aid for endoscopic detection of malignant disease. J. Thorac. Cardiovasc. Surg. 1961, 42, 623–629. [Google Scholar] [CrossRef]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporphyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [Green Version]
- Kessel, D.; Thomas, J. Dougherty: An Appreciation. Photochem. Photobiol. 2020, 96, 454–457. [Google Scholar] [CrossRef] [Green Version]
- Sivasubramanian, M.; Chuang, Y.C.; Lo, L.-W. Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules 2019, 24, 520. [Google Scholar] [CrossRef] [Green Version]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Ożog, L.; David Aebisher, D. Singlet oxygen lifetime and diffusion measurements. Eur. J. Clin. Exp. Med. 2018, 16, 123–126. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle Ross, W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Metal-Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Babu, B.; Mack, J.; Nyokong, T. Sn(iv) N-confused porphyrins as photosensitizer dyes for photodynamic therapy in the near IR region. Dalton Trans. 2020, 49, 15180–15183. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wu, J.; Pang, X.; Jiang, Y.; Wang, P.; Leung, A.W.; Gao, L.; Jiang, S.; Xu, C. Discovery and Development of Natural Products and their Derivatives as Photosensitizers for Photodynamic Therapy. Curr. Med. Chem. 2017, 25, 839–860. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Liu, K.; Li, Y.; Wang, X.; Xie, X.; Jiao, X.; Tang, B. A light-activatable photosensitizer for photodynamic therapy based on a diarylethene derivative. Chem. Commun. 2021, 57, 8320–8323. [Google Scholar] [CrossRef]
- Pye, H.; Stamati, I.; Yahioglu, G.; Butt, M.A.; Deonarain, M. Antibody-Directed Phototherapy (ADP). Antibodies 2013, 2, 270–305. [Google Scholar] [CrossRef]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef] [PubMed]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Agel, M.R.; Engelhardt, K.H.; Pinnapireddy, S.R.; Agel, S.; Duse, L.; Preis, E.; Wojcik, M.; Bakowsky, U. Improvement of Pulmonary Photodynamic Therapy: Nebulisation of Curcumin-Loaded Tetraether Liposomes. Pharmaceutics 2021, 13, 1243. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.H.; Lord, M.S. Curcumin-loading-dependent stability of PEGMEMA-based micelles affects endocytosis and exocytosis in colon carcinoma cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliver Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Zhao, Y.; Jiang, J.; Li, T.; Zeng, X.; Yang, P.; Evans, C.E.; Cai, J. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale 2017, 9, 16365–16374. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.; Swamy, M.; Sinniah, U.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Kayani, Z.; Vais, R.D.; Soratijahromi, E.; Mohammadi, S.; Sattarahmady, N. Curcumin-gold-polyethylene glycol nanoparticles as a nanosensitizer for photothermal and sonodynamic therapies: In vitro and animal model studies. Photodiagn. Photodyn. Ther. 2021, 33, 102139. [Google Scholar] [CrossRef]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109810. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Kong, Z.L.; Kuo, H.P.; Johnson, A.; Wu, L.C.; Chang, K. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocolloid. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Sorolla, A.; Sorolla, A.M.; Wang, E.; Ceña, V. Peptides, proteins and nanotechnology: A promising synergy for breast cancer targeting and treatment. Expert Opin. Drug Deliv. 2020, 11, 1597–1613. [Google Scholar] [CrossRef]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake, and improved in vivo bioavailability. Colloids Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jourghanian, P.; Ghaffari, S.; Ardjmand, M.; Haghighat, S.; Mohammadnejad, M. Sustained release Curcumin loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Chen, S.; Zhou, J.; Dou, Y.; Song, L.; Che, L.; Zhou, X.; Chen, X.; Jia, Y.; Zhang, J.; et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials 2013, 34, 5344–5358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef]
- Reddy, D.N.K.; Kumar, R.; Wang, S.P.; Huang, F.Y. Curcumin-C3 Complexed with α-, β-cyclodextrin Exhibits Antibacterial and Antioxidant Properties Suitable for Cancer Treatments. Curr. Drug Metab. 2019, 20, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating Nanogels as an Effective Anticancer Formulation for Intracellular Uptake. Mol. Cell Pharmacol. 2015, 7, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-Responsive, Plasmonic Nanogel for Dual Delivery of Curcumin and Photothermal Therapy for Cancer Treatment. Front. Chem. 2021, 8, 602941. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases therapy. Biomed. Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Peng, Y.; Tan, H.; Li, M.; Li, W. Curcumin nanocrystallites are an ideal nanoplatform for cancer chemotherapy. Front Nanosci. Nanotech. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin Nanoformulations with Metal Oxide Nanomaterials for Biomedical Applications. Nanomaterials 2021, 1, 460. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. A biomolecule-assisted one-pot synthesis of zinc oxide nanoparticles and its bioconjugate with curcumin for potential multifaceted therapeutic applications. New J. Chem. 2019, 43, 11934–11948. [Google Scholar] [CrossRef]
- Globocan 2020: New Global Cancer Data. 17 December 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 20 July 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Reddy, R.M.; Kakarala, M.; Wicha, M.S. Clinical Trial Design for Testing the Stem Cell Model for the Prevention and Treatment of Cancer. Cancers 2011, 3, 2696–2708. [Google Scholar] [CrossRef]
- Park, C.H.; Hahm, E.R.; Park, S.; Kim, H.K.; Yang, C.H. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005, 579, 2965–2971. [Google Scholar] [CrossRef] [Green Version]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 77–785. [Google Scholar] [CrossRef] [Green Version]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Zhang, X.; Zheng, X.; Chen, Y.; Xuan, Z.; Huang, P. Curcumin suppresses LGR5(+) colorectal cancer stem cells by inducing autophagy and via repressing TFAP2A-mediated ECM pathway. J. Nat. Med. 2021, 75, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Frumkina, A.; Schwarzenbach, C.; Kaina, B. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients 2021, 13, 2385. [Google Scholar] [CrossRef]
- Zarrabi, A.; Zarepour, A.; Khosravi, A.; Alimohammadi, Z.; Thakur, V.K. Synthesis of Curcumin Loaded Smart pH-Responsive Stealth Liposome as a Novel Nanocarrier for Cancer Treatment. Fibers 2021, 9, 19. [Google Scholar] [CrossRef]
- Garufi, A.; Giorno, E.; Gilardini Montani, M.S.; Pistritto, G.; Crispini, A.; Cirone, M.; D’Orazi, G. p62/SQSTM1/Keap1/NRF2 Axis Reduces Cancer Cells Death-Sensitivity in Response to Zn(II)–Curcumin Complex. Biomolecules 2021, 11, 348. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Shpakova, V.; Sudnitsyna, J.; Panteleev, M.; Makhoul, S.; Gambaryan, S.; Jurk, K. Curcumin at Low Doses Potentiates and at High Doses Inhibits ABT-737-Induced Platelet Apoptosis. Int. J. Mol. Sci. 2021, 22, 5405. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Horvath, D.; Seliga, R.; Wagnieres, G. Influence of Oxidative Stress on Time-Resolved Oxygen Detection by [Ru(Phen)3]2+ In Vivo and In Vitro. Molecules 2021, 26, 485. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Development and Evaluation of Paclitaxel and Curcumin Dry Powder for Inhalation Lung Cancer Treatment. Pharmaceutics 2021, 13, 9. [Google Scholar] [CrossRef]
- Wan Mohd Tajuddin, W.N.B.; Abas, F.; Othman, I.; Naidu, R. Molecular Mechanisms of Antiproliferative and Apoptosis Activity by 1,5-Bis(4-Hydroxy-3-Methoxyphenyl)1,4-Pentadiene-3-one (MS13) on Human Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7424. [Google Scholar] [CrossRef]
- World Health Organization. Breast Cancer. 26 March 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 20 July 2021).
- National Cancer Institute. Advances in Breast Cancer Research. 9 April 2021. Available online: https://www.cancer.gov/types/breast/research (accessed on 20 July 2021).
- Yang, K.; Liao, Z.; Wu, Y.; Li, M.; Guo, T.; Lin, J.; Li, Y.; Hu, C. Curcumin and Glu-GNPs Induce Radiosensitivity against Breast Cancer Stem-Like Cells. BioMed Res. Int. 2020, 2020, 3189217. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef] [Green Version]
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 299–312. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Sudeep, H.V.; Chandrappa, S.; Raj, A.; Naveen, P.; Shyamaprasad, K. Efficacy of a Standardized Turmeric Extract Comprised of 70% Bisdemothoxy-Curcumin (REVERC3) Against LPS-Induced Inflammation in RAW264.7 Cells and Carrageenan-Induced Paw Edema. J. Inflamm. Res. 2021, 14, 859–868. [Google Scholar] [CrossRef]
- Huang, T.; Chen, Z.; Fang, L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells. Oncol. Rep. 2013, 29, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, Q.; Duan, P.; Yang, L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2018, 40, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer. (Review). Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef]
- Shahabipour, F.; Caraglia, M.; Majeed, M.; Derosa, G.; Maffioli, P.; Sahebkar, A. Naturally occurring anti-cancer agents targeting EZH2. Cancer Lett. 2017, 400, 325–335. [Google Scholar] [CrossRef]
- Guan, X.; Shi, A.; Zou, Y.; Sun, M.; Zhan, Y.; Dong, Y.; Fan, Z. EZH2-Mediated microRNA-375 Upregulation Promotes Progression of Breast Cancer via the Inhibition of FOXO1 and the p53 Signaling Pathway. Front. Genet. 2021, 12, 633756. [Google Scholar] [CrossRef]
- Gallardo, M.; Kemmerling, U.; Aguayo, F.; Bleak, T.C.; Muñoz, J.P.; Calaf, G.M. Curcumin rescues breast cells from epithelial−mesenchymal transition and invasion induced by anti−miR−34a. Int. J. Oncol. 2020, 56, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Silva de Sá, I.; Peron, A.P.; Leimann, F.V.; Bressan, G.N.; Krum, B.N.; Fachinetto, R.; Pinela, J.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem. Toxicol. 2019, 125, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef]
- Damyeh, M.S.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Barkat, M.A.; Harshita Rizwanullah, M.; Pottoo, F.H.; Beg, S.; Akhter, S.; Ahmad, F.J. Therapeutic Nanoemulsion: Concept to Delivery. Curr. Pharm. Des. 2020, 26, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Shouqair, D.; Ramesh, K.V.R.N.S.; Khaleel, T.; Amin, M.; Boateng, J.; Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 2020, 576, 118952. [Google Scholar] [CrossRef]
- Machado, F.C.; Adum de Matos, R.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the use of nanocarrier systems to deliver the magical molecule; Curcumin and its derivatives. J. Control. Release 2016, 225, 1–30. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Jayarama Bhat, G.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control. Release 2018, 290, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Menyailo, M.E.; Bokova, U.A.; Ivanyuk, E.E.; Khozyainova, A.A.; Denisov, E.V. Metastasis Prevention: Focus on Metastatic Circulating Tumor Cells. Mol. Diagn. Ther. 2021, 25, 549–562. [Google Scholar] [CrossRef]
- Raschpichler, M.; Preis, E.; Pinnapireddy, S.R.; Baghdan, E.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, F.; Attaran-Kakhki, N.; Sazgarnia, A. The synergistic effect of photodynamic therapy and photothermal therapy in the presence of gold-gold sulfide nanoshells conjugated Indocyanine green on HeLa cells. Photodiagn. Photodyn. Ther. 2017, 17, 48–55. [Google Scholar] [CrossRef]
- Hu, J.J.; Cheng, Y.J.; Zhang, X.Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.N.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. 2020, 10, 9765. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef] [PubMed]
- Ashkbar, A.; Rezaei, F.; Attari, F.; Ashkevarian, S. Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci. Rep. 2020, 10, 21206. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Arvanitidou, M.; Mavroidi, B.; Hatzidimitriou, A.G.; Politopoulos, K.; Alexandratou, E.; Pelecanou, M.; Sagnou, M. A novel curcumin gallium complex as photosensitizer in photodynamic therapy: Synthesis, structural and physicochemical characterization, photophysical properties and in vitro studies against breast cancer cells. J. Mol. Struct. 2021, 1240, 130485. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33, Erratum in 2021, 71, 359. [Google Scholar] [CrossRef]
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Webb-Vargas, Y.; Wheeler, W.; Gail, M.H. Proportion of U.S. trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International patterns and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, featuring survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- De Matos, R.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. Biomed. Res. Int. 2018, 2018, 4057959. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Patel, J.; Amrutiya, J.; Bhatt, P.; Javia, A.; Jain, M.; Misra, A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Pereira, M.C. PLGA Based Drug Carrier and Pharmaceutical Applications: The Most Recent Advances. Pharmaceutics 2020, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Mu, T.; Yuan, Y.; Yang, W.; Zhang, Y.; Chen, Q.; Bian, M.; Pan, Y.; Xiang, Q.; Chen, Z.; et al. Effects of Notch Signaling Pathway in Cervical Cancer by Curcumin Mediated Photodynamic Therapy and Its Possible Mechanisms in Vitro and in Vivo. J. Cancer 2019, 10, 4114–4122. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, M.; Anusewicz, D.; Bednarek, A.K. Functional Gene Expression Differentiation of the Notch Signaling Pathway in Female Reproductive Tract Tissues-A Comprehensive Review with Analysis. Front. Cell Dev. Biol. 2020, 8, 592616. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Skin Cancer Types. This Page Was Updated on 22 July 2021. Available online: https://www.cancercenter.com/cancer-types/skin-cancer/types (accessed on 8 August 2021).
- Key Statistics for Basal and Squamous Cell Skin Cancers. American Cancer Society. 12 January 2021. Available online: http://www.cancer.org/cancer/skincancer-basalandsquamouscell/detailedguide/skin-cancer-basal-and-squamous-cell-key-statistics (accessed on 8 August 2021).
- American Cancer Society. Cancer Facts and Figures. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 8 August 2021).
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 5, 1989–2007. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Tzellos, T.G.; Kechagias, N.; Patrikidou, A.; Xirou, P.; Kitikidou, K.; Bourlidou, E.; Vahtsevanos, K.; Antoniades, K. Cutaneous squamous cell carcinoma (SCC) of the head and neck: Risk factors of overall and recurrence-free survival. Eur. J. Cancer 2010, 46, 1563–1572. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Maubec, E. Update on the Management of Cutaneous Squamous Cell Carcinoma. Acta Dermato-Venereol. 2020, 100, adv00143. [Google Scholar] [CrossRef] [PubMed]
- Najjar, T.; Meyers, A.D.; Monroe, M.M.; Alam, M.; Baibak, L.M.; Campbell, W.J.; Caputy, G.; de la Torre, J.I.; DeBacker, C.; Dryden, R.M.; et al. Cutaneous Squamous Cell Carcinoma. Medscape. Emedicine, Updated: 8 July 2020. Available online: https://emedicine.medscape.com/article/1965430-overview#a6 (accessed on 8 August 2021).
- Byeon, H.K.; Ku, M.; Yang, J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bander, T.S.; Nehal, K.S.; Lee, E.H. Cutaneous Squamous Cell Carcinoma: Updates in Staging and Management. Derm. Clin. 2019, 37, 241–251. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. Epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hober, C.; Jamme, P.; Desmedt, E.; Greliak, A.; Mortier, L. Dramatic response of refractory metastatic squamous cell carcinoma of the skin with cetuximab/pembrolizumab. Ther. Adv. Med. Oncol. 2021, 13, 17588359211015493. [Google Scholar] [CrossRef]
- Cowey, C.L.; Robert, N.J.; Espirito, J.L.; Davies, K.; Frytak, J.; Lowy, I.; Fury, M.G. Clinical outcomes among unresectable, locally advanced, and metastatic cutaneous squamous cell carcinoma patients treated with systemic therapy. Cancer Med. 2020, 20, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Chen, Y.H.; Meng, T.; Qu, L.H.; Zhang, Z.Y.; Zhang, X. Comparative efficacy and safety of radiotherapy/cetuximab versus radiotherapy/chemotherapy for locally advanced head and neck squamous cell carcinoma patients: A systematic review of published, primarily non-randomized, data. Ther. Adv. Med. Oncol. 2020, 12, 1758835920975355. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Q.; Zheng, Z.; Liu, Z.; Meng, L.; Dong, L.; Jiang, X. Status of Treatment and Prophylaxis for Radiation-Induced Oral Mucositis in Patients with Head and Neck Cancer. Front. Oncol. 2021, 11, 642575. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Li, L.; Li, X. Photodynamic therapy-induced apoptosis of keloid fibroblasts is mediated by radical oxygen species in vitro. Clin. Lab. 2015, 61, 1257–1266. [Google Scholar] [CrossRef]
- Cohen, D.K.; Lee, P.K. Photodynamic Therapy for Non-Melanoma Skin Cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Griffin, L.L.; Lear, J.T. Photodynamic Therapy and Non-Melanoma Skin Cancer. Cancers 2016, 8, 98. [Google Scholar] [CrossRef]
- Steeb, T.; Schlager, J.G.; Kohl, C.; Ruzicka, T.; Heppt, M.V.; Berking, C. Laser-assisted photodynamic therapy for actinic keratosis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 947–956. [Google Scholar] [CrossRef]
- Gu, X.; Zhao, S.; Shen, M.; Su, J.; Chen, X. Laser-assisted photodynamic therapy vs. conventional photodynamic therapy in non-melanoma skin cancers: Systematic review and meta-analysis of randomized controlled trials. Photodermatol. Photoimmunol. Photomed. 2021. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Karrer, S. Photodynamische Therapie—Trends und neue Entwicklungen [Photodynamic therapy-trends and new developments]. Hautarzt 2021, 72, 27–33. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, Q.; Zhang, P.; Guo, W.W.; Zhang, L.Z.; Jiang, G. Demethoxycurcumin in combination with ultraviolet radiation B induces apoptosis through the mitochondrial pathway and caspase activation in A431 and HaCaT cells. Tumour Biol. 2017, 39, 1010428317706216. [Google Scholar] [CrossRef] [Green Version]
- Abdel Fadeel, D.A.; Kamel, R.; Fadel, M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: In-vitro/in-vivo studies and histopathological examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef]
- Luo, H.; Lu, L.; Liu, N.; Li, Q.; Yang, X.; Zhang, Z. Curcumin loaded sub-30 nm targeting therapeutic lipid nanoparticles for synergistically blocking nasopharyngeal cancer growth and metastasis. J. Nanobiotechnol. 2021, 19, 224. [Google Scholar] [CrossRef]
- Fadel, M.; Kassab, K.; Abd El Fadeel, D.A.; Nasr, M.; El Ghoubary, N.M. Comparative enhancement of curcumin cytotoxic photodynamic activity by nanoliposomes and gold nanoparticles with pharmacological appraisal in HepG2 cancer cells and Erlich solid tumor model. Drug Dev. Ind. Pharm. 2018, 44, 1809–1816. [Google Scholar] [CrossRef]
- Yamazaki, S.; Sekiguchi, A.; Uchiyama, A.; Fujiwara, C.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; Akai, R.; et al. Apelin/APJ signalling suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model. Sci. Rep. 2020, 10, 1349. [Google Scholar] [CrossRef]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global prevalence and incidence of pressure injuries in hospitalized adult patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef]
- Coelho, V.H.M.; Alvares, L.D.; Carbinatto, F.M.; de Aquino Junior, A.E.; Angarita, D.P.R.; Bagnato, V.S. Phtodynamic Therapy, Laser Therapy and Cellulose Membrane for the Healing of Venous Ulcers: Results of a Pilot Study. J. Nurs. Care 2017, 6, 387. [Google Scholar] [CrossRef]
- Mussttaf, R.A.; Jenkins, D.F.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound healing effects of curcumin: A short review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Ebrahiminaseri, A.; Sadeghizadeh, M.; Moshaii, A.; Asgaritarghi, G.; Safari, Z. Combination treatment of dendrosomal nanocurcumin and low-level laser therapy develops proliferation and migration of mouse embryonic fibroblasts and alter TGF-β, VEGF, TNF-α and IL-6 expressions involved in wound healing process. PLoS ONE 2021, 16, e0247098. [Google Scholar] [CrossRef]
- Bomar, L.; Senithilnathan, A.; Ahn, C. Systemic Therapies for Advanced Melanoma. Dermatol. Clin. 2019, 37, 409–423. [Google Scholar] [CrossRef]
- Queirolo, P.; Boutros, A.; Tanda, E.; Spagnolo, F.; Quaglino, P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: A model of cancer immunotherapy. Semin. Cancer Biol. 2019, 59, 290–297. [Google Scholar] [CrossRef]
- Poklepovic, A.S.; Luke, J.J. Considering adjuvant therapy for stage II melanoma. Cancer 2020, 126, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Corrigendum to “Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)—In vitro studies”. Biomed. Pharmacother. 2020, 132, 110883, Erratum in 2021, 139, 111694. [Google Scholar] [CrossRef]
- Mohammadi, S.; Soratijahromi, E.; Dehdari Vais, R.; Sattarahmady, N. Phototherapy and Sonotherapy of Melanoma Cancer Cells. Using Nanoparticles of Selenium-Polyethylene Glycol-Curcumin as a Dual-Mode Sensitizer. J. Biomed. Phys. Eng. 2020, 10, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Vetha, S.B.S.; Oh, P.S.; Kim, S.H.; Jeong, H.J. Curcuminoids encapsulated liposome nanoparticles as a blue light emitting diode induced photodynamic therapeutic system for cancer treatment. J. Photochem. Photobiol. B 2020, 205, 111840. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.G.; Oh, J.H.; Hong, S.J. Species-specific role of gene-adjacent retroelements in human and mouse gastric carcinogenesis. Int. J. Cancer 2018, 142, 1520–1527. [Google Scholar] [CrossRef]
- Hung, K.F.; Yang, T.; Kao, S.Y. Cancer stem cell theory: Are we moving past the mist? J. Chin. Med. Assoc. 2019, 82, 814–818. [Google Scholar] [CrossRef]
- Rhyu, M.G.; Oh, J.H.; Kim, T.H.; Kim, J.S.; Rhyu, Y.A.; Hong, S.J. Periodic Fluctuations in the Incidence of Gastrointestinal Cancer. Front. Oncol. 2021, 11, 558040. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vetha, B.S.S.; Kim, E.M.; Oh, P.S.; Kim, S.H.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Curcumin Encapsulated Micellar Nanoplatform for Blue Light Emitting Diode Induced Apoptosis as a New Class of Cancer Therapy. Macromol. Res. 2019, 27, 1179–1184. [Google Scholar] [CrossRef]
- Şueki, F.; Ruhi, M.K.; Gülsoy, M. The effect of curcumin in antitumor photodynamic therapy: In vitro experiments with Caco-2 and PC-3 cancer lines. Photodiagn. Photodyn. Ther. 2019, 27, 95–99. [Google Scholar] [CrossRef]
- De Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110853. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Polychronidis, G.; Heger, U.; Frongia, G.; Mehrabi, A.; Hoffmann, K. Incidence trends and survival prediction of hepatoblastoma in children: A population-based study. Cancer Commun. 2019, 39, 62. [Google Scholar] [CrossRef] [Green Version]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016, 36, 3363–3372. [Google Scholar] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing role of P-glycoprotein in tumor microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kiguchi, K.; Mikami, M.; Aoki, D.; Iwamori, M. Involvement of the MDR1 gene and glycolipids in anticancer drug-resistance of human ovarian carcinoma-derived cells. Hum. Cell 2019, 32, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, S.; Liu, C.; He, J.; Li, T.; Fu, C.; Meng, X.; Shao, H. Enhanced Photothermal-Photodynamic Therapy by Indocyanine Green and Curcumin-Loaded Layered MoS2 Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomed. 2021, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Lin, J.J.; Shaw, A.T. Resisting Resistance: Targeted Therapies in Lung Cancer. Trends Cancer 2016, 2, 350–364. [Google Scholar] [CrossRef] [Green Version]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, R.; He, X.; Wang, J.; Wang, M.; Qian, Y.; Wang, S. Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles. Int. J. Nanomed. 2016, 12, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Tang, X.; Qiu, X.; Jiang, K.; Wu, J.; Hu, Y. Activatable photodynamic destruction of cancer cells by NIR dye/photosensitizer loaded liposomes. Chem. Commun. 2015, 51, 3340–3342. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Tao, D.; Dong, Z.; Chen, Q.; Chao, Y.; Liu, Z.; Chen, M. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 2017, 127, 13–24. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Zhang, Y.; Zhou, S.; Cai, H.H.; Li, T.; Jin, H.; Cai, J.; Zhou, H.; Pi, J. GE11 Peptide Conjugated Liposomes for EGFR-Targeted and Chemophotothermal Combined Anticancer Therapy. Bioinorg. Chem. Appl. 2021, 2021, 5534870. [Google Scholar] [CrossRef]
- Baghdan, E.; Duse, L.; Schüer, J.J.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy. Eur. J. Pharm. Sci. 2019, 132, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Lee, A.; Arasaratnam, M.; Chan, D.L.H.; Khasraw, M.; Howell, V.M.; Wheeler, H. Anti-epidermal growth factor receptor therapy for glioblastoma in adults. Cochrane Database Syst. Rev. 2020, 5, CD013238. [Google Scholar] [CrossRef]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagn. Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, C.M.D.; Monteleoni, L.F.; Calmon, M.F.; Candido, N.M.; Provazzi, P.J.S.; Lino, V.S.; Rabachini, T.; Sichero, L.; Villa, L.L.; Quintana, S.M.; et al. Antiviral activity of curcumin-nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16. Artif. Cells Nanomed. Biotechnol. 2020, 48, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
| References | Type of Study | Type of Light and Curcumin | Total Energy (J) Applied | Analyzed Parameters | Conclusions |
|---|---|---|---|---|---|
| [94] | In vitro experimental 4T1 mouse breast cancer cells. | Blue light 450 nm. Curcumin nanodrugs (Cur NDs) | Blue light (P = 640 mW) with a wavelength of 450 nm at a fixed distance of 13 cm for 1 min. | In vitro cytotoxicity. Intracellular ROS production was analyzed using an intracellular ROS kit. c-Jun N-terminal kinase (JNK); p-JNK; mitogen-activated protein kinase (MAPK); caspase-3 (Casp 3); Bcl-2–associated X (Bax); Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). | This study proved that Cur NDs could be a full of promise PS for speeding up the performance and reliability of PDT against breast cancer, with very good prospects for use in clinical practice. |
| [98] | In vitro breast cancer model, MCF-7 cells. | LED 440 (±10) nm, with 420 mW output power, 2.52 W total power, with 209 W/cm2 irradiance. Curcumin-nanoemulsion (CNE). | 80 J/cm2 fluency, set at 6.4 s/application | Caspases 3 and 7 activity. Estimation of intracellular reactive oxygen species production by 2′-7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) technique. | Curcumin-nanoemulsion and PDT increased the activity of capsases 3 and 7, had a phototoxic effect with a significant reduction in MCF-7 cell proliferation and stimulated ROS release; this association has great prospects for breast cancer therapy. |
| [102] | In vitro experimental model of circulating tumor cells (CTCs) with human breast cancer cells (MDA-MB-231, ATCC HTB-26). | Blue light (447 nm, 100 mW). CUR-NPs. | MDA-MB-231 cells were laser irradiated for 30 min under flow conditions (5 cm s−1). | Cell viability assay Morphology of nanoparticles Photodynamic inactivation Scanning electron microscopy of circulating breast cancer cells. CLSM micrographs showing cellular curcumin accumulation and photodynamic effect of curcumin loaded nanoparticles (CUR-NPs) on circulating MDA-MB-231 cells. | Apoptosis and necrosis of metastatic malignant cells were demonstrated by this experimental study in vitro on human breast cancer cells, using CUR-PLGA NPs and 30 min laser irradiation (447 nm and 100 mW) under continuous flow conditions. Results open new perspectives in clinical oncology for targeting metastases. |
| [103] | In vitro MCF-7 Human Breast Cancer Cell-Line. | LED 430-nm GaAlAs, CW. In vitro release of Curcumin Nanostructured Lipid Carriers (CUR-NLCs) formulas. | Irradiation protocol: blue light (430 nm) for 5 min (power 100 mW), spot size radius 4 cm, irradiance 2 mW/cm2, fluence 6 J/cm2). | Determination of encapsulation efficiency and drug loading percentages by spectrophotometry measurements. Morphology changes by transmission electron microscopy. Dark and photo-cytotoxicity studies of MCF-7 cells survival. | Carriers of nanostructured lipids loaded with curcumin and olive oil used in conjunction with PDT have increased the potency of penetrating breast cancer cells and the cytotoxic activity. The results of the study suggest that CUR-NLCs in low doses after exposure to blue light have a significant anticancer effect in breast cancer. |
| [109] | In vivo on the breast cancer model in Female Balb/c mice (6 to 8 weeks). | Blue diode laser at 450nm, CW for PDT, and 808 nm in NIR range for PTT. An external magnetic field was applied for appropriate delivery of the drug. Curcumin on silica-coated Fe3O4 nanoparticles. | In vivo experiment: with female Balb/c tumorized mice, divided into 6 groups: (I) PBS injection (control group). (II) Curcumin plus irradiation with a blue diode laser at 450 nm with 150 mW/cm2 for 3 min (CUR + PDT group). (III) Blue diode laser for 3 min followed by NIR laser with 0.5W/cm2 for 7 min (Blue + NIR lasers group). (IV) injection of 40 µL NC (NC group). (V) injection of 40 µL of NC solution containing 20 µg curcumin (0.46 mg/mL) plus irradiation with NIR laser at 808 nm for 7 min (NC + PTT). (VI) injection of 40 µL of NC containing 20 µg curcumin plus irradiations with two lasers with up-mentioned intensities and exposure times, while a rigid magnet was fixed on the tumor to maintain the injected NC in the tumor position (NC + PDT + PTT group). | Analysis of expression of apoptotic proteins Bax and Caspase 3. In vitro toxicity of Fe3O4/ SiO2 NPs after 24 and 48 h. In vitro release of curcumin from the NCs. Antitumor effect of nanocomposite plus PDT and PTT approach in vivo. | In the group treated with NC+PDT+PTT the tumor volume was significantly reduced and the expression of proapoptotic proteins Bax and Caspase 3 increased significantly compared to the control group, without weight loss, no adverse effects on vital organs in the mice autopsy images. Method could replace chemotherapy for triple-negative breast cancers. |
| [110] | In vitro study of human breast adenocarcinoma MCF-7 cells. | 5 min irradiation at 450 nm and 100 mW/cm2. Ga(III)-curcumin complex. | 6 mW/cm2 and the exposure times were 167 s, 334 s, 501 s, 1002 s, and 6012 s yielding 1, 2, 3, 6 and 10 J/cm2 fluence, respectively. | Photophysical and photochemical studies (UV-Visible absorption and fluorescence; ROS production; in vitro cytotoxicity assay. Dark cytotoxicity. | Administration of the Ga (III) -curcumin complex studied on MCF-7 breast cancer cells has shown that metal complexation increases its photodynamic effect compared to simple curcumin. |
| References | Type of Study | Type of Light and Curcumin | Total Energy (J) Applied | Analyzed Parameters | Conclusions |
|---|---|---|---|---|---|
| [145] | In vitro A431 -human cell line model (epidermoid carcinoma cell line) and HaCaT cells (spontaneously transformed aneuploid immortal keratinocyte cell line from adult human skin) | Ultraviolet radiation B (UVB) Demethoxycurcumin (DMC) | UVB (10–100 mJ/cm2) | Inhibition of tumor cell growth. Enhancement of apoptosis in cells. Apoptosis-associated proteins including Bcl-2, Mcl-1, Bax, nuclear factor-κB (p65), p-p65, p53, caspase-3, caspase-9, and cytochrome C. Measurement of ROS (which increased significantly). Analysis of mitochondrial potential (which decreased: important depolarization occurred). | PDT by ultraviolet B radiation and DMC have experimentally succeeded in causing apoptosis in skin cancer cells. DMC may be a promising photosensitizer for PDT to eradicate skin cancer cells. |
| [146] | In-vitro/In-vivo studies and histopathological examination on a human skin cancer cell line (A431) | Blue light (410 nm); PEGylated lipid nanocarriers (PLN) loaded with curcumin (Cur). | In vitro 300 mW/cm2 for 4 min by blue light. In vivo LED (420 nm) for 10 min at a fluence of 90 mW/cm2 | Fluorescence intensity measured by confocal laser microscopy. Histopathological studies. In-vitro cytotoxicity. | This in vitro study with Cur-loaded PLN together with blue light proved a significantly higher cytotoxicity than the control sample against human epidermoid squamous cell carcinoma cell line (A431). In vivo study showed a significant improvement in skin carcinoma after photodynamic therapy and Cur-loaded PEGylated lipid nanoparticles. Beneficial effects of this safe and economical method, bring hope in the treatment of cancer. |
| [155] | In vitro experiments on mouse embryonic fibroblasts (MEFs) cells | Diode laser device with a wavelength of 450 nm and an output power of 75 mW. Dendrosomal Nano-Curcumin (DNC) | Cells were irradiated for 224 s (for getting a dose of 17.9 J, with an energy density of 0.63 J/cm2), and 337 s (for getting a dose of 26.9 J, with an energy density of 0.95 J/cm2). For other doses, the time was set in the same way. | - RNA extraction was quantified by spectrophotometry - cDNA synthesis - TGF-β, VEGF, TNF-α, IL-6 and glyceraldehydes 3-phosphate dehydrogenase (GAPDH). In vitro migration assay for cell motility; cell cycle analysis by flow cytometry; quantitation of DNA content stained. Measurements of intracellular ROS. | Results revealed a notable proliferation of mouse embryonic fibroblasts after the combination of DNC + LLLT (450 nm) at a dose of 0.95 J/cm2. Simultaneous exposure to DNC +LLLT enriched S-phase entry and increased proliferation as well as significant migration of MEF cells in the denuded area, up-regulating growth factors (TGF-β, VEGF) and shortening the inflammatory phase by modulating cytokines (TNF-α, IL-6). Combined therapy (DNC + LLLT) also highlights the modulating role of nanocurcumin on the production and excessive accumulation of ROS generated by laser action. |
| [159] | In vitro studies on curcumin + PDT on melanotic (A375) and amelanotic melanoma (C32) cell lines. | Lamp with polarized light with power density set to 20 mW/cm2, blue light (380–500 nm), including maximum absorption of curcumin (410 nm). Curcumin dissolved in dimethyl sulfoxide (DMSO). | Irradiation time = 5 min (6 J/cm2). | MTT cell viability assay. Cell death evaluation by neutral comet assay (NCA). Fluorescent staining of actin filaments Caspase-3 immunocytochemical staining. Holotomographic microscopy studies. Cell viability and phototoxicity. | PDT + curcumin increased the number of apoptotic and necrotic cells compared to the control without irradiation, it induced overexpression of caspase-3 and DNA cleavage and low cell proliferation due to reorganization of the actin cytoskeleton. PDT together with curcumin can be an effective way to induce apoptosis in melanoma. |
| [160] | Experimental study on malignant melanoma C540 (B16/F10) cell line. | 808-nm laser. Ultrasound (US). Nanoparticles of selenium- polyethylene glycol-curcumin (Se-PEG-Cur). | Output power = 1000 mW Power density = 1.0 W/cm2. Irradiation time = 10 min. US output power of 1.0 W/cm2; Frequency of 1MHz; Irradiation time = 1 min. | Detection of intracellular ROS Viability of C540 (B16/F10) cells. Fluorescence intensity (FI). | Se-PEG-Cur can be a very good photosensitizer for phototherapy plus sonotherapy in the destruction of melanoma cancer cells through thermal and ROS-generating effects. |
| [162] | Experiments on melanoma (MugMel2), squamous cell carcinoma (SCC-25), and normal human keratinocytes (HaCaT) cell lines. | Blue light (380–500 nm); 20 mW/cm2. Liposomal Curcumin. | Irradiation time = 2 min; 2.5 J/cm2 | Impact of Liposomal Curcumin on Cells Lines’ Apoptosis. Bax and Bcl-2 Expression. Cell Viability Assay. Wound-Healing Assay. | Experimental study demonstrated a significant improvement in the bioavailability and stability of liposomal encapsulated curcumin as a potent apoptotic photosensitizer in squamous cell carcinoma (SCC-25) and melanoma (MugMel2). Low phototoxicity was observed in normal cutaneous keratinocyte HaCaT cells after PDT treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. https://doi.org/10.3390/pharmaceutics13101562
Ailioaie LM, Ailioaie C, Litscher G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics. 2021; 13(10):1562. https://doi.org/10.3390/pharmaceutics13101562
Chicago/Turabian StyleAilioaie, Laura Marinela, Constantin Ailioaie, and Gerhard Litscher. 2021. "Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer" Pharmaceutics 13, no. 10: 1562. https://doi.org/10.3390/pharmaceutics13101562
APA StyleAilioaie, L. M., Ailioaie, C., & Litscher, G. (2021). Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics, 13(10), 1562. https://doi.org/10.3390/pharmaceutics13101562