Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
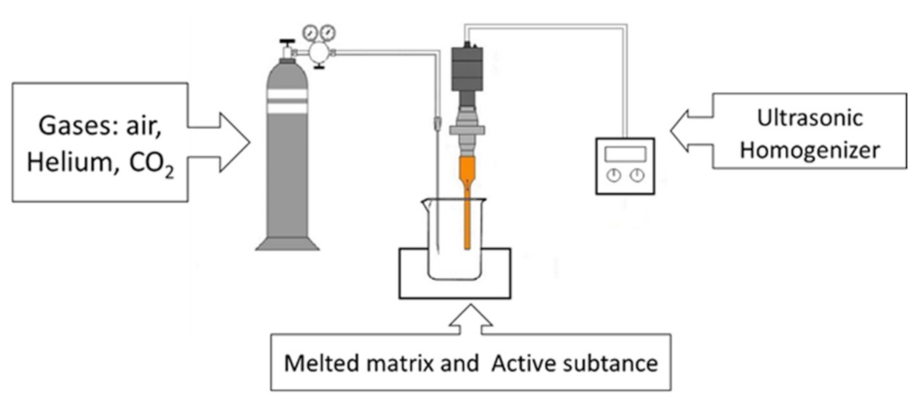
2.2.1. Development of the Ultrasonic Foaming Equipment
2.2.2. Optimization of Process Parameters
2.2.3. Preparation of Floating Acyclovir-Loaded Samples
2.2.4. Determination of the Density of Samples
2.2.5. Contact Angle Measurement
2.2.6. SEM Analysis and Chemical Element Analysis
2.2.7. Determination of Gas Content
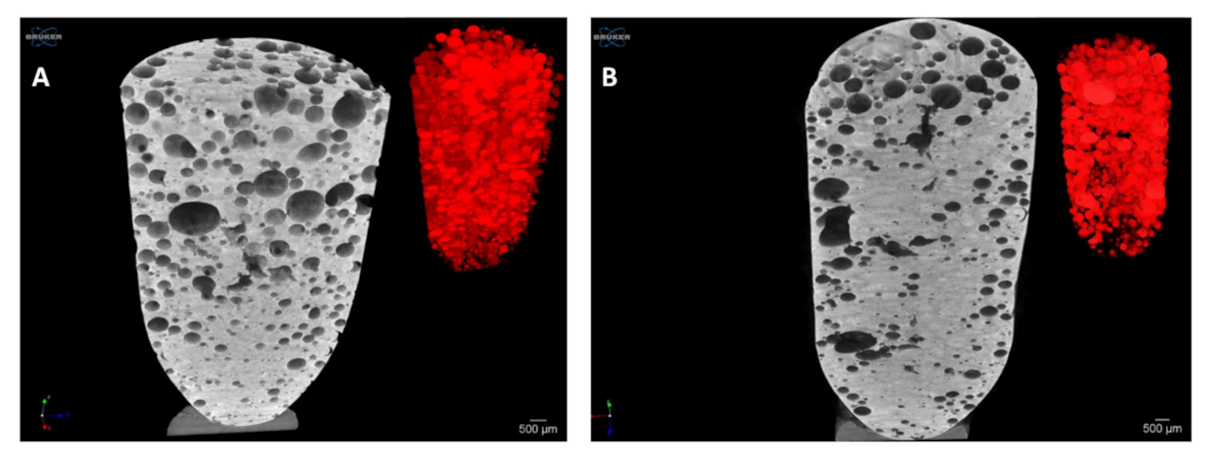
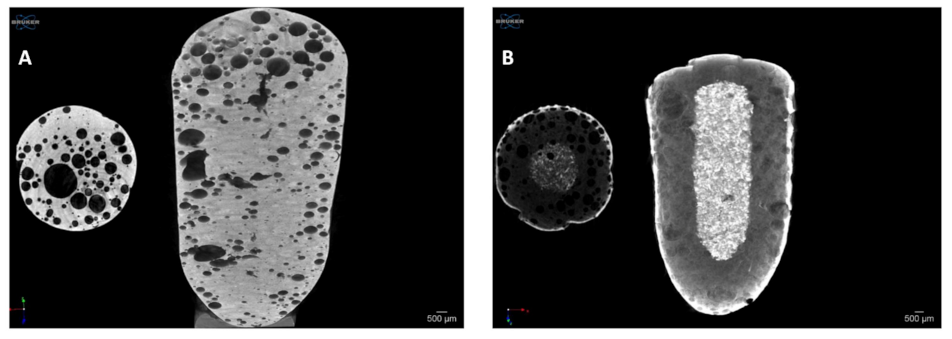
2.2.8. Microtomography and Size Distribution of Foam Bubbles
2.2.9. Dissolution Test
2.2.10. Mathematical Analysis of the Drug Release Profiles
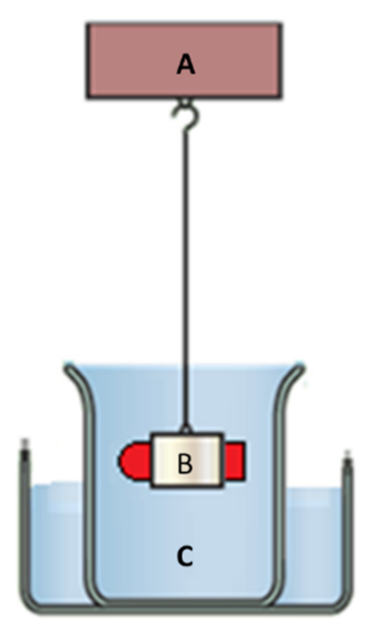
2.2.11. Floating Strength Determination
2.2.12. Ex Vivo Mucoadhesion Studies
2.2.13. Statistical Analysis
3. Results
3.1. Optimization of Process Parameters
3.2. Determination of the Density of Samples
3.3. SEM Analysis and Chemical Element Analysis
3.4. Contact Angle Measurement
3.5. Microtomography and Size Distribution of Foam Bubbles
3.6. Dissolution Test
3.7. Floating Force Determination
3.8. Ex Vivo Mucoadhesion Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Pal, P.; Sharma, V.; Singh, L. A review on floating type gastro retentive drug delivery system. Int. Res. J. Pharm. 2012, 3, 37–43. [Google Scholar]
- Hao, S.; Wang, B.; Wang, Y. Density-dependent gastroretentive microparticles motion in human gastric emptying studied using computer simulation. Eur. J. Pharm. Sci. 2015, 70, 72–81. [Google Scholar] [CrossRef]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Tiwari, R.; Sharun, K.; Dhama, K.; Harapan, H. Antivirals for COVID-19: A critical review. Clin. Epidemiol. Glob. Heal. 2021, 9, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Dalavi, V.V.; Patil, J.S. Gastroretentive drug delivery system of an antiretroviral agent. Int. J. PharmTech Res. 2009, 1, 1678–1684. [Google Scholar]
- El Gamal, S.S.; Naggar, V.F.; Allam, A.N. Optimization of acyclovir oral tablets based on gastroretention technology: Factorial design analysis and physicochemical characterization studies. Drug Dev. Ind. Pharm. 2011, 37, 855–867. [Google Scholar] [CrossRef]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Garg, B.; Chaturvedi, S.C.; Arora, S.; Mandsaurwale, R.; Kapil, R.; Singh, B. Formulation development of gastroretentive tablets of lamivudine using the floating-bioadhesive potential of optimized polymer blends. J. Pharm. Pharmacol. 2012, 64, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef] [Green Version]
- de Souza, M.P.C.; Sábio, R.M.; Ribeiro, T.d.C.; dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.; Singh, D.; Pathak, K. Bifunctional capsular dosage form: Novel fanicular cylindrical gastroretentive system of clarithromycin and immediate release granules of ranitidine HCl for simultaneous delivery. Int. J. Pharm. 2014, 461, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Huang, J.; Luo, Z.; Li, J.; Wang, L.; Di, L. Mucoadhesive improvement of alginate microspheres as potential gastroretentive delivery carrier by blending with Bletilla striata polysaccharide. Int. J. Biol. Macromol. 2020, 156, 1191–1201. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Ahmed, T.A.; Lila, A.E.A.; Samy, A.M.; Kaseem, A.A.; Nutan, M.T.H. Mucoadhesive controlled release microcapsules of indomethacin: Optimization and stability study. J. Microencapsul. 2010, 27, 377–386. [Google Scholar] [CrossRef]
- Patil, S.; Rathi, M.; Misra, A. Applications of Polymers in Gastric Drug Delivery, 2nd. ed; Misra, A., Shahiwala, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lohani, A.; Chaudhary, G. Mucoadhesive microspheres: A novel approach to increase gastroretention. Chron. Young Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Louis, M.M.; Badawy, A.A.; Nessem, D.I.; Abd Elmalak, N.S. Drotaverine hydrochloride gastroretentive floating mini-tablets: Formulation, in-vitro and in-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101733. [Google Scholar] [CrossRef]
- Simons, F.J.; Wagner, K.G. Modeling, design and manufacture of innovative floating gastroretentive drug delivery systems based on hot-melt extruded tubes. Eur. J. Pharm. Biopharm. 2019, 137, 196–208. [Google Scholar] [CrossRef]
- Vasvári, G.; Haimhoffer, Á.; Horváth, L.; Budai, I.; Trencsényi, G.; Béresová, M.; Dobó-Nagy, C.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Development and characterisation of gastroretentive solid dosage form based on melt foaming. AAPS PharmSciTech 2019, 20, 290. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Vasvári, G.; Trencsényi, G.; Béresová, M.; Budai, I.; Czomba, Z.; Rusznyák, Á.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Process optimization for the continuous production of a gastroretentive dosage form based on melt foaming. AAPS PharmSciTech 2021, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Mankovits, T.; Budai, I.; Balogh, G.; Gábora, A.; Kozma, I.; Varga, T.; Manó, S.; Kocsis, I. Structural analysis and its statistical evaluation of a closed-cell metal foam. Int. Rev. Appl. Sci. Eng. 2014, 5, 135–143. [Google Scholar] [CrossRef]
- Mukherjee, M.; García-Moreno, F.; Jiménez, C.; Rack, A.; Banhart, J. Microporosity in aluminium foams. Acta Mater. 2017, 131, 156–168. [Google Scholar] [CrossRef]
- Baker, V.S. Acyclovir for SARS-CoV-2: An ClinicalPractice old drug with a new purpose. Clin. Pract. 2021, 18, 1584–1592. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Samaha, D.; Shehayeb, R.; Kyriacos, S. Modeling and comparison of dissolution profiles of diltiazem modified-release formulations. Dissolution Technol. 2009, 16, 41–46. [Google Scholar] [CrossRef]
- Diós, P.; Nagy, S.; Pál, S.; Pernecker, T.; Kocsis, B.; Budán, F.; Horváth, I.; Szigeti, K.; Bölcskei, K.; Máthé, D.; et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015, 96, 196–206. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Romero-Azogil, L.; Benito, E.; Lucas, R.; García-Martín, M.G.; De-Paz, M.V. In-depth study into polymeric materials in low-density gastroretentive formulations. Pharmaceutics 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Peppas, N.A.; McGinity, J.W. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J. Control. Release 2006, 115, 121–129. [Google Scholar] [CrossRef]
- Zhang, P.; Shadambikar, G.; Almutairi, M.; Bandari, S.; Repka, M.A. Approaches for developing acyclovir gastro-retentive formulations using hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2020, 60, 102002. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradation of polyethers (polyethylene glycol, polypropylene glycol, polytetramethylene glycol, and others). In Biopolymers Online; Matsumura, S., Steinbüchel, A., Eds.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Litwack, G. Lipids. In Human Biochemistry, 2nd ed.; Elsevier: Los Angeles, CA, USA, 2018; pp. 199–255. [Google Scholar]
- Delahaije, R.J.B.M.; Lech, F.J.; Wierenga, P.A. Investigating the effect of temperature on the formation and stabilization of ovalbumin foams. Food Hydrocoll. 2019, 91, 263–274. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Niu, C.; Wang, Q.; Ma, T. Enhancement of gallium phase-change heat transfer by copper foam and ultrasonic vibration. J. Enhanc. Heat Transf. 2020, 27, 71–84. [Google Scholar] [CrossRef]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.O.; Kim, J.Y.; Ha, J.M.; Chi, S.C.; Rhee, Y.S.; Park, C.W.; Park, E.S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013, 83, 460–467. [Google Scholar] [CrossRef]
- Bahri-Najafi, R.; Mostafavi, A.; Tavakoli, N.; Taymouri, S.; Shahraki, M.-M. Preparation and in vitro-in vivo evaluation of acyclovir floating tablets. Res. Pharm. Sci. 2017, 12, 128. [Google Scholar] [CrossRef]
- Lewis, L.; Fowle, A.; Bittiner, S.; Bye, A.; Isaacs, P. Human gastrointestinal absorption of acyclovir from tablet duodenal infusion and sipped solution. Br. J. Clin. Pharmacol. 1986, 21, 459–462. [Google Scholar] [CrossRef] [Green Version]
- Raval, J.A.; Patel, M.M. Formulation and characterization of gastroretentive discs containing famotidine. Braz. Arch. Biol. Technol. 2011, 54, 293–300. [Google Scholar] [CrossRef]





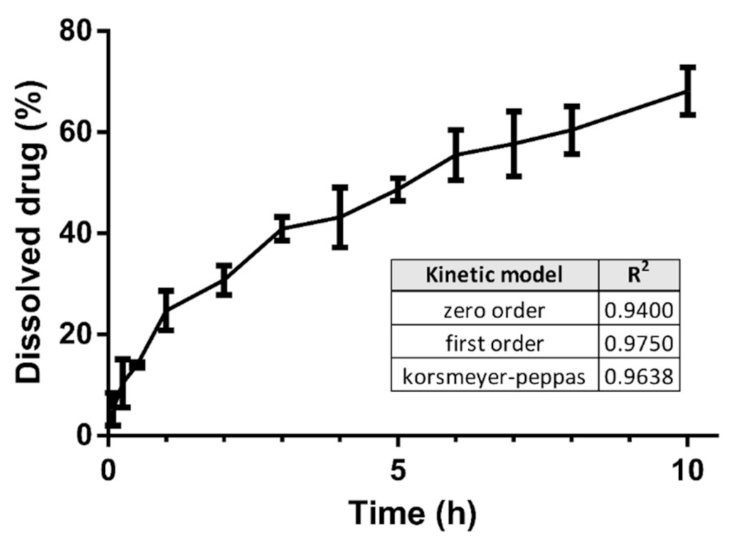


| Model | Equations [27,28] | Graphic | |
|---|---|---|---|
| Zero-order | (2) | The graphic of the drug-dissolved fraction versus time is linear. | |
| First-order | (3) | The graphic of the decimal logarithm of the released amount of drug versus time is linear. | |
| Korsmeyer–Peppas model | (4) | The graphic of the released drug versus the square root of time should form a straight line. | |
| Type of Gas | Contact Angle (° ± SD) |
|---|---|
| Air | 11.74° ± 2.01 |
| Carbon dioxide | 17.89° ± 1.63 |
| Helium | 18.66° ± 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haimhoffer, Á.; Fenyvesi, F.; Lekli, I.; Béresová, M.; Bak, I.; Czagány, M.; Vasvári, G.; Bácskay, I.; Tóth, J.; Budai, I. Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology. Pharmaceutics 2021, 13, 1571. https://doi.org/10.3390/pharmaceutics13101571
Haimhoffer Á, Fenyvesi F, Lekli I, Béresová M, Bak I, Czagány M, Vasvári G, Bácskay I, Tóth J, Budai I. Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology. Pharmaceutics. 2021; 13(10):1571. https://doi.org/10.3390/pharmaceutics13101571
Chicago/Turabian StyleHaimhoffer, Ádám, Ferenc Fenyvesi, István Lekli, Mónika Béresová, István Bak, Máté Czagány, Gábor Vasvári, Ildikó Bácskay, Judit Tóth, and István Budai. 2021. "Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology" Pharmaceutics 13, no. 10: 1571. https://doi.org/10.3390/pharmaceutics13101571
APA StyleHaimhoffer, Á., Fenyvesi, F., Lekli, I., Béresová, M., Bak, I., Czagány, M., Vasvári, G., Bácskay, I., Tóth, J., & Budai, I. (2021). Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology. Pharmaceutics, 13(10), 1571. https://doi.org/10.3390/pharmaceutics13101571












