Estimation of the Difference in Colistin Plasma Levels in Critically Ill Patients with Favorable or Unfavorable Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Subjects
2.2. Determination of the Plasma Colistin Concentrations
2.3. Bacterial Isolates and Detection of Resistant Genes
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Clinical Outcome
3.3. Clinical Prognosis Scales
3.4. Site of Infection and Microbiological Isolation
3.5. Management with Colistin and Concomitant Antimicrobial Therapy
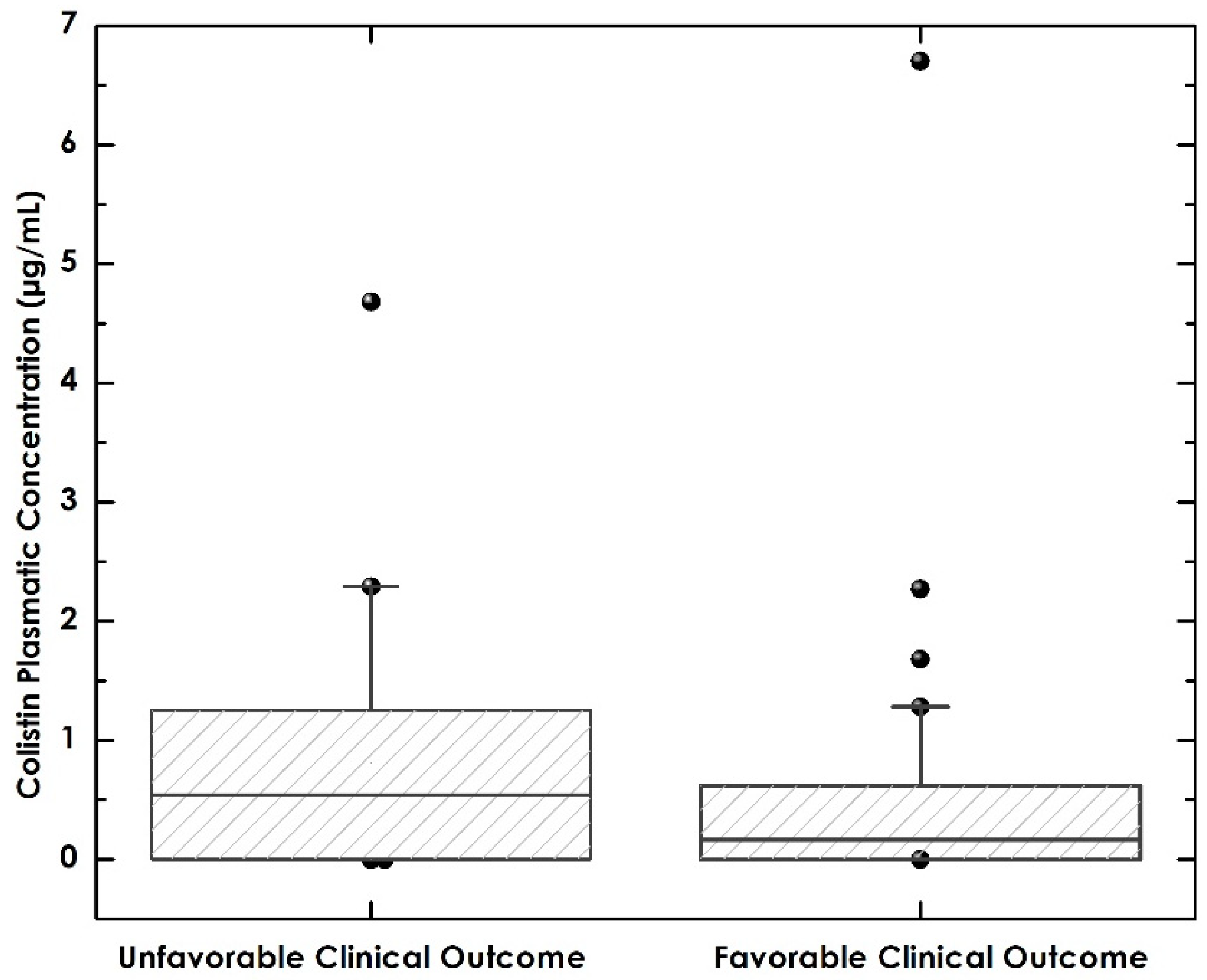
3.6. Relationship between Colistin Levels and Favorable Clinical Outcomes and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tillotson, G.S.; Zinner, S.H. Burden of antimicrobial resistance in an era of decreasing susceptibility. Expert Rev. Anti-Infect. Ther. 2017, 15, 663–676. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations/the Review on Antimicrobial Resistance Chaired; Wellcometrust: London, UK, 2014; p. 20. [Google Scholar]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control. 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberjé, E.J.M.; Tanke, M.A.C.; Jeurissen, P.P.T. Antimicrobial stewardship initiatives throughout europe: Proven value for money. Infect. Dis. Rep. 2017, 9, 6800. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States. 2013; p. 114. Available online: www.CDC.gov (accessed on 3 June 2021).
- WHO. Antimicro Bial Resistanceglobal Reporton Surveillance. 2014, p. 256. Available online: www.who.int (accessed on 5 January 2021).
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N.; Gales, A.C.; Silva, J.B.; Pignatari, A.C. SENTRY antimicrobial surveillance program report: Latin American and Brazilian results for 1997 through 2001. Braz. J. Infect. Dis. 2004, 8, 25–79. [Google Scholar] [CrossRef] [Green Version]
- Buitrago Gutierrez, G. Relationship Between Consumption of Antibiotics and Bacterial Resistance in Colombian Institutions. 2009, p. 143. Available online: https://repositorio.unal.edu.co/handle/unal/11356 (accessed on 10 March 2021).
- Pacheco, T.; Bustos, R.H.; González, D.; Garzón, V.; García, J.C.; Ramírez, D. An approach to measuring colistin plasma levels regarding the treatment of multidrug-resistant bacterial infection. Antibiotics 2019, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Arias Ramos, D.; Hoyos Pulgarín, J.A.; Moreno Gómez, G.A.; Alzate, J.A.; Olaya Gómez, J.C.; Cortés Bonilla, I.; Vargas Mosquera, C. Geographic mapping of Enterobacteriaceae with extended-spectrum β-lactamase (ESBL) phenotype in Pereira, Colombia. BMC Infect. Dis. 2020, 20, 540. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef]
- Martis, N.; Leroy, S.; Blanc, V. Colistin in multi-drug resistant Pseudomonas aeruginosa blood-stream infections: A narrative review for the clinician. J. Infect. 2014, 69, 1–12. [Google Scholar] [CrossRef]
- Reina, R.; Estenssoro, E.; Sáenz, G.; Canales, H.S.; Gonzalvo, R.; Vidal, G.; Martins, G.; Das Neves, A.; Santander, O.; Ramos, C. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: A prospective cohort study. Intensive Care Med. 2005, 31, 1058–1065. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Jiménez-Jiménez, F.J.; Barrero-Almodóvar, A.E.; García-Garmendia, J.L.; Bernabeu-Wittel, I.M.; Gallego-Lara, S.L.; Madrazo-Osuna, J. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: A comparison with imipenem-susceptible VAP. Clin. Infect. Dis. 2003, 36, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef]
- Kunin, C.M.; Bugg, A. Binding of polymyxin antibiotics to tissues: The major determinant of distribution and persistence in the body. J. Infect. Dis. 1971, 124, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coulthard, K.; Milne, R.; Nation, R.L.; Conway, S.; Peckham, D.; Etherington, C.; Turnidge, J. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 2003, 52, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Mejías, M.E.; Pichardo-Guerrero, C.; Márquez-Rivas, F.J.; Martín-Lozano, D.; Prados, T.; Pachón, J. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Markantonis, S.L.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 2009, 53, 4907–4910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- García, J.C.; Arias, S. Dosis de colistina en multirresistencia: Reporte de caso. Iatreia 2018, 31, 412–418. [Google Scholar]
- Parker, S.L.; Sime, F.B.; Roberts, J.A. Optimizing dosing of antibiotics in critically ill patients. Curr. Opin. Infect. Dis. 2015, 28, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Wootton, M.; Holt, H.A.; Macgowan, A.P. Development of a novel assay method for colistin sulphomethate. Clin. Microbiol. Infect. 2005, 11, 243–244. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.H. Microbiological and chemical analysis of polymyxin B and polymyxin E (colistin) sulphates. Analyst 1980, 105, 1068–1075. [Google Scholar] [CrossRef]
- Niece, K.L.; Akers, K.S. Preliminary method for direct quantification of colistin methanesulfonate by attenuated total reflectance Fourier transform infrared spectroscopy. Antimicrob. Agents Chemother. 2015, 59, 5542–5547. [Google Scholar] [CrossRef] [Green Version]
- Zabidi, M.S.; Abu Bakar, R.; Musa, N.; Yusuf, W.N.W. Analytical methodologies for measuring colistin levels in pharmacokinetic studies. J. Liquid Chromatogr. Related Technol. 2020, 43, 671–686. [Google Scholar] [CrossRef]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K.; Valentine, J. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 2002, 46, 3304–3307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K.; Johnson, D.W. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001, 761, 167–175. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Gerber, J.P.; Milne, R.W. Determination of colistin in human plasma, urine and other biological samples using LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 862, 205–212. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Markopoulou, C.K.; Koundourellis, J.E.; Loukas, Y.L. Validation of a novel LC-MS/MS method for the quantitation of colistin A and B in human plasma. J. Sep. Sci. 2011, 34, 37–45. [Google Scholar] [CrossRef]
- Barco, S.; Castagnola, E.; Mesini, A.; Tripodi, G.; Cangemi, G. Potential pitfalls in LC-MS/MS quantification of colistin for therapeutic drug monitoring of patients treated with colistimethate. J. Pharm. Biomed. Anal. 2019, 170, 193–195. [Google Scholar] [CrossRef]
- Gikas, E.; Bazoti, F.N.; Katsimardou, M.; Anagnostopoulos, D.; Papanikolaou, K.; Inglezos, I.; Skoutelis, A.; Daikos, G.L.; Tsarbopoulos, A. Determination of colistin A and colistin B in human plasma by UPLC-ESI high resolution tandem MS: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2013, 83, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European dose recommendations for intravenous colistin: How do they perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Benattar, Y.D.; Omar, M.; Zusman, O.; Yahav, D.; Zak-Doron, Y.; Altunin, S.; Elbaz, M.; Daitch, V.; Granot, M.; Leibovici, L.; et al. The effectiveness and safety of high-dose colistin: Prospective cohort study. Clin. Infect. Dis. 2016, 63, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Ma, Z.; Yang, G.; Yang, J.; Cheng, J. A Simple HPLC method for the separation of colistimethate sodium and colistin sulphate. J. Chromatogr. Sep. Tech. 2011, 1, 105. [Google Scholar] [CrossRef] [Green Version]
- da Cunha-Pino, A. Desenvolvimento de Uma Técnica de HPLC Para a Quantificação de Colistina em Plasma Humano e a Sua Monitorização Sérica em Doentes Internados no CHUC. 2016. Available online: https://estudogeral.uc.pt/bitstream/10316/36563/1/DM%20Raquel%20Pinho.pdf (accessed on 1 September 2021).
- Hanai, Y.; Matsuo, K.; Kosugi, T.; Kusano, A.; Ohashi, H.; Kimura, I.; Hirayama, S.; Nanjo, Y.; Ishii, Y.; Sato, T.; et al. Rapid, simple, and clinically applicable high-performance liquid chromatography method for clinical determination of plasma colistin concentrations. J. Pharm. Health Care Sci. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Køppenn, B.; Bencic, N.; Melander, C. Characterization of colistimethate sodium (cms). Patent WO2014195405, 11 December 2014. [Google Scholar]
- Liu, X.; Yu, Z.; Wang, Y.; Wu, H.; Bian, X.; Li, X.; Fan, Y.; Guo, B.; Zhang, J. Therapeutic drug monitoring of polymyxin B by LC-MS/MS in plasma and urine. Bioanalysis 2020, 12, 845–855. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 14 May 2021).
- Monteiro, J.; Widen, R.H.; Pignatari, A.C.; Kubasek, C.; Silbert, S. Rapid detection of carbapenemase genes by multiplex real-time PCR. J. Antimicrob. Chemother. 2012, 67, 906–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garonzik, S.; Li, J.; Thamlikitkul, V.; Paterson, D.; Shoham, S.; Jacob, J.; Silveira, F.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnik, N.D.; Sridharan, K.; Jadhav, S.P.; Kadam, P.P.; Naidu, R.K.; Namjoshi, R.D.; Gupta, V.; Gore, M.S.; Surase, P.V.; Mehta, P.R.; et al. Pharmacokinetics of colistin in critically ill patients with multidrug-resistant Gram-negative bacilli infection. Eur. J. Clin. Pharmacol. 2013, 69, 1429–1436. [Google Scholar] [CrossRef]
- Vicari, G.; Bauer, S.R.; Neuner, E.A.; Lam, S.W. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin. Infect. Dis. 2012, 56, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.T.; Al Omran, S.; Al Aithan, A.S.; Al Sultan, M. Efficacy and safety of low-dose colistin in the treatment for infections caused by multidrug-resistant gram-negative bacteria. J. Clin. Pharm. Ther. 2014, 39, 272–276. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Wi, Y.M.; Kwon, Y.J.; Kim, S.R.; Chang, S.-H.; Cho, S. Association between colistin dose and development of nephrotoxicity*. Crit. Care Med. 2015, 43, 1187–1193. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Karvanen, M.; Iosifidis, E.; Jansson, B.; Plachouras, D.; Cars, O.; Roilides, E. Serum and cerebrospinal fluid levels of colistin in pediatric patients. Antimicrob. Agents Chemother. 2010, 54, 3985–3987. [Google Scholar] [CrossRef] [Green Version]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrentraut, S.F.; Muenster, S.; Kreyer, S.; Theuerkauf, N.U.; Bode, C.; Steinhagen, F.; Ehrentraut, H.; Schewe, J.-C.; Weber, M.; Putensen, C.; et al. Extensive therapeutic drug monitoring of colistin in critically Ill patients reveals undetected risks. Microorganisms 2020, 8, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorlí, L. Impacto de la Monitorización de Los Niveles Plasmáticos de Colistina en la Práctica Clínica Diaria; Universitat Autònoma de Barcelona: Barcelona, Spain, 2016. [Google Scholar]
- Rivera-Espinar, F.; Machuca, I.; Tejero, R.; Rodríguez, J.; Mula, A.; Marfil, E.; Cano, Á.; Gutiérrez-Gutiérrez, B.; Rodríguez, M.; Pozo, J.C.; et al. Impact of KPC Production and high-level meropenem resistance on all-cause mortality of ventilator-associated pneumonia in association with Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e02164-19. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.R.P.; Villar, S.S.; Ramos, J.C.R.; Pintado, V. Infections caused by carbapenemase-producing Enterobacteriaceae: Risk factors, clinical features and prognosis. Enferm. Infecc. Microbiol. Clin. 2014, 32, 41–48. [Google Scholar] [CrossRef]
- Celis, G.; Velez, P.; Mena, W.; Atiencia, H.; Morales, D.; Vélez, J. Factores de riesgo predictores de mortalidad por infección enterobacterias productoras de carbapenemasas. Rev. Fac. Cienc. Médicas 2014, 39, 60–68. [Google Scholar]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious diseases society of America guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef] [PubMed]
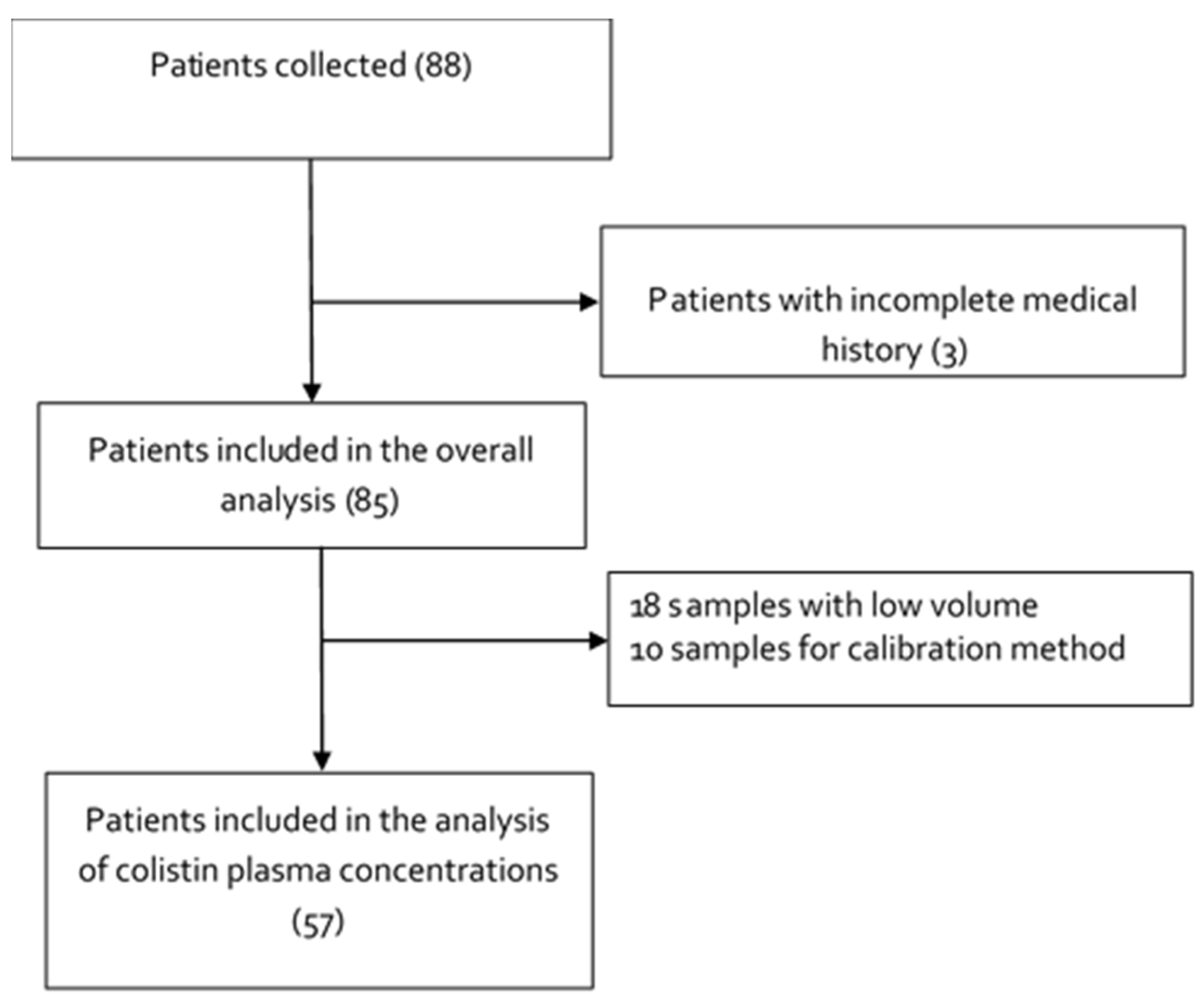
| Variables | Included Patients (n = 85) | Favorable Outcome n = 50 | Unfavorable Outcome n = 35 | p-Value |
|---|---|---|---|---|
| Sex, (%), male | 64 (72.72%) 21 (27.28%) | 40 (80%) | 21 (60%) | 0.0438 |
| Age (years), median (IQR) | 59 (45–70) | 59 (45–69.25) | 60 (34–72) | 0.8934 |
| Site of infection (%) | ||||
| Urinary tract | 24 (28.23%) | 15 (30%) | 9 (25.7%) | 0.6657 |
| Respiratory tract | 20 (23.52%) | 9 (18%) | 11 (31.42%) | 0.1509 |
| Abdominal | 17 (20%) | 10 (20%) | 7 (20%) | 0.9999 |
| Blood | 13 (15.29%) | 8 (16%) | 5 (14.28%) | 0.8289 |
| Bone and joints | 7 (8.23%) | 4 (8%) | 3 (8.57%) | 0.9249 |
| Skin | 2 (2.35%) | 2 (4%) | 0 (0%) | NS |
| CNS | 2 (2.35%) | 2 (4%) | 0 (0%) | 0.2312 |
| BMI, median (IQR) | 25.56 (21.37–29.04) | 25.27 (22.01–28.10) | 25.63 (21.08–29.86) | 0.5890 |
| SOFA score day 1, median (IQR) | 4 (2–6) | 3.5 (2–4) | 4 (3–8) | 0.0131 |
| SOFA score day 7, median (IQR) | 2 (1–4.75) | 2 (1–3) | 4.5 (3–8) | 0.0000 |
| APACHE II, median (IQR) | 10 (6.5–14) | 8 (6–11) | 12 (8–19) | 0.0158 |
| CCI, median (IQR) | 2 (1–4) | 2 (1–4) | 4 (0–6) | 0.0405 |
| Days of hospitalization, median (IQR) | 30 (23–43) | 29 (21.75–39.25) | 32 (25–45) | 0.2698 |
| GFR (mL/min) day 1, median (IQR) | 93.5 (60–120.75) | 95 (62–122) | 89.5 (33–120) | 0.4853 |
| GFR (mL/min) day 7, median (IQR) | 73 (35–103) | 77 (37–116) | 60 (28–103) | 0.3309 |
| Mortality (%) | 14 (16.47%) | 2 (4%) | 12 (34.28%) | 0.0002 |
| Microorganisms found in isolation | n, (%) | Favorable n, (%) | Unfavorable n, (%) | |
| Pseudomonas aeruginosa | 52 (61.17%) | 34 (68%) | 18 (51.42%) | |
| Klebsiella pneumoniae | 12 (14.11%) | 4 (8%) | 8 (22.85%) | |
| Acinetobacter baumannii | 1 (1.1%) | 1 (2%) | 0 (0%) | |
| Klebsiella oxytoca | 3 (3.52%) | 1 (2%) | 2 (5.71%) | |
| Escherichia coli | 3 (3.52%) | 2 (4%) | 1 (2.85%) | |
| Enterobacter cloacae | 9 (10.58%) | 6 (12%) | 3 (8.57%) | |
| Providencia rettgeri | 1 (1.1%) | 0 (0%) | 1 (2.85%) | |
| Pseudomonas putida | 4 (4.7%) | 2 (4%) | 2 (5.71%) | |
| Colistin (%) Loading dose 1 mg/Kg | 49 (57.64%) | 25 (50%) | 24 (65.71%) |
| Microorganism | Outcome | Antibiotic Family n (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactam | Polymyxins | Aminoglycoside | Quinolones | Glycylcyclines | ||||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | ||
| P. aeruginosa n = 30 | Favorable n = 10 | 0 (0) | 0 (0) | 10 (100) | 2 (20) | 4 (40) | 4 (40) | 2 (20) | 0 (0) | 8 (80) | 2 (20) | 0 (0) | 8 (80) | 0 (0) | 0 (0) | 9 (90) |
| Unfavorable n = 14 | 0 (0) | 0 (0) | 14 (100) | 1 (7.14) | 6 (42.8) | 7 (50) | 1 (7.1) | 0 (0) | 13 (92.9) | 2 (14.3) | 0 (0) | 11 (78.6) | 0 (0) | 0 (0) | 11 (78.6) | |
| K. pneumoniae n = 5 | Favorable n = 3 | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 0 (0) | 1 (33.3) | 2 (66.7) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Unfavorable n = 2 | 0 (0) | 0 (0) | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | |
| P. putida n = 2 | Favorable n = 1 | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| Unfavorable n = 1 | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |
| A. baumannii n = 1 | Favorable | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) |
| E. cloacae n = 1 | Favorable | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| E. coli n = 1 | Favorable | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| C. freundii n = 1 | Unfavorable | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| Microbiological Isolation | % of Prevalence | % blaKPC | % MBL | % blaKPC + blaVIM | None | |
|---|---|---|---|---|---|---|
| blaVIM | Others | |||||
| Acinetobacter baumannii | 1.13% | - | - | - | - | |
| Pseudomomonas aeruginosa | 59.1% | 10% | 6.7% | - | 57% | - |
| Escherichia coli | 3.4% | 33% | - | - | - | - |
| Enterobacter cloacae | 10.2% | 11% | - | - | - | - |
| Klebsiella oxytoca | 1.13% | - | - | - | - | - |
| Klebsiella Oxytoca + E. coli | 1.13% | - | - | - | - | - |
| Klebsiella pneumoniae | 13.6% | 80% | - | - | - | - |
| Klebsiella pneumoniae + Pseudomonas aeruginosa | 1.13% | - | - | - | - | - |
| Providencia rettgeri | 1.13% | - | - | - | - | - |
| Pseudomonas sp. | 1.13% | - | - | - | - | - |
| Pseudomonas + Enterobacter cloacae | 1.13% | - | - | - | - | - |
| Pseudomonas putida | 3.4% | - | 25% | - | 25% | - |
| Antibiotic | n, (%) | Favorable n, (%) | Unfavorable n, (%) |
|---|---|---|---|
| Doripenem | 54 (63.5%) | 35 (70%) | 19 (54.28%) |
| Meropenem | 23 (27%) | 9 (18%) | 14 (40%) |
| Tigecycline | 7 (8.2%) | 5 (10%) | 2 (5.71%) |
| Fosfomycin | 1 (1.1%) | 1 (2%) | 0 (0%) |
| Primary Outcome | General Population n = 57 | Favorable n = 34 | Unfavorable n = 23 | p-Value |
|---|---|---|---|---|
| Colistin levels, Median, IQR | 0.51 (0–0.78) | 0.16 (0–0.62) | 0.54 (0–1.25) | 0.1670 |
| Secondary outcome | Low colistin levels n = 53 | Normal colistin levels n = 2 | High colistin levels n = 2 | |
| 30 days mortality, n, % | 10 (18.86%) | 0 (0%) | 0 (0%) | |
| Acute kidney injury, n, % | 11 (20.75) | - | 1 (50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria, J.; Garzón, V.; Pacheco, T.; Avila, M.-P.; Garcia, J.-C.; Jaimes, D.; Torres, A.; Bustos, R.-H.; Escobar-Perez, J.; Abril, D. Estimation of the Difference in Colistin Plasma Levels in Critically Ill Patients with Favorable or Unfavorable Clinical Outcomes. Pharmaceutics 2021, 13, 1630. https://doi.org/10.3390/pharmaceutics13101630
Sanabria J, Garzón V, Pacheco T, Avila M-P, Garcia J-C, Jaimes D, Torres A, Bustos R-H, Escobar-Perez J, Abril D. Estimation of the Difference in Colistin Plasma Levels in Critically Ill Patients with Favorable or Unfavorable Clinical Outcomes. Pharmaceutics. 2021; 13(10):1630. https://doi.org/10.3390/pharmaceutics13101630
Chicago/Turabian StyleSanabria, Jose, Vivian Garzón, Tatiana Pacheco, Maria-Paula Avila, Julio-Cesar Garcia, Diego Jaimes, Angela Torres, Rosa-Helena Bustos, Javier Escobar-Perez, and Deisy Abril. 2021. "Estimation of the Difference in Colistin Plasma Levels in Critically Ill Patients with Favorable or Unfavorable Clinical Outcomes" Pharmaceutics 13, no. 10: 1630. https://doi.org/10.3390/pharmaceutics13101630






