Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Tegaserod Characterization
In Vitro Measurement of AChE and BuChE Activity
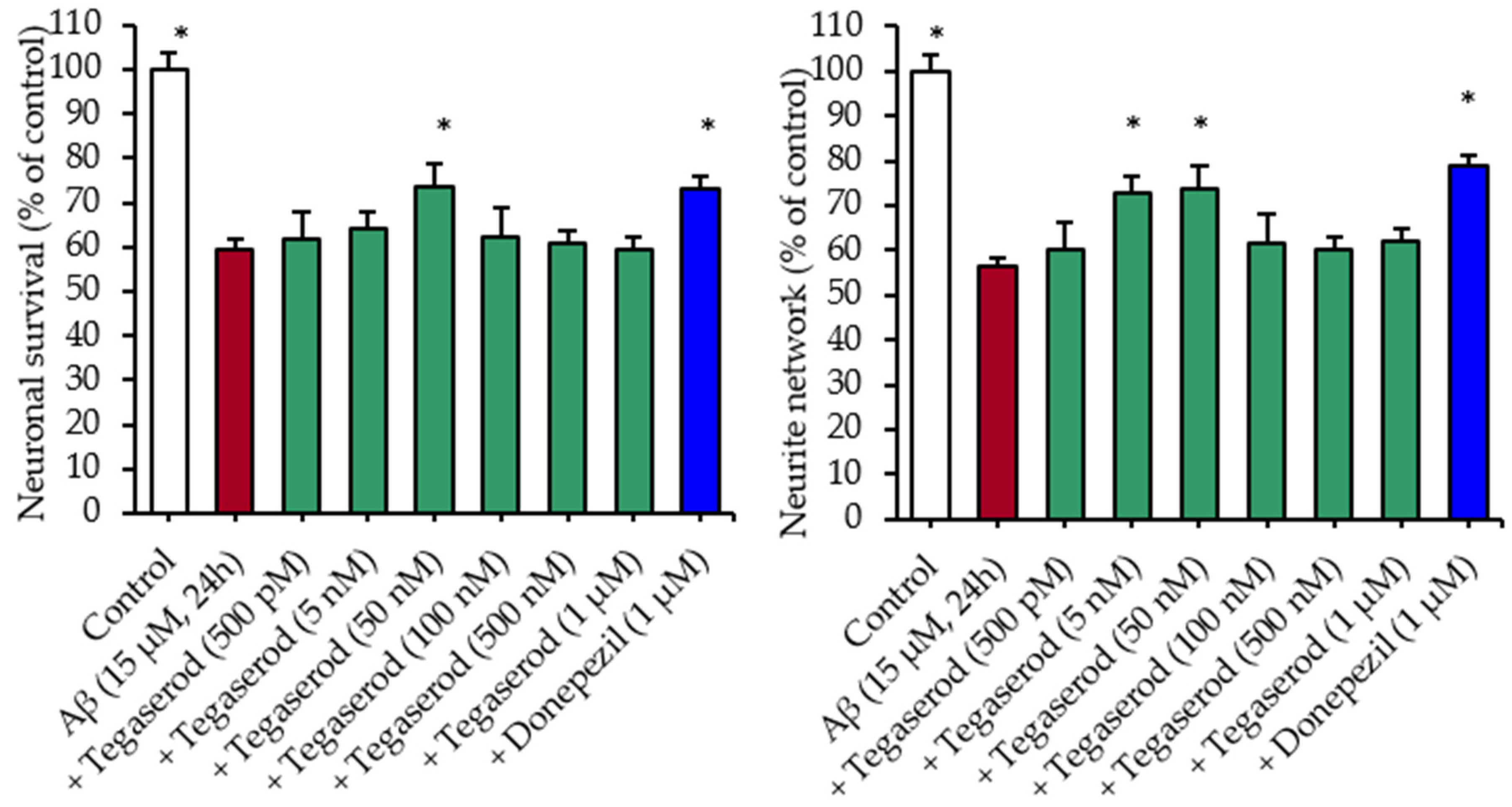
Effects of Tegaserod with Soluble Aβ Peptides in a Primary Culture of Hippocampal Neurons: Survival and Neurite Network Evaluation
hERG Inhibition Assay
Thermodynamic Solubility Determination at pH 7.4
Blood-Brain Barrier (PAMPA-BBB) and Gastrointestinal Tract Parallel Artificial Membrane Permeability (PAMPA-GIT) Assays
Calculated Physicochemical Properties of Tegaserod
2.2.2. Encapsulation of Tegaserod into Nanoemulsions
Nanoemulsions Formulation Process
Physicochemical Characterization of the Nanoemulsions
Stability Studies
Tegaserod Content Analysis by High-Performance Liquid Chromatography (HPLC)
2.2.3. Functionalization of Tegaserod-Loaded Nanoemulsions with Peptide-22
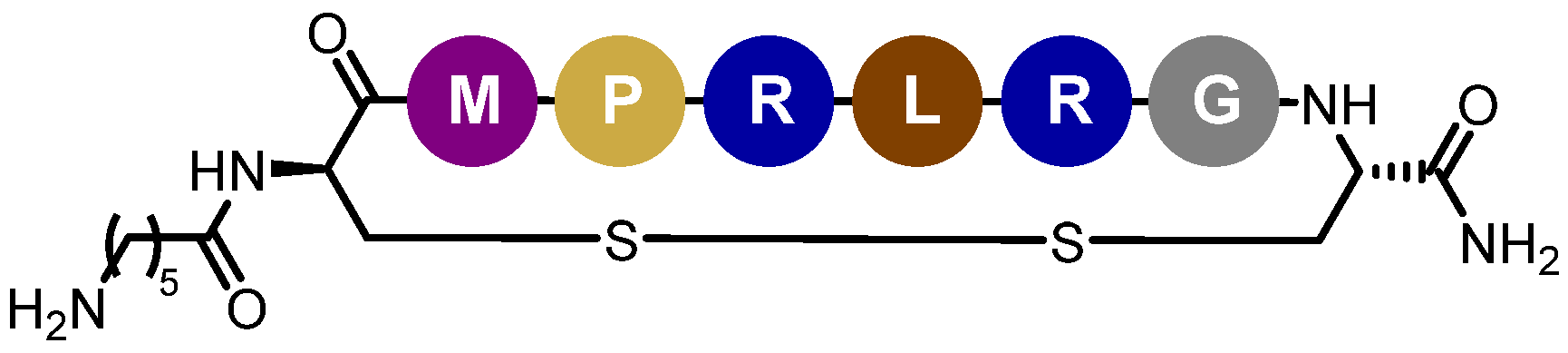
Determination of Peptide-22 Critical Aggregation Concentration
Peptide-22 Adsorption by Incubation with NEs Suspension
Study of Nanoemulsions/Peptide-22 Interactions through Isothermal Titration Calorimetry (ITC)
Quantification of Peptide-22 Adsorption
Adsorption Stability under Dilution
2.2.4. Biopharmaceutical Assessment
In Vitro Tegaserod Release Study
In Vitro Hemolysis Assay
2.2.5. Statistical Analysis
3. Results
3.1. Tegaserod Characterization
3.1.1. Tegaserod Pharmacological Characterization
3.1.2. Tegaserod Physicochemical Characterization
3.1.3. Tegaserod Profile
3.2. Encapsulation of Tegaserod into Nanoemulsions
3.2.1. Characterization of Nanoemulsions
3.2.2. Stability Study of Tg-NEs over Time
3.3. Functionalization of Tegaserod-Loaded Nanoemulsions with Peptide-22
3.3.1. Peptide-22 Adsorption on Tg-NEs
3.3.2. Adsorption Stability of the Peptide-22 under Dilution
3.4. Biopharmaceutical Assessment of Tg-NEs and Tg-NEs-P22
3.4.1. In Vitro Release of Tegaserod
3.4.2. In Vitro Hemolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Dementia Collaborators Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [CrossRef] [Green Version]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Claeysen, S.; Bockaert, J.; Giannoni, P. Serotonin: A New Hope in Alzheimer’s Disease? ACS Chem. Neurosci. 2015, 6, 940–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochet, M.; Donneger, R.; Cassier, E.; Gaven, F.; Lichtenthaler, S.F.; Marin, P.; Bockaert, J.; Dumuis, A.; Claeysen, S. 5-HT4 Receptors Constitutively Promote the Non-Amyloidogenic Pathway of APP Cleavage and Interact with ADAM10. ACS Chem. Neurosci. 2013, 4, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic Treatments with a 5-HT4 Receptor Agonist Decrease Amyloid Pathology in the Entorhinal Cortex and Learning and Memory Deficits in the 5xFAD Mouse Model of Alzheimer’s Disease. Neuropharmacology 2017, 126, 128–141. [Google Scholar] [CrossRef]
- Giannoni, P.; Gaven, F.; de Bundel, D.; Baranger, K.; Marchetti-Gauthier, E.; Roman, F.S.; Valjent, E.; Marin, P.; Bockaert, J.; Rivera, S.; et al. Early Administration of RS 67333, a Specific 5-HT4 Receptor Agonist, Prevents Amyloidogenesis and Behavioral Deficits in the 5XFAD Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, C.; Dallemagne, P.; Lecoutey, C.; Claeysen, S.; Rochais, C. Therapeutic Modulators of the Serotonin 5-HT4 Receptor: A Patent Review (2014-Present). Expert Opin. Ther. Pat. 2020, 30, 495–508. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Tyler, K.; Maceachern, S.J.; Balemba, O.B.; Johnson, A.C.; Brooks, E.M.; Zhao, H.; Swain, G.M.; Moses, P.L.; Galligan, J.J.; et al. Activation of Colonic Mucosal 5-HT4 Receptors Accelerates Propulsive Motility and Inhibits Visceral Hypersensitivity. Gastroenterology 2012, 142, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Canavan, C.; West, J.; Card, T. The Epidemiology of Irritable Bowel Syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Novartis Media Release—Novartis Suspends US Marketing and Sales of Zelnorm in Response to Request from FDA. Available online: https://www.fiercebiotech.com/biotech/press-release-novartis-suspends-us-marketing-and-sales-of-zelnorm-r-response-to-request (accessed on 12 July 2021).
- Roberson, E.N.; Wald, A. Functional Gastrointestinal Disorders and Irritable Bowel Syndrome. In GI/Liver Secrets, 4th ed.; McNally, P.R., Ed.; Mosby: Maryland Heights, MO, USA, 2010; pp. 439–445. ISBN 978-0-323-06397-5. [Google Scholar]
- Loughlin, J.; Quinn, S.; Rivero, E.; Wong, J.; Huang, J.; Kralstein, J.; Earnest, D.L.; Seeger, J.D. Tegaserod and the Risk of Cardiovascular Ischemic Events: An Observational Cohort Study. J. Cardiovasc. Pharmacol. Ther 2010, 15, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; May, H.T.; Bair, T.L.; Muhlestein, J.B.; Horne, B.D.; Carlquist, J.F. Lack of Association of Tegaserod with Adverse Cardiovascular Outcomes in a Matched Case-Control Study. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 170–175. [Google Scholar] [CrossRef]
- US WorldMeds, LLC, US agent for NDA applicant, Sloan Pharma. In Proceedings of the FDA Joint Meeting of the Gastrointestinal Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee Briefing Document, Silver Springs, MD, USA, 17–18 December 2018.
- Liu, W.; Stachura, P.; Xu, H.C.; Umesh Ganesh, N.; Cox, F.; Wang, R.; Lang, K.S.; Gopalakrishnan, J.; Häussinger, D.; Homey, B.; et al. Repurposing the Serotonin Agonist Tegaserod as an Anticancer Agent in Melanoma: Molecular Mechanisms and Clinical Implications. J. Exp. Clin. Cancer Res. 2020, 39, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, Q.; Zhang, X.; Li, L.; Xu, X.; Xu, X.; Li, X.; Wang, Z.; Lin, Y.; Li, X.; et al. Zelnorm, an Agonist of 5-Hydroxytryptamine 4-Receptor, Acts as a Potential Antitumor Drug by Targeting JAK/STAT3 Signaling. Investig. New Drugs 2020, 38, 311–320. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Jiang, Y.; Zhou, H.; Li, A.; Wei, Y.; Bao, Z.; Wang, D.; Zhao, J.; Chen, X.; et al. Tegaserod Maleate Inhibits Esophageal Squamous Cell Carcinoma Proliferation by Suppressing the Peroxisome Pathway. Front. Oncol 2021, 11, 683241. [Google Scholar] [CrossRef]
- Liu, M.-T.; Kuan, Y.-H.; Wang, J.; Hen, R.; Gershon, M.D. 5-HT4 Receptor-Mediated Neuroprotection and Neurogenesis in the Enteric Nervous System of Adult Mice. J. Neurosci. 2009, 29, 9683–9699. [Google Scholar] [CrossRef]
- Bushman, J.; Mishra, B.; Ezra, M.; Gul, S.; Schulze, C.; Chaudhury, S.; Ripoll, D.; Wallqvist, A.; Kohn, J.; Schachner, M.; et al. Tegaserod Mimics the Neurostimulatory Glycan Polysialic Acid and Promotes Nervous System Repair. Neuropharmacology 2014, 79, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.-C.; Shen, Y.-Q.; Loers, G.; Jakovcevski, I.; Schachner, M. Tegaserod, a Small Compound Mimetic of Polysialic Acid, Promotes Functional Recovery after Spinal Cord Injury in Mice. Neuroscience 2014, 277, 356–366. [Google Scholar] [CrossRef]
- Jourdan, J.-P.; Since, M.; El Kihel, L.; Lecoutey, C.; Corvaisier, S.; Legay, R.; Sopková-de Oliveira Santos, J.; Cresteil, T.; Malzert-Fréon, A.; Rochais, C.; et al. Benzylphenylpyrrolizinones with Anti-Amyloid and Radical Scavenging Effects, Potentially Useful in Alzheimer’s Disease Treatment. ChemMedChem 2017, 12, 913–916. [Google Scholar] [CrossRef] [Green Version]
- De Pascale, M.; Iacopetta, D.; Since, M.; Corvaisier, S.; Vie, V.; Paboeuf, G.; Hennequin, D.; Perato, S.; De Giorgi, M.; Sinicropi, M.S.; et al. Synthesis of Pyridoclax Analogues: Insight into Their Druggability by Investigating Their Physicochemical Properties and Interactions with Membranes. ChemMedChem 2020, 15, 136–154. [Google Scholar] [CrossRef]
- Guyon, L.; Groo, A.-C.; Malzert-Fréon, A. Relevant Physicochemical Methods to Functionalize, Purify, and Characterize Surface-Decorated Lipid-Based Nanocarriers. Mol. Pharm. 2021, 18, 44–64. [Google Scholar] [CrossRef]
- Burks, S.R.; Kersch, C.N.; Witko, J.A.; Pagel, M.A.; Sundby, M.; Muldoon, L.L.; Neuwelt, E.A.; Frank, J.A. Blood–Brain Barrier Opening by Intracarotid Artery Hyperosmolar Mannitol Induces Sterile Inflammatory and Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2021, 118, e2021915118. [Google Scholar] [CrossRef]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Chu, C.; Walczak, P.; Searson, P.C. Modeling Hyperosmotic Blood–Brain Barrier Opening within Human Tissue-Engineered in Vitro Brain Microvessels. J. Cereb. Blood Flow Metab. 2020, 40, 1517–1532. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic Opening of the Blood–Brain Barrier: Principles, Mechanism, and Therapeutic Applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef]
- Huang, R.; Boltze, J.; Li, S. Strategies for Improved Intra-Arterial Treatments Targeting Brain Tumors: A Systematic Review. Front. Oncol. 2020, 10, 1443. [Google Scholar] [CrossRef]
- Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J.N.; Bigio, I.J. Inconsistent Blood Brain Barrier Disruption by Intraarterial Mannitol in Rabbits: Implications for Chemotherapy. J. Neurooncol. 2011, 104, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Fukui, M.; Nishio, S.; Kitamura, K.; Nagara, H. Hyperosmotic Blood-Brain Barrier Disruption in Brains of Rats with an Intracerebrally Transplanted RG-C6 Tumor. J. Neurosurg. 1987, 66, 256–263. [Google Scholar] [CrossRef]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-Human Trial of Blood–Brain Barrier Opening in Amyotrophic Lateral Sclerosis Using MR-Guided Focused Ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef] [Green Version]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood-Brain Barrier Shuttle Peptides: An Emerging Paradigm for Brain Delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef] [Green Version]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics across the Blood-Brain Barrier. Ann. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef]
- Molino, Y.; David, M.; Varini, K.; Jabès, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL Receptor-Targeting Peptide Vectors for in Vitro and in Vivo Cargo Transport across the Blood-Brain Barrier. FASEB J. 2017, 31, 1807–1827. [Google Scholar] [CrossRef]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Michaelis, M.; Kreuter, J.; Langer, K. Covalent Linkage of Apolipoprotein E to Albumin Nanoparticles Strongly Enhances Drug Transport into the Brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Spencer, B.; Marr, R.A.; Gindi, R.; Potkar, R.; Michael, S.; Adame, A.; Rockenstein, E.; Verma, I.M.; Masliah, E. Peripheral Delivery of a CNS Targeted, Metalo-Protease Reduces Aβ Toxicity in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2011, 6, e16575. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, N.C.; D’Orsi, L.; Sambri, I.; Nusco, E.; Monaco, C.; Spampanato, C.; Polishchuk, E.; Saccone, P.; De Leonibus, E.; Ballabio, A.; et al. A Highly Secreted Sulphamidase Engineered to Cross the Blood-Brain Barrier Corrects Brain Lesions of Mice with Mucopolysaccharidoses Type IIIA. EMBO Mol. Med. 2013, 5, 675–690. [Google Scholar] [CrossRef]
- Séguy, L.; Groo, A.-C.; Goux, D.; Hennequin, D.; Malzert-Fréon, A. Design of Non-Haemolytic Nanoemulsions for Intravenous Administration of Hydrophobic APIs. Pharmaceutics 2020, 12, 1141. [Google Scholar] [CrossRef]
- Malcor, J.-D.; Payrot, N.; David, M.; Faucon, A.; Abouzid, K.; Jacquot, G.; Floquet, N.; Debarbieux, F.; Rougon, G.; Martinez, J.; et al. Chemical Optimization of New Ligands of the Low-Density Lipoprotein Receptor as Potential Vectors for Central Nervous System Targeting. J. Med. Chem. 2012, 55, 2227–2241. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-Mediated Peptide-22-Conjugated Nanoparticles for Dual-Targeting Therapy of Brain Glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef]
- Chen, C.; Duan, Z.; Yuan, Y.; Li, R.; Pang, L.; Liang, J.; Xu, X.; Wang, J. Peptide-22 and Cyclic RGD Functionalized Liposomes for Glioma Targeting Drug Delivery Overcoming BBB and BBTB. ACS Appl. Mater. Interfaces 2017, 9, 5864–5873. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Callizot, N.; Combes, M.; Steinschneider, R.; Poindron, P. Operational Dissection of β-Amyloid Cytopathic Effects on Cultured Neurons. J. Neurosci. Res. 2013, 91, 706–716. [Google Scholar] [CrossRef]
- Bard, B.; Martel, S.; Carrupt, P.-A. High Throughput UV Method for the Estimation of Thermodynamic Solubility and the Determination of the Solubility in Biorelevant Media. Eur. J. Pharm. Sci. 2008, 33, 230–240. [Google Scholar] [CrossRef]
- Carrier, M.-N.; Garinot, O.; Vitzling, C. Stability and Compatibility of Tegaserod from Crushed Tablets Mixed in Beverages and Foods. Am. J. Health Syst. Pharm. 2004, 61, 1135–1142. [Google Scholar] [CrossRef]
- Yang, B.; Gao, M.J.; Duan, G.L. Ion-Pair RP-LC of Tegaserod Maleate and Its Impurities in Pharmaceutical Formulations and in Dissolution Studies. Chroma 2006, 63, 431–436. [Google Scholar] [CrossRef]
- ICH Secretariat. In Proceedings of the International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Guideline, Validation of Analytical Prodecedures: Text and Methodology Q2(R1); ICH Secretariat: Genewa, Switzerland, 2005.
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef]
- Beattie, D.T.; Smith, J.A.M.; Marquess, D.; Vickery, R.G.; Armstrong, S.R.; Pulido-Rios, T.; McCullough, J.L.; Sandlund, C.; Richardson, C.; Mai, N.; et al. The 5-HT4 Receptor Agonist, Tegaserod, Is a Potent 5-HT2B Receptor Antagonist in Vitro and in Vivo. Br. J. Pharmacol 2004, 143, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Tsubouchi, T.; Kunimatsu, T.; Tsujimoto, S.; Kiyoshi, A.; Katsura, Y.; Oku, S.; Chihara, K.; Mine, Y.; Yamada, T.; Shimizu, I.; et al. The in Vitro Pharmacology and Non-Clinical Cardiovascular Safety Studies of a Novel 5-HT4 Receptor Agonist, DSP-6952. Eur. J. Pharmacol 2018, 826, 96–105. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent Progress in the Identification of Selective Butyrylcholinesterase Inhibitors for Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An Important New Target in Alzheimer’s Disease Therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Dallemagne, P.; Rochais, C. 5-HT4R Modulators: A Patent Landscape. Pharm. Pat. Anal. 2021, 10, 179–181. [Google Scholar] [CrossRef]
- Rochais, C.; Lecoutey, C.; Hamidouche, K.; Giannoni, P.; Gaven, F.; Cem, E.; Mignani, S.; Baranger, K.; Freret, T.; Bockaert, J.; et al. Donecopride, a Swiss Army Knife with Potential against Alzheimer’s Disease. Br. J. Pharmacol. 2020, 177, 1988–2005. [Google Scholar] [CrossRef]
- Lecoutey, C.; Hedou, D.; Freret, T.; Giannoni, P.; Gaven, F.; Since, M.; Bouet, V.; Ballandonne, C.; Corvaisier, S.; Fréon, A.M.; et al. Design of Donecopride, a Dual Serotonin Subtype 4 Receptor Agonist/Acetylcholinesterase Inhibitor with Potential Interest for Alzheimer’s Disease Treatment. Proc. Natl. Acad. Sci. USA 2014, 111, E3825–E3830. [Google Scholar] [CrossRef] [Green Version]
- Kalepu, S.; Nekkanti, V. Insoluble Drug Delivery Strategies: Review of Recent Advances and Business Prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel-Dingemanse, S. Clinical Pharmacokinetics of Tegaserod, a Serotonin 5-HT(4) Receptor Partial Agonist with Promotile Activity. Clin. Pharmacokinet. 2002, 41, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Hasler, W.L.; Schoenfeld, P. Safety Profile of Tegaserod, a 5-HT4 Receptor Agonist, for the Treatment of Irritable Bowel Syndrome. Drug Saf. 2004, 27, 619–631. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Refusal Assessment Report for Zelnorm. 2007. Available online: https://www.ema.europa.eu/en/documents/assessment-report/zelnorm-epar-refusal-public-assessment-report_en.pdf (accessed on 12 July 2021).
- Preeti Venkataraman, M.D. FDA Introductive Remarks—Gastrointestinal Drugs Advisory Committe, NDA 021200, Zelnorm: For the Treatment of Irritable Bowel Syndrome Constipation (IBS-C) in Females <65 Years; FDA: Montgomery, MD, USA, 2018. [Google Scholar]
- Appel, S.; Kumle, A.; Hubert, M.; Duvauchelle, T. First Pharmacokinetic-Pharmacodynamic Study in Humans with a Selective 5-Hydroxytryptamine4 Receptor Agonist. J. Clin. Pharmacol. 1997, 37, 229–237. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals Corporation. NDA 21-200, Clinical Pharmacology and Biopharmaceutics Review; Novartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2000. [Google Scholar]
- Di, L.; Kerns, E.H.; Bezar, I.F.; Petusky, S.L.; Huang, Y. Comparison of Blood-Brain Barrier Permeability Assays: In Situ Brain Perfusion, MDR1-MDCKII and PAMPA-BBB. J. Pharm. Sci. 2009, 98, 1980–1991. [Google Scholar] [CrossRef]
- Perrin, M.J.; Subbiah, R.N.; Vandenberg, J.I.; Hill, A.P. Human Ether-a-Go-Go Related Gene (HERG) K+ Channels: Function and Dysfunction. Prog. Biophys. Mol. Biol. 2008, 98, 137–148. [Google Scholar] [CrossRef]
- Tack, J.; Camilleri, M.; Chang, L.; Chey, W.D.; Galligan, J.J.; Lacy, B.E.; Müller-Lissner, S.; Quigley, E.M.M.; Schuurkes, J.; De Maeyer, J.H.; et al. Systematic Review: Cardiovascular Safety Profile of 5-HT(4) Agonists Developed for Gastrointestinal Disorders. Aliment. Pharmacol. Ther. 2012, 35, 745–767. [Google Scholar] [CrossRef]
- Busti, A.J.; Murillo, J.R.; Cryer, B. Tegaserod-Induced Myocardial Infarction: Case Report and Hypothesis. Pharmacotherapy 2004, 24, 526–531. [Google Scholar] [CrossRef]
- Chan, K.Y.; de Vries, R.; Leijten, F.P.J.; Pfannkuche, H.-J.; van den Bogaerdt, A.J.; Danser, A.H.J.; MaassenVanDenBrink, A. Functional Characterization of Contractions to Tegaserod in Human Isolated Proximal and Distal Coronary Arteries. Eur. J. Pharmacol. 2009, 619, 61–67. [Google Scholar] [CrossRef]
- Anton, N.; Hallouard, F.; Attia, M.F.; Vandamme, T.F. Nano-emulsions for Drug Delivery and Biomedical Imaging. In Intracellular Delivery III; Prokop, A., Weissig, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 273–300. ISBN 978-3-319-43523-7. [Google Scholar]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation. In Nanoemulsions; Elsevier: Amsterdam, The Netherland, 2018; pp. 3–20. ISBN 978-0-12-811838-2. [Google Scholar]
- Singh, S.S.; Patel, H.; Sharma, K. Estimation of Tegaserod in Human Plasma by High-Performance Liquid Chromatography–Tandem Mass Spectroscopy and Its Application to Bioequivalence Study. Anal. Chim. Acta 2006, 557, 229–235. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Reciprocal Competition between Lipid Nanocapsules and P-Gp for Paclitaxel Transport across Caco-2 Cells. Eur. J. Pharm. Sci. 2010, 40, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, R.; Chan, C.Y.S.; Curley, P.; Sharp, J.; Chiong, J.; Rannard, S.; Siccardi, M.; Owen, A. Inhibitory Effects of Commonly Used Excipients on P-Glycoprotein in Vitro. Mol. Pharm. 2018, 15, 4835–4842. [Google Scholar] [CrossRef]
- Matougui, N.; Groo, A.-C.; Umerska, A.; Cassisa, V.; Saulnier, P. A Comparison of Different Strategies for Antimicrobial Peptides Incorporation onto/into Lipid Nanocapsules. Nanomedicine 2019, 14, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Lau, B.L.T. Biomolecule-Nanoparticle Interactions: Elucidation of the Thermodynamics by Isothermal Titration Calorimetry. Biochim. Biophys. Acta 2016, 1860, 945–956. [Google Scholar] [CrossRef]
- Omanovic-Miklicanin, E.; Manfield, I.; Wilkins, T. Application of Isothermal Titration Calorimetry in Evaluation of Protein–Nanoparticle Interactions. J. Therm. Anal. Calorim. 2016, 127, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Gazaille, C.; Sicot, M.; Akiki, M.; Lautram, N.; Dupont, A.; Saulnier, P.; Eyer, J.; Bastiat, G. Characterization of Biological Material Adsorption to the Surface of Nanoparticles without a Prior Separation Step: A Case Study of Glioblastoma-Targeting Peptide and Lipid Nanocapsules. Pharm. Res. 2021, 38, 681–691. [Google Scholar] [CrossRef]
- Fan, W.; Yu, Z.; Peng, H.; He, H.; Lu, Y.; Qi, J.; Dong, X.; Zhao, W.; Wu, W. Effect of Particle Size on the Pharmacokinetics and Biodistribution of Parenteral Nanoemulsions. Int. J. Pharm 2020, 586, 119551. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M.L. Electrical Properties of the Red Blood Cell Membrane and Immunohematological Investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Relevance of the Colloidal Stability of Chitosan/PLGA Nanoparticles on Their Cytotoxicity Profile. Int. J. Pharm. 2009, 381, 130–139. [Google Scholar] [CrossRef]
- Wadhwa, R.; Aggarwal, T.; Thapliyal, N.; Kumar, A.; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red Blood Cells as an Efficient in Vitro Model for Evaluating the Efficacy of Metallic Nanoparticles. 3 Biotech. 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, D.; Kolanowska, K.; Gajek, A.; Gomez-Ramirez, R.; de la Mata, J.; Pedziwiatr-Werbicka, E.; Klajnert, B.; Waczulikova, I.; Bryszewska, M. Interaction of Cationic Carbosilane Dendrimers and Their Complexes with SiRNA with Erythrocytes and Red Blood Cell Ghosts. Biochim. Biophys. Acta Biomembr. 2014, 1838, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]





| Time (min) | Flow (mL/min) | Phase A (%) | Phase B (%) |
|---|---|---|---|
| 0 | 0.6 | 10 | 90 |
| 0.2 | 0.6 | 10 | 90 |
| 1.0 | 0.6 | 50 | 50 |
| 1.1 | 0.6 | 50 | 50 |
| 1.2 | 0.6 | 10 | 90 |
| Conpound | eqBuChE | hAChE | |
|---|---|---|---|
| % inhibition at 1.10−5 M | IC50 | % inhibition at 1.10−5 M | |
| Tacrine | 98% | 3.2 ± 0.6 nM | - |
| Donepezil | - | - | 97% |
| Tegaserod | 78% | 4.2 ± 0.9 µM | 2% |
 | Values |
|---|---|
| Molecular weight (g/mol) | 301.39 |
| pKa1, pKa2 and pKa3 | 1.94, 8.50 and 15.24 |
| LogP | 2.95 |
| Thermodynamic solubility at pH 7.4 (µM) | 151.5 ± 8.6 |
| PAMPA gastro intestinal tract permeability assay at pH 5: Pe (nm/s) | 0.0 ± 0.0 |
| PAMPA gastro intestinal tract permeability assay at pH 6: Pe (nm/s) | 0.0 ± 0.0 |
| PAMPA gastro intestinal tract permeability assay at pH 7.4: Pe (nm/s) | 0.0 ± 0.0 |
| PAMPA blood–brain barrier permeability assay: Pe (nm/s) | 81.9 ± 13.2 |
| Formulation | Hydrodynamic Diameter (nm) | PDI | Ζ Potential (mV) | pH | Osmolarity (mOsm) | EE (%) |
|---|---|---|---|---|---|---|
| Blank-NEs | 51.6 ± 2.0 | 0.170 ± 0.014 | −6.9 ± 2.4 | 7.2 ± 0.1 | 283 ± 10 | - |
| Tg-NEs | 47.2 ± 2.1 | 0.167 ± 0.015 | 1.8 ± 4.0 | 7.4 ± 0.1 | 312 ± 10 | 91 ± 10 |
| Physicochemical Characteristics | Tg-NEs | Tg-NEs-P222 | Tg-NEs-P226 |
|---|---|---|---|
| Hydrodynamic diameter (nm) | 47.2 ± 2.1 | 47.8 ± 1.1 | 50.2 ± 3.5 |
| PDI | 0.167 ± 0.015 | 0.152 ± 0.018 | 0.147 ± 0.016 |
| ζ potential (mV) | 1.8 ± 4.0 | 4.8 ± 11.0 | 5.6 ± 1.7 |
| Adsorption efficiency by SEC/HPLC (%) | - | 20 ± 3 | 45 ± 4 |
| Peptide molecules/nanodroplet | - | 115 ± 15 | 740 ± 80 |
| Dilution Factor | Peptide Molecules/Nanodroplet | |||
|---|---|---|---|---|
| 1:1 | 1:10 | 1:100 | 1:200 | |
| [P22] = 2 mg/mL | 115 | 115 | 45 | 50 |
| [P22] = 6 mg/mL | 510 | 645 | 550 | 540 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Séguy, L.; Guyon, L.; Maurel, M.; Verdié, P.; Davis, A.; Corvaisier, S.; Lisowski, V.; Dallemagne, P.; Groo, A.-C.; Malzert-Fréon, A. Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment. Pharmaceutics 2021, 13, 1626. https://doi.org/10.3390/pharmaceutics13101626
Séguy L, Guyon L, Maurel M, Verdié P, Davis A, Corvaisier S, Lisowski V, Dallemagne P, Groo A-C, Malzert-Fréon A. Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment. Pharmaceutics. 2021; 13(10):1626. https://doi.org/10.3390/pharmaceutics13101626
Chicago/Turabian StyleSéguy, Line, Léna Guyon, Manon Maurel, Pascal Verdié, Audrey Davis, Sophie Corvaisier, Vincent Lisowski, Patrick Dallemagne, Anne-Claire Groo, and Aurélie Malzert-Fréon. 2021. "Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment" Pharmaceutics 13, no. 10: 1626. https://doi.org/10.3390/pharmaceutics13101626
APA StyleSéguy, L., Guyon, L., Maurel, M., Verdié, P., Davis, A., Corvaisier, S., Lisowski, V., Dallemagne, P., Groo, A.-C., & Malzert-Fréon, A. (2021). Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment. Pharmaceutics, 13(10), 1626. https://doi.org/10.3390/pharmaceutics13101626







