Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of the MNPs
2.3. Analysis of the MNPs’ Colloidal Properties
2.4. Determination of the Magnetic Core Size and Shape by Trasmission Electron Microscopy
2.5. Determination of the Magnetic Core’s Crystalline Structure by X-ray Driffraction
2.6. Drug Loading Procedure and Quantification
2.7. Cell Culture
2.8. In Vitro Cell Viability Assay
2.9. Mouse Generation and Husbandry
2.10. Preparation of the Eye Drops for the Topical Application of the MNPs
2.11. Topical Application of the MNP-Based Eye Drops under the Influence of a Magnetic Field
2.12. Electroretinography
2.13. Histological Analysis by Light Microscopy and TEM
2.14. Ocular Biodistribution of the MNPs
2.15. Statistical Analysis
3. Results
3.1. MNP Screening for Optimal Drug Loading
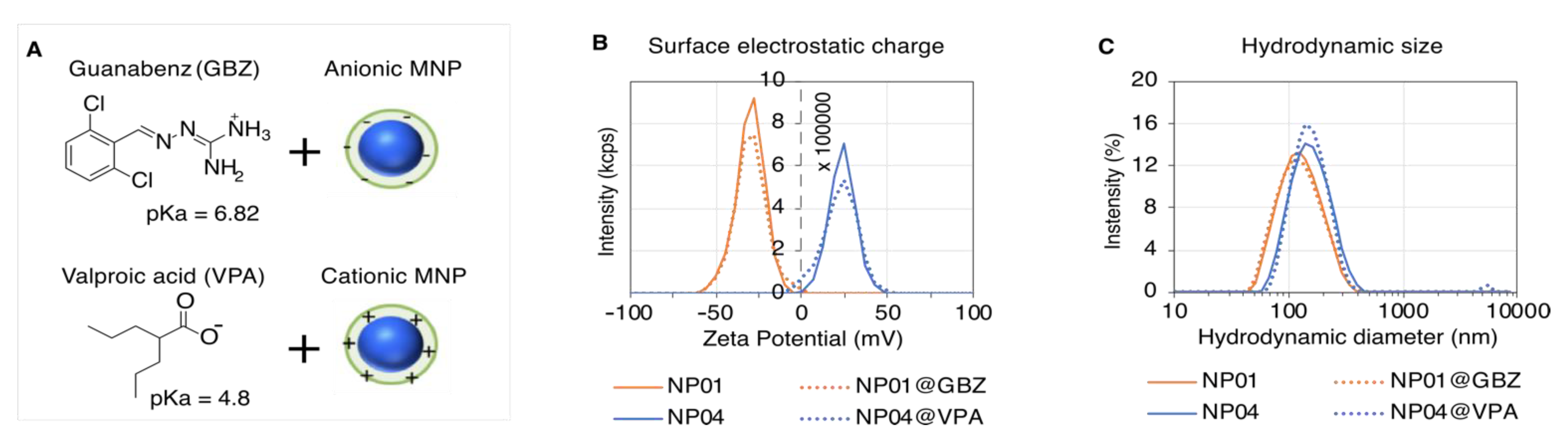
3.2. Physicochemical Characterization of the Selected MNPs
3.3. Formulation of the Drug-Loaded MNP-Based Eye Drops
3.4. Biocompatibility of the MNP-Based Therapeutics In Vitro
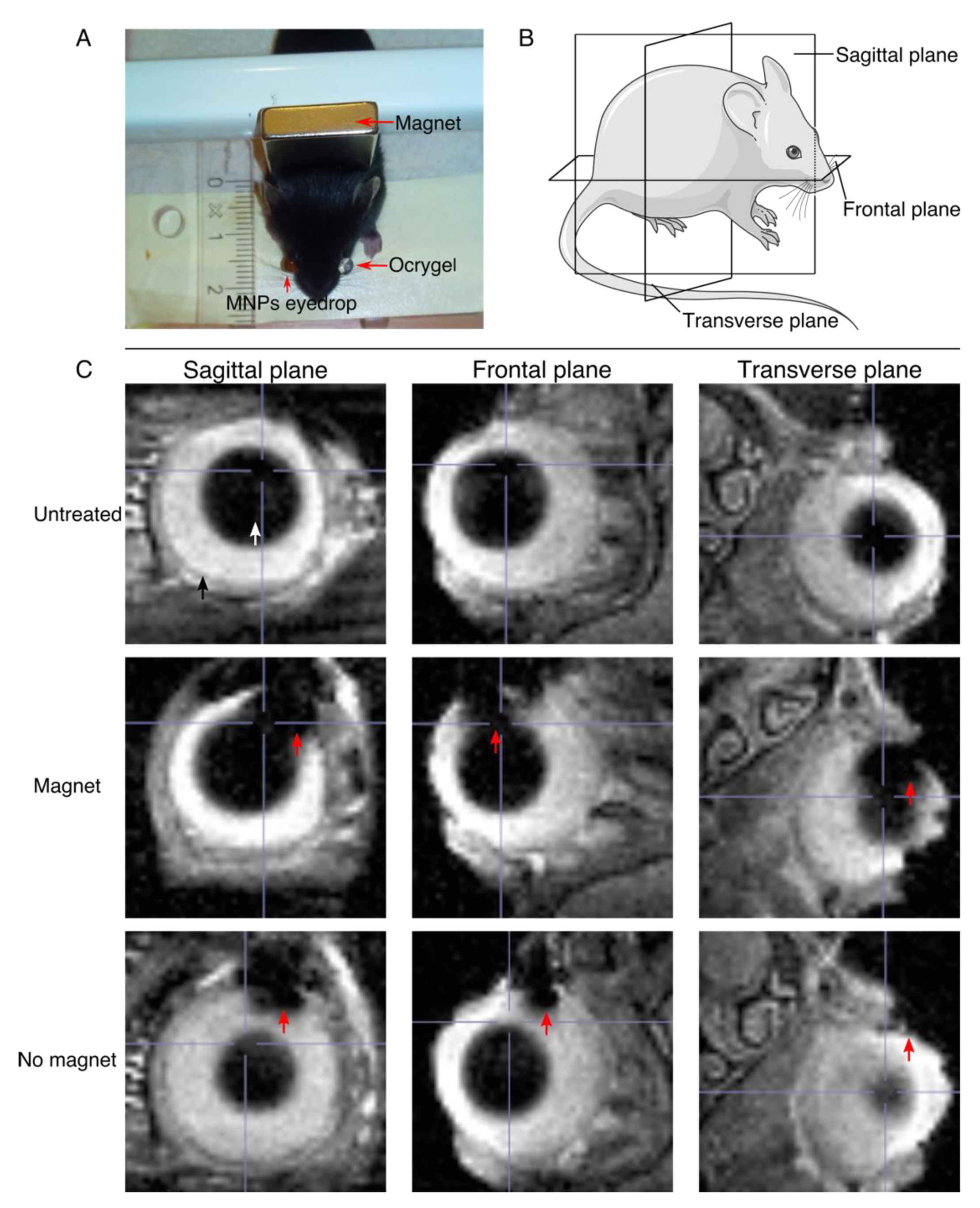
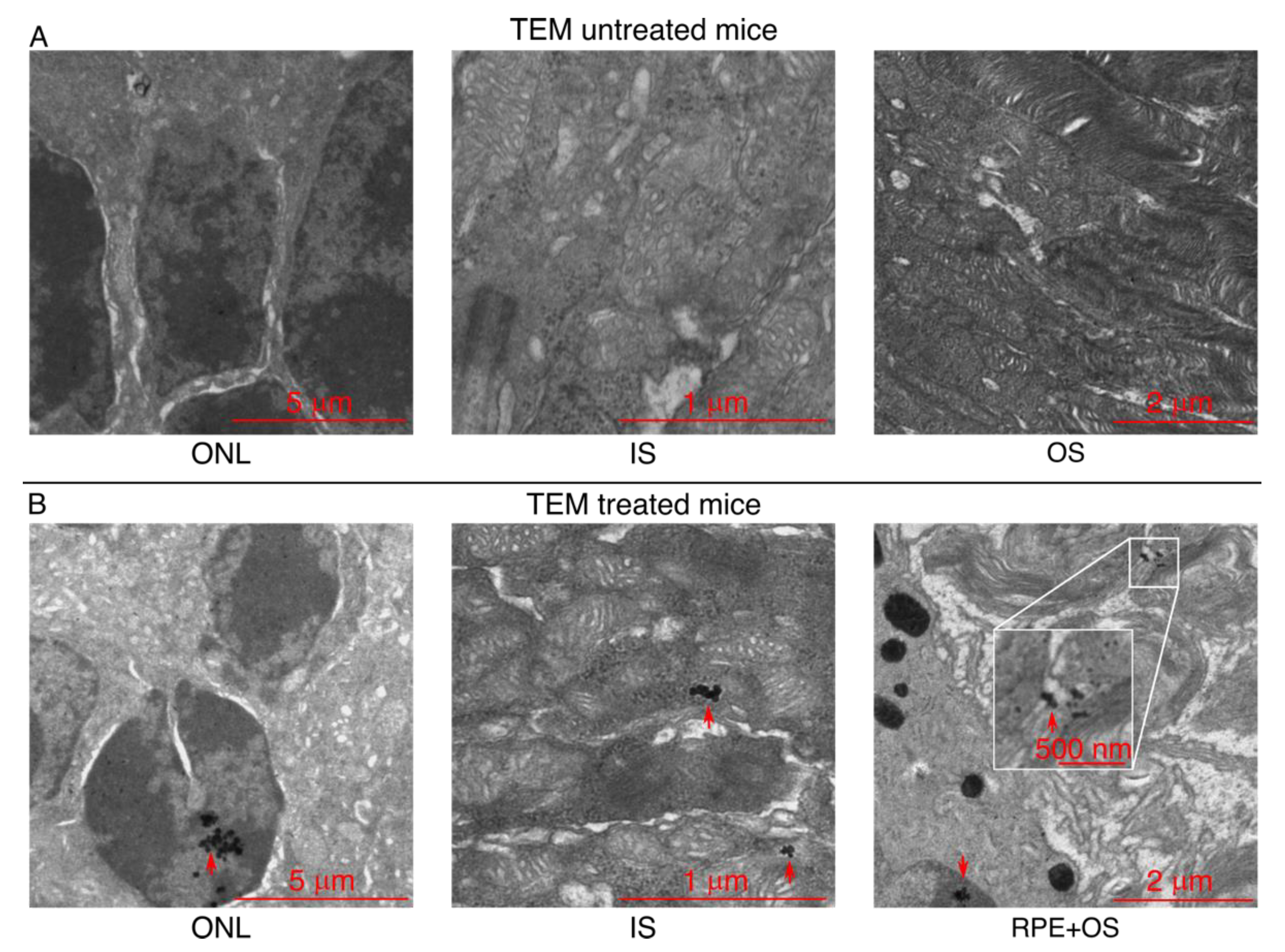
3.5. Upon Topical Eye Drop Administration, MNPs Are Successfully Transported to the Retina with a Magnetic Push
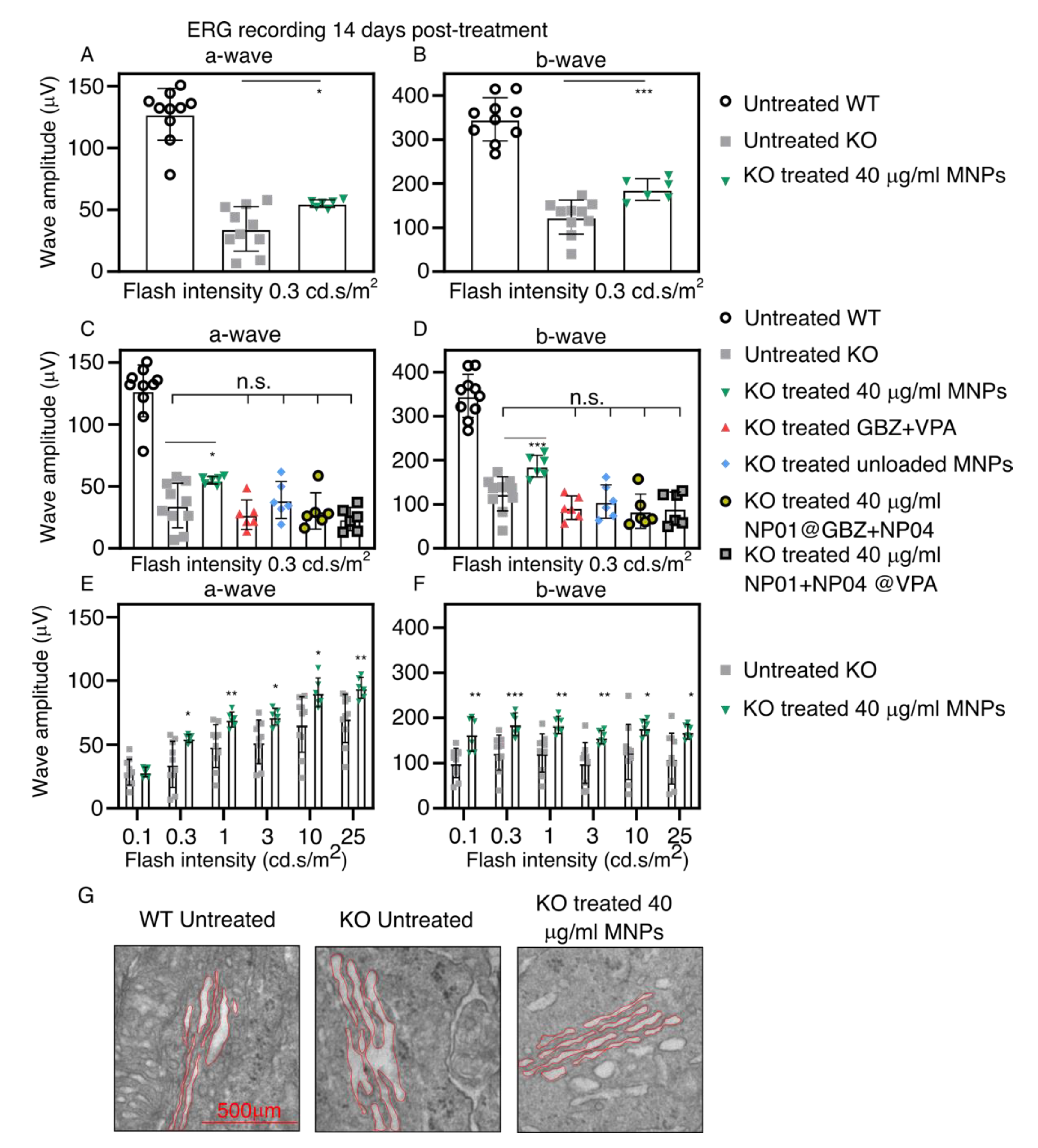
3.6. Non-Invasive, Magnetically Assisted GBZ/VPA Delivery Improves Retinal Function in Bbs KO Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Anglade, E.; Whitcup, S.M. The Diagnosis and Management of Uveitis. Drugs 1995, 49, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T. Risks of Intravitreous Injection: A Comprehensive Review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Shikari, H.; Samant, P.M. Intravitreal injections: A review of pharmacological agents and techniques. J. Clin. Ophthalmol. Res. 2016, 4, 51. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226. [Google Scholar] [CrossRef]
- Niederlova, V.; Modrak, M.; Tsyklauri, O.; Huranova, M.; Stepanek, O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum. Mutat. 2019, 40, 2068–2087. [Google Scholar] [CrossRef]
- Hamel, C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.; Beales, P.L. Making sense of cilia in disease: The human ciliopathies. Am. J. Med. Genet. Part C Semin. Med. Genet. 2009, 151C, 281–295. [Google Scholar] [CrossRef]
- Forsythe, E.; Kenny, J.; Bacchelli, C.; Beales, P.L. Managing Bardet–Biedl Syndrome—Now and in the Future. Front. Pediatr. 2018, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Cognard, N.; Scerbo, M.J.; Obringer, C.; Yu, X.; Costa, F.; Haser, E.; Le, D.; Stoetzel, C.; Roux, M.J.; Moulin, B.; et al. Comparing the Bbs10 complete knockout phenotype with a specific renal epithelial knockout one highlights the link between renal defects and systemic inactivation in mice. Cilia 2015, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Brun, A.; Yu, X.; Obringer, C.; Ajoy, D.; Haser, E.; Stoetzel, C.; Roux, M.J.; Messaddeq, N.; Dollfus, H.; Marion, V. In vivo phenotypic and molecular characterization of retinal degeneration in mouse models of three ciliopathies. Exp. Eye Res. 2019, 186, 107721. [Google Scholar] [CrossRef]
- Mockel, A.; Obringer, C.; Hakvoort, T.B.M.; Seeliger, M.; Lamers, W.H.; Stoetzel, C.; Dollfus, H.; Marion, V. Pharmacological Modulation of the Retinal Unfolded Protein Response in Bardet-Biedl Syndrome Reduces Apoptosis and Preserves Light Detection Ability. J. Biol. Chem. 2012, 287, 37483–37494. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Hola, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Bassetto, M.; Poulhès, F.; Sapet, C.; Bonvin, E.; le Clech, M.; Zelphati, O. Magnetofection Technology: Magnetic-nanoparticles Based Transfection Method for Gene Therapy. In Advances in Nanotechnology; Bartul, Z., Trenor, J., Eds.; Nova Science Publisher Inc.: New York, NY, USA, 2020; Volume 24, pp. 77–123. [Google Scholar]
- Yanai, A.; Hafeli, U.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused Magnetic Stem Cell Targeting to the Retina Using Superparamagnetic Iron Oxide Nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Kennerley, A.J.; Hughes, R.; Fagnano, E.; Richardson, J.; Paul, M.; Murdoch, C.; Wright, F.; Payne, C.; Lythgoe, M.F.; et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun. 2015, 6, 8009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Mousavikhamene, Z.; Abdekhodaie, M.J.; Ahmadieh, H. Facilitation of transscleral drug delivery by drug loaded magnetic polymeric particles. Mater. Sci. Eng. C 2017, 79, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Giannaccini, M.; Monte, M.D.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the Somatostatin Analog Octreotide with Magnetic Nanoparticles for Intraocular Delivery: A Possible Approach for the Treatment of Diabetic Retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 144. [Google Scholar] [CrossRef]
- Dengler, M.; Saatchi, K.; Dailey, J.P.; Matsubara, J.; Mikelberg, F.S.; Häfeli, U.O.; Yeung, S.N. Targeted delivery of magnetic cobalt nanoparticles to the eye following systemic administration. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2010; Volume 1311, pp. 329–336. [Google Scholar] [CrossRef]
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic Targeting of Growth Factors Using Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 707. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, M.R.; Jiang, H.; Shen, M.; Cui, L.; Bhattacharya, S.K. Detection of magnetic particles in live DBA/2J mouse eyes using magnetomotive optical coherence tomography. Eye Contact Lens 2010, 36, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.L.; Goldberg, R.A.; Kunzevitzky, N.J.; Dillon, J.R. Magnetic Eye Shields and Methods of Treatment and Diagnosis Using the Same. U.S. Patent 10,327,945, 25 June 2019. [Google Scholar]
- Dailey, J.P. Magnetized Scleral Buckle for Use with Silicone Magnetic Fluids in the Treatment of Retnal Diseases. U.S. Patent 6,547,714, 15 April 2003. [Google Scholar]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Swiderski, R.E.; Rahmouni, K.; Nishimura, D.Y.; Mullins, R.; Agassandian, K.; Philp, A.R.; Searby, C.C.; Andrews, M.P.; Thompson, S.; et al. A knockin mouse model of the Bardet Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc. Natl. Acad. Sci. USA 2007, 104, 19422–19427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattapallil, M.J.; Wawrousek, E.F.; Chan, C.-C.; Zhao, H.; Roychoudhury, J.; Ferguson, T.A.; Caspi, R.R. TheRd8Mutation of theCrb1Gene Is Present in Vendor Lines of C57BL/6N Mice and Embryonic Stem Cells, and Confounds Ocular Induced Mutant Phenotypes. Investig. Opthalmology Vis. Sci. 2012, 53, 2921–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeap, S.P.; Ahmad, A.L.; Ooi, B.S.; Lim, J. Electrosteric Stabilization and Its Role in Cooperative Magnetophoresis of Colloidal Magnetic Nanoparticles. Langmuir 2012, 28, 14878–14891. [Google Scholar] [CrossRef]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R.; et al. Clinical experiences with magnetic drug targeting: A phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Berger, R.; Bissey, J.-C.; Kliava, J.; Daubric, H.; Estournes, C. Temperature dependence of superparamagnetic resonance of iron oxide nanoparticles. J. Magn. Magn. Mater. 2001, 234, 535–544. [Google Scholar] [CrossRef]
- Ghosh, S.; Jiang, W.; McClements, J.D.; Xing, B. Colloidal Stability of Magnetic Iron Oxide Nanoparticles: Influence of Natural Organic Matter and Synthetic Polyelectrolytes. Langmuir 2011, 27, 8036–8043. [Google Scholar] [CrossRef]
- Tóth, I.Y.; Illés, E.; Bauer, R.A.; Nesztor, D.; Szekeres, M.; Zupkó, I.; Tombácz, E. Designed Polyelectrolyte Shell on Magnetite Nanocore for Dilution-Resistant Biocompatible Magnetic Fluids. Langmuir 2012, 28, 16638–16646. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.H.; Invergo, B.; Shimomura, K.; Takahashi, J.S.; Troy, J.B. Interpretation of the mouse electroretinogram. Doc. Ophthalmol. 2007, 115, 127–136. [Google Scholar] [CrossRef] [Green Version]
- De Majumdar, S.; Subinya, M.; Korward, J.; Pettigrew, A.; Scherer, D.; Xu, H. A Low Concentration of Tacrolimus/Semifluorinated Alkane (SFA) Eyedrop Suppresses Intraocular Inflammation in Experimental Models of Uveitis. Curr. Mol. Med. 2017, 17, 211–220. [Google Scholar] [CrossRef]
- Kam, J.H.; Lynch, A.; Begum, R.; Cunea, A.; Jeffery, G. Topical cyclodextrin reduces amyloid beta and inflammation improving retinal function in ageing mice. Exp. Eye Res. 2015, 135, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.K.; Sutariya, V.; Kanwar, J.R.; Pathak, Y.V. (Eds.) Drug Delivery for the Retina and Posterior Segment Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 241–269. [Google Scholar] [CrossRef]
- Lajunen, T.; Hisazumi, K.; Kanazawa, T.; Okada, H.; Seta, Y.; Yliperttula, M.; Urtti, A.; Takashima, Y. Topical drug delivery to retinal pigment epithelium with microfluidizer produced small liposomes. Eur. J. Pharm. Sci. 2014, 62, 23–32. [Google Scholar] [CrossRef]
- Zahn, D.; Klein, K.; Radon, P.; Berkov, D.; Erokhin, S.; Nagel, E.; Eichhorn, M.; Wiekhorst, F.; Dutz, S. Investigation of magnetically driven passage of magnetic nanoparticles through eye tissues for magnetic drug targeting. Nanotechnology 2020, 31, 495101. [Google Scholar] [CrossRef] [PubMed]
- Raju, H.B.; Hu, Y.; Vedula, A.; Dubovy, S.R.; Goldberg, J.L. Evaluation of Magnetic Micro- and Nanoparticle Toxicity to Ocular Tissues. PLoS ONE 2011, 6, e17452. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Pedreschi, E.; Di Colo, G. Is dialysis a reliable method for studying drug release from nanoparticulate systems?—A case study. Int. J. Pharm. 2012, 434, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, H.; Yao, S.; Tu, X.; Su, M.; Zhou, L. Magnetic targeted nanoparticles based on β-cyclodextrin and chitosan for hydrophobic drug delivery and a study of their mechanism. RSC Adv. 2017, 7, 29025–29034. [Google Scholar] [CrossRef] [Green Version]
- Sen, M.; Bassetto, M.; Poulhes, F.; Zelphati, O.; Ueffing, M.; Arango-Gonzalez, B. Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics 2021, 13, 225. [Google Scholar] [CrossRef]
- Bassetto, M.; Sen, M.; Poulhes, F.; Arango-Gonzalez, B.; Bonvin, E.; Sapet, C.; Ueffing, M.; Zelphati, O. New Method for Efficient siRNA Delivery in Retina Explants: Reverse Magnetofection. Bioconjugate Chem. 2021, 32, 1078–1093. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Padgett, K.R.; E Rodriguez, J.; Goldberg, J.L. Investigation of nanoparticles using magnetic resonance imaging after intravitreal injection. Clin. Exp. Ophthalmol. 2012, 40, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular Nanoparticle Toxicity and Transfection of the Retina and Retinal Pigment Epithelium. Nanomedicine 2008, 4, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Russell, P.; Martiniello-Wilks, R.; Rasko, J.E.J.; Khatri, A. Concise review: Nanoparticles and cellular carriers-allies in cancer imaging and cellular gene therapy? Stem Cells 2010, 28, 1686–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, D.; Wang, Z.; Dong, W.-F.; Zhang, X.; Zheng, X.; Xiao, X.-A.; Wang, Y.-S.; Zhao, X.; Zhang, M.; Li, J.; et al. Facile Synthesis of Core-shell Magnetic Mesoporous Silica Nanoparticles for pH-sensitive Anticancer Drug Delivery. Chem. Biol. Drug Des. 2015, 86, 1548–1553. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Lee, J.; Kim, T.; Kim, H.; Yu, T.; Song, I.-C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Sarwar, A.; Preciado, D.; Depireux, D. Shaping Magnetic Fields to Direct Therapy to Ears and Eyes. Annu. Rev. Biomed. Eng. 2014, 16, 455–481. [Google Scholar] [CrossRef] [Green Version]

| MNPs | Drug | Coating | Zeta Potential (mV) | Hydrodynamic Size (nm) | PDI | Drug Loaded on MNPs Surface | Drug Loading Efficiency (%) |
|---|---|---|---|---|---|---|---|
| NP01 | GBZ | Inorganic+Organic | −29.7 ± 9.50 | 141 ± 64 | 0.167 | 3.99 ± 1.99 μM | 31.50 ± 15.69 |
| NP02 | Inorganic | −44.8 ± 6.6 | 248 ± 83 | 0.105 | / | / | |
| NP03 | Organic | −35.0 ± 8.7 | 125 ± 53 | 0.171 | 3.73 ± 2.42 μM | 29.49 ± 19.14 | |
| NP04 | VPA | Proprietary polymer | 33.0 ± 8.03 | 169 ± 63 | 0.133 | 1.18 ± 0.31 mM | 11.81 ± 3.14 |
| NP05 | Inorganic+polymer | 37.6 ± 8.93 | 195 ± 75 | 0.161 | 0.25 ± 0.09 mM | 2.50 ± 0.79 | |
| NP06 | Inorganic+organic | 30.3 ± 11.1 | 213 ± 70 | 0.110 | 0.05 ± 0.23 mM | 0.53 ± 2.35 | |
| NP07 | Inorganic+organic | 36.3 ± 7.60 | 209 ± 110 | 0.162 | 0.96 ± 0.16 mM | 9.62 ± 1.60 | |
| NP08 | Inorganic+organic | 34.8 ± 4.74 | 207 ± 108 | 0.184 | 1.11 ± 0.14 mM | 11.05 ± 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassetto, M.; Ajoy, D.; Poulhes, F.; Obringer, C.; Walter, A.; Messadeq, N.; Sadeghi, A.; Puranen, J.; Ruponen, M.; Kettunen, M.; et al. Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice. Pharmaceutics 2021, 13, 1650. https://doi.org/10.3390/pharmaceutics13101650
Bassetto M, Ajoy D, Poulhes F, Obringer C, Walter A, Messadeq N, Sadeghi A, Puranen J, Ruponen M, Kettunen M, et al. Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice. Pharmaceutics. 2021; 13(10):1650. https://doi.org/10.3390/pharmaceutics13101650
Chicago/Turabian StyleBassetto, Marco, Daniel Ajoy, Florent Poulhes, Cathy Obringer, Aurelie Walter, Nadia Messadeq, Amir Sadeghi, Jooseppi Puranen, Marika Ruponen, Mikko Kettunen, and et al. 2021. "Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice" Pharmaceutics 13, no. 10: 1650. https://doi.org/10.3390/pharmaceutics13101650
APA StyleBassetto, M., Ajoy, D., Poulhes, F., Obringer, C., Walter, A., Messadeq, N., Sadeghi, A., Puranen, J., Ruponen, M., Kettunen, M., Toropainen, E., Urtti, A., Dollfus, H., Zelphati, O., & Marion, V. (2021). Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice. Pharmaceutics, 13(10), 1650. https://doi.org/10.3390/pharmaceutics13101650






