Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Medical Devices
2.1.2. Medications
- Valium® (Diazepam) 10 mg/2mL (Roche, Rosny-Sous-Bois, France; batch F1126F01, expiring 9/2020), diluted to 0.2 mg/mL in a 5% glucose solution (B. Braun, Saint Cloud, Germany).
- NOVORAPID® (Insulin aspart) 100 UI/mL (Novo Nordisk, Courbevoie, France; batch HS65E14, expiring 1/2020), diluted to 0.1 UI/mL in a 0.9% sodium chloride solution (Versylene®, Fresenius Kabi, Louviers, France). Insulin aspart will henceforth be referred to as insulin.
- Paracetamol B BRAUN® (paracetamol) 10 mg/mL (B. Braun, Saint Cloud, France; batch 18105452, expiring 2/2020 and 18141450, expiring 3/2020), diluted to 1 mg/mL in a 0.9% sodium chloride solution (Versylene®, Fresenius Kabi, Louviers, France).
2.1.3. Reagents
2.2. Methods
2.2.1. Study Design
2.2.2. Evaluation of Drug Loss Caused by Individual Catheters
2.2.3. Sorption Studies of Complete Infusion Lines
2.3. Analysis
Analysis of Catheters Inner Surface
- Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
- Surface Zeta Potential
- Quantitative Analysis
- Expression of the Results
- Statistical Analysis
3. Results
3.1. Catheter’s Surface Characterization
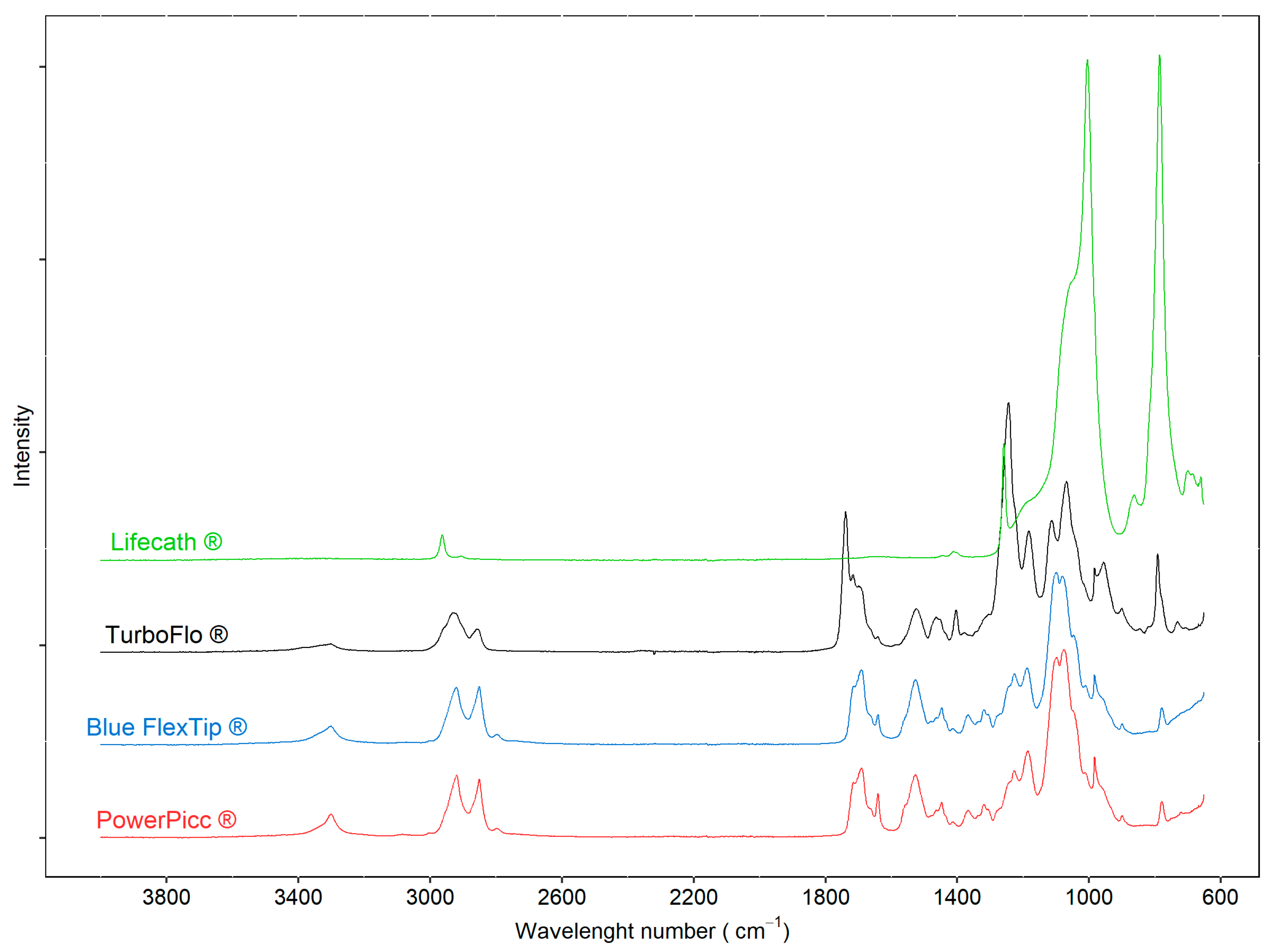
3.1.1. ATR-FTIR
3.1.2. Surface Zeta Potential
3.2. Drug loss Studies
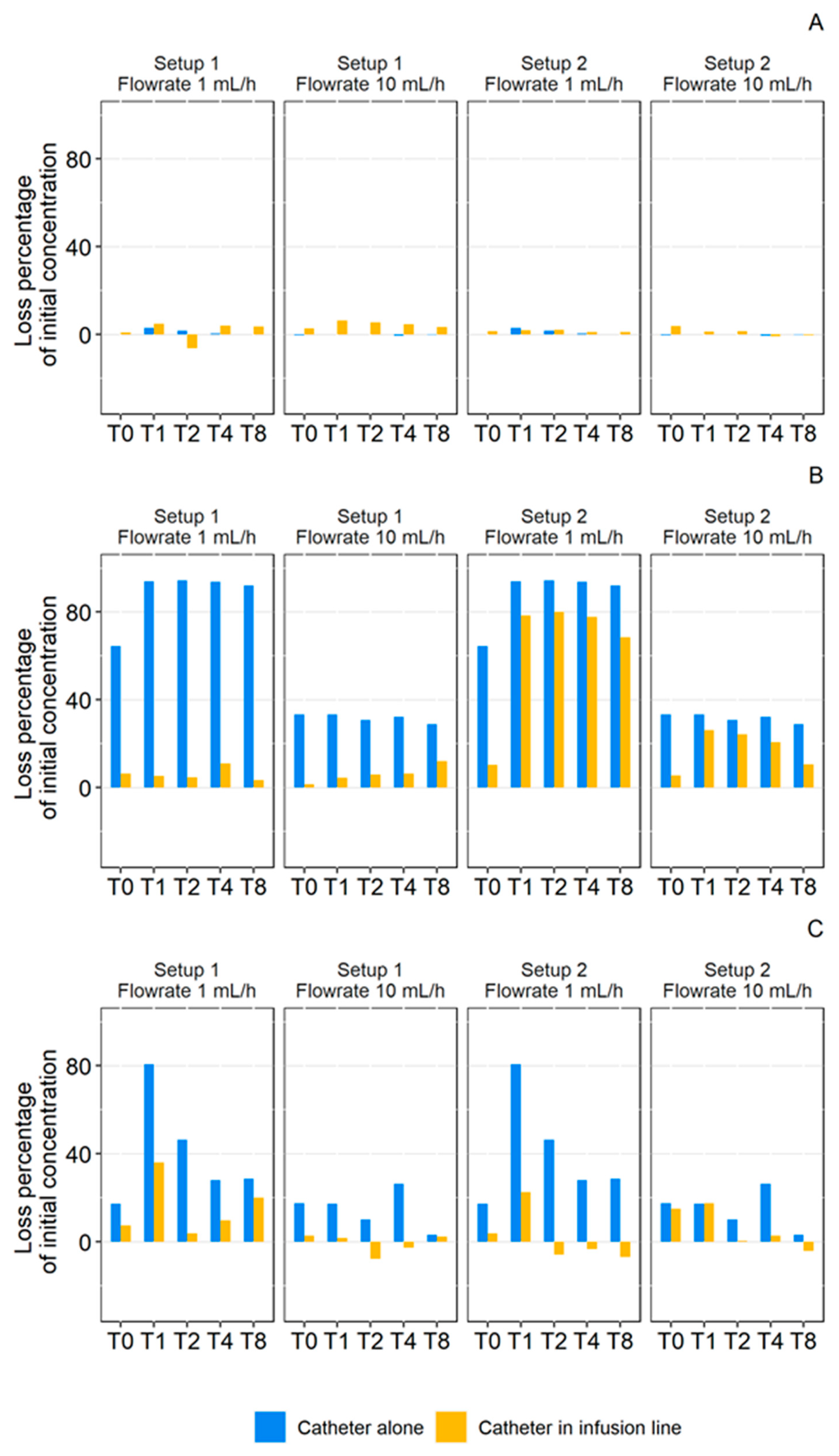
3.2.1. Individual Catheters
- 1 mL/ Dynamic Condition
- 10 mL/h Dynamic Condition
- Effect Size (ES)
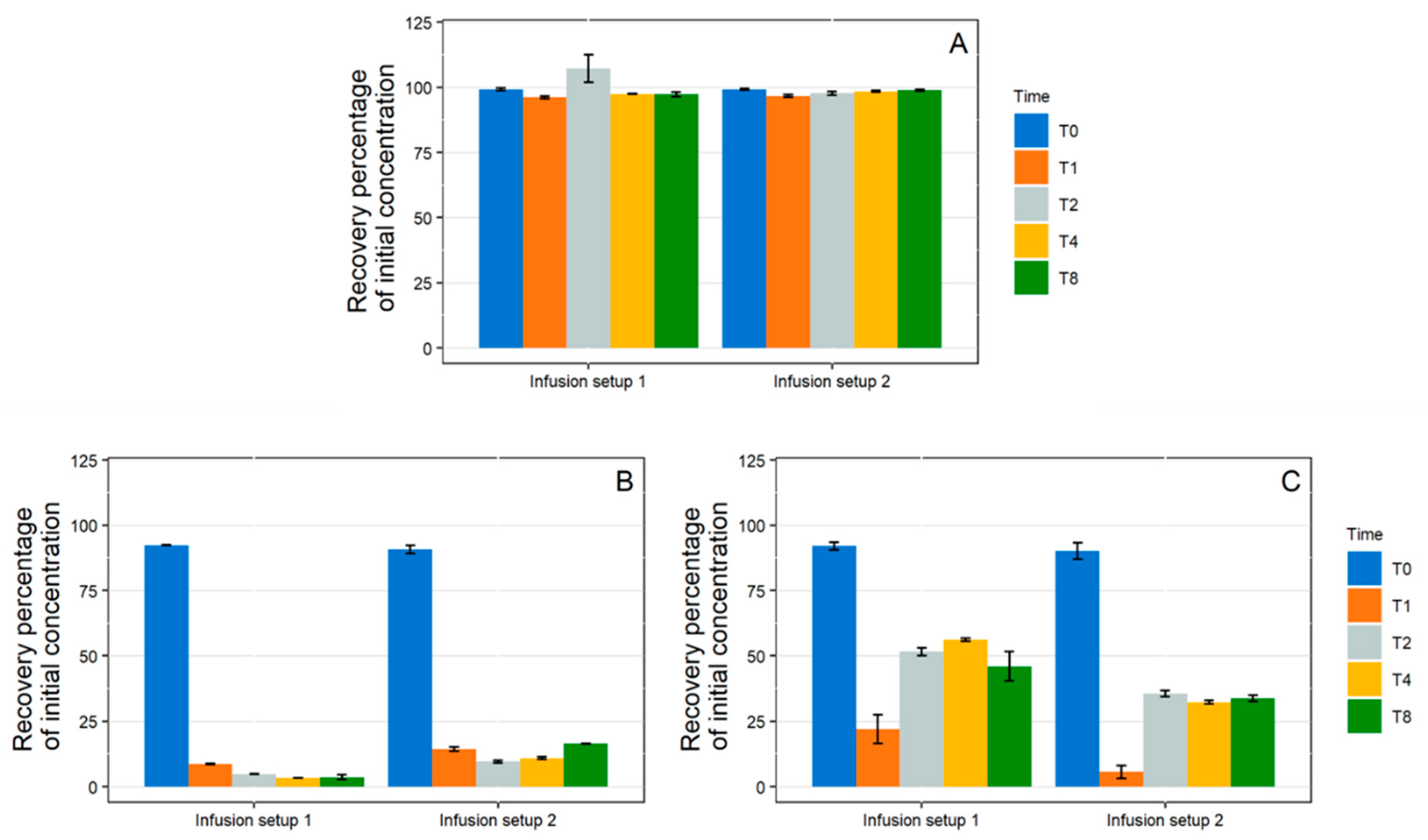
3.2.2. Complete Infusion Setup
- Medical Device Selection
- 1 mL/h Dynamic Condition
- 10 mL/h Dynamic Condition
- Setup Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterfreund, R.A.; Philip, J.H. Critical Parameters in Drug Delivery by Intravenous Infusion. Expert Opin. Drug Deliv. 2013, 10, 1095–1108. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Lazúen-Muros, M.; Ruiz-Samblás, C.; Navas-Iglesias, N. Leachables from Plastic Materials in Contact with Drugs. State of the Art and Review of Current Analytical Approaches. Int. J. Pharm. 2020, 583, 119332. [Google Scholar] [CrossRef]
- Laschi, A.; Sehnal, N.; Alarcon, A.; Barcelo, B.; Caire-Maurisier, F.; Delaire, M.; Feuilloley, M.; Genot, S.; Lacaze, C.; Pisarik, L.; et al. Container-Content Compatibility Studies: A Pharmaceutical Team’s Integrated Approach. PDA J. Pharm. Sci. Technol. 2009, 63, 285–293. [Google Scholar]
- Masse, M.; Maton, M.; Genay, S.; Blanchemain, N.; Barthélémy, C.; Décaudin, B.; Odou, P. In Vitro Assessment of the Influence of Intravenous Extension Set Materials on Insulin Aspart Drug Delivery. PLoS ONE 2018, 13, e0201623. [Google Scholar] [CrossRef]
- Maiguy-Foinard, A.; Blanchemain, N.; Barthélémy, C.; Décaudin, B.; Odou, P. Influence of a Double-Lumen Extension Tube on Drug Delivery: Examples of Isosorbide Dinitrate and Diazepam. PLoS ONE 2016, 11, e0154917. [Google Scholar]
- Zahid, N.; Taylor, K.M.G.; Gill, H.; Maguire, F.; Shulman, R. Adsorption of Insulin onto Infusion Sets Used in Adult Intensive Care Unit and Neonatal Care Settings. Diabetes Res. Clin. Pract. 2008, 80, e11–e13. [Google Scholar] [CrossRef]
- Jakobsson, T.; Shulman, R.; Gill, H.; Taylor, K. The Impact of Insulin Adsorption onto the Infusion Sets in the Adult Intensive Care Unit. J. Diabetes Sci. Technol. 2009, 3, 213–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.-E.; You, S.; Jeon, S.; Hwang, S.-J. Diazepam Sorption to PVC- and Non-PVC-Based Tubes in Administration Sets with Quantitative Determination Using a High-Performance Liquid Chromatographic Method. Int. J. Pharm. 2016, 506, 414–419. [Google Scholar] [CrossRef]
- Jin, S.-E.; Jeon, S.; Byon, H.-J.; Hwang, S.-J. Evaluation of Tacrolimus Sorption to PVC- and Non-PVC-Based Tubes in Administration Sets: Pump Method vs. Drip Method. Int. J. Pharm. 2017, 528, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Knopp, J.L.; Hardy, A.R.; Vergeer, S.; Chase, J.G. Modelling Insulin Adsorption in Intravenous Infusion Sets in the Intensive Care Unit. IFAC J. Syst. Control 2019, 8, 100042. [Google Scholar] [CrossRef]
- Treleano, A.; Wolz, G.; Brandsch, R.; Welle, F. Investigation into the Sorption of Nitroglycerin and Diazepam into PVC Tubes and Alternative Tube Materials during Application. Int. J. Pharm. 2009, 369, 30–37. [Google Scholar] [CrossRef]
- Tokhadze, N.; Chennell, P.; Bernard, L.; Lambert, C.; Pereira, B.; Mailhot-Jensen, B.; Sautou, V. Impact of Alternative Materials to Plasticized PVC Infusion Tubings on Drug Sorption and Plasticizer Release. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Muynck, C.; Vandenbossche, G.M.R.; Colardyn, F.; Remon, J.P. Sorption of Isosorbide Dinitrate to Central Venous Catheters. J. Pharm. Pharmacol. 1993, 45, 139–141. [Google Scholar] [CrossRef]
- Ley, S.C.; Ammann, J.; Herder, C.; Dickhaus, T.; Hartmann, M.; Kindgen-Milles, D. Insulin Adsorption to Catheter Materials Used for Intensive Insulin Therapy in Critically Ill Patients: Polyethylene Versus Polyurethane–Possible Cause of Variation in Glucose Control? Open Crit. Care Med. J. 2014, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wildgruber, M.; Lueg, C.; Borgmeyer, S.; Karimov, I.; Braun, U.; Kiechle, M.; Meier, R.; Koehler, M.; Ettl, J.; Berger, H. Polyurethane versus Silicone Catheters for Central Venous Port Devices Implanted at the Forearm. Eur. J. Cancer 2016, 59, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Al Salloum, H.; Saunier, J.; Aymes-Chodur, C.; Barakat, H.; Yagoubi, N. Impact of the Nature and Concentration of Plasticizers on the Ability of PVC to Sorb Drug. Int. J. Pharm. 2015, 496, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Davies, M.C.; Melia, C.D.; Denyer, S.P.; Derrick, M.R. Uptake of Drugs by Catheters: The Influence of the Drug Molecule on Sorption by Polyurethane Catheters. Biomaterials 1996, 17, 1469–1472. [Google Scholar] [CrossRef]
- Basle, Y.L.; Chennell, P.; Sautou, V. A Sorption Study between Ophthalmic Drugs and Multi Dose Eyedroppers in Simulated Use Conditions. Pharm. Technol. Hosp. Pharm. 2017, 2, 181. [Google Scholar] [CrossRef]
- Arruda, W.O.; Brito Filho, D.; Rosa, S.L.; Fontoura, P.S.; Cardoso, M.D.A. Factors Affecting Diazepam Availability from Intravenous Admixture Solutions. Arq. Neuropsiquiatr. 1989, 47, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, N.A.; Cline, S.; Hyneck, M.L.; Berardi, R.R.; Ho, N.F.; Flynn, G.L. Factors Affecting Diazepam Infusion: Solubility, Administration-Set Composition, and Flow Rate. Am. J. Hosp. Pharm. 1981, 38, 1449–1454. [Google Scholar] [CrossRef]
- Mollmann, S.H.; Jorgensen, L.; Bukrinsky, J.T.; Elofsson, U.; Norde, W.; Frokjaer, S. Interfacial Adsorption of Insulin: Conformational Changes and Reversibility of Adsorption. Eur. J. Pharm. Sci. 2006, 27, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, S.H.; Bukrinsky, J.T.; Frokjaer, S.; Elofsson, U. Adsorption of Human Insulin and AspB28 Insulin on a PTFE-like Surface. J. Colloid Interface Sci. 2005, 286, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Knopp, J.L.; Bishop, K.; Lerios, T.; Chase, J.G. Capacity of Infusion Lines for Insulin Adsorption: Effect of Flow Rate on Total Adsorption. J. Diabetes Sci. Technol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Tokhadzé, N.; Chennell, P.; Cueff, R.; Sautou, V. Do Bevacizumab Solutions Interact with Silicone or Polyurethane Catheters during an Infusion through Implantable Venous Access Ports? J. R. Soc. Interface 2019, 16, 20180721. [Google Scholar] [CrossRef]
- Yliruusi, J.K.; Uotila, J.A.; Kristoffersson, E.R. Effect of Tubing Length on Adsorption of Diazepam to Polyvinyl Chloride Administration Sets. Am. J. Hosp. Pharm. 1986, 43, 2789–2794. [Google Scholar] [CrossRef]
- Ilium, L.; Bundgaard, H.; Davis, S.S. A Constant Partition Model for Examining the Sorption of Drugs by Plastic Infusion Bags. Int. J. Pharm. 1983, 17, 183–192. [Google Scholar] [CrossRef]
- Atkinson, H.C.; Duffull, S.B. Prediction of Drug Loss from PVC Infusion Bags. J. Pharm. Pharmacol. 1991, 43, 374–376. [Google Scholar] [CrossRef]
- Jenke, D.R. Modeling of Solute Sorption by Polyvinyl Chloride Plastic Infusion Bags. J. Pharm. Sci. 1993, 82, 1134–1139. [Google Scholar] [CrossRef]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as Vectors for Bioaccumulation of Hydrophobic Organic Chemicals in the Marine Environment: A State-of-the-Science Review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Bundgaard, H. Sorption of Drugs by Plastic Infusion Bags. Int. J. Pharm. 1982, 10, 339–351. [Google Scholar] [CrossRef]
- Kowaluk, E.A.; Roberts, M.S.; Polack, A.E. Factors Affecting the Availability of Diazepam Stored in Plastic Bags and Administered through Intravenous Sets. Am. J. Hosp. Pharm. 1983, 40, 417–423. [Google Scholar] [CrossRef]
- Hacker, C.; Verbeek, M.; Schneider, H.; Steimer, W. Falsely Elevated Cyclosporin and Tacrolimus Concentrations over Prolonged Periods of Time Due to Reversible Adsorption to Central Venous Catheters. Clin. Chim. Acta 2014, 433, 62–68. [Google Scholar] [CrossRef]
- Hoehne, M.; Samuel, F.; Dong, A.; Wurth, C.; Mahler, H.-C.; Carpenter, J.F.; Randolph, T.W. Adsorption of Monoclonal Antibodies to Glass Microparticles. J. Pharm. Sci. 2011, 100, 123–132. [Google Scholar] [CrossRef]
- Bee, J.S.; Chiu, D.; Sawicki, S.; Stevenson, J.L.; Chatterjee, K.; Freund, E.; Carpenter, J.F.; Randolph, T.W. Monoclonal Antibody Interactions with Micro- and Nanoparticles: Adsorption, Aggregation, and Accelerated Stress Studies. J. Pharm. Sci. 2009, 98, 3218–3238. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Thirumangalathu, R.; Randolph, T.W.; Carpenter, J.F. IgG1 Aggregation and Particle Formation Induced by Silicone–Water Interfaces on Siliconized Borosilicate Glass Beads: A Model for Siliconized Primary Containers. J. Pharm. Sci. 2013, 102, 852–865. [Google Scholar] [CrossRef]
- Majumdar, S.; Ford, B.M.; Mar, K.D.; Sullivan, V.J.; Ulrich, R.G.; D’souza, A.J.M. Evaluation of the Effect of Syringe Surfaces on Protein Formulations. J. Pharm. Sci. 2011, 100, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Summary of Products Characteristics: BLINCYTO. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003731/human_med_001921.jsp&mid=WC0b01ac058001d124 (accessed on 29 June 2018).
- Hauk, A.; Jurkiewicz, E.; Pahl, I.; Loewe, T.; Menzel, R. Filtration Membranes—Scavengers for Leachables? Eur. J. Pharm. Sci. 2018, 120, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Gasch, J.; Leopold, C.S.; Knoth, H. Drug Retention by Inline Filters—Effect of Positively Charged Polyethersulfone Filter Membranes on Drug Solutions with Low Concentration. Eur. J. Pharm. Sci. 2011, 44, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Besheer, A. Protein Adsorption to In-Line Filters of Intravenous Administration Sets. J. Pharm. Sci. 2017, 106, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, X.; Bian, C.; Wu, S.; Chen, M.; Wang, W.; Wang, J.; Cheng, L. Hydrophobic Drug Adsorption Loss to Syringe Filters from a Perspective of Drug Delivery. J. Pharmacol. Toxicol. Methods 2019, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sahnoune, M.; Tokhadzé, N.; Devémy, J.; Dequidt, A.; Goujon, F.; Chennell, P.; Sautou, V.; Malfreyt, P. Understanding and Characterizing the Drug Sorption to PVC and PE Materials. ACS Appl. Mater. Interfaces 2021, 13, 18594–18603. [Google Scholar] [CrossRef]






| Product Code | Material | Length (cm) | Inner Diameter (cm) | |
|---|---|---|---|---|
| Syringe | ||||
| Plastipak®, Becton-Dickinson (Le Pont de Claix, France) | 300865 | Barrel: polypropylene Plunger rod: polypropylene Seal: synthetic isoprene | Barrel: 13.3 | Barrel: 2.65 |
| Catheters | ||||
| Blue FlexTip® central venous catheter, Teleflex Medical (Le Faget, France) | CV-04301 | Polyurethane | 20.0 | 0.13 |
| Power Picc®, BARD Medical (Voisins Le bretonneux, France) | 6175118 | Polyurethane | 40.0 | 0.094 |
| Turbo-Flo®, COOK Medical® (Paris, France) | G12987 | Polyurethane | 40.0 | 0.12 |
| Lifecath®, VYGON (Ecouen, France) | 2191.50 | Silicone | 40.0 | 0.095 |
| Extension sets | ||||
| CAIR LGL (Lissieu, France) | PN10318-1 | Polyvinyl chloride | 2000.0 | 0.25 |
| CAIR LGL (Lissieu, France) | RPB5320 | Outer layer: Polyvinyl chloride Inner Layer: Polyethylene | 2000.0 | 0.25 |
| Infusion Setup 1 | Infusion Setup 2 | |||
|---|---|---|---|---|
| Medical Device | Manufacturer | Material | Manufacturer | Material |
| Syringe | Plastipak® (Becton-Dickinson) | PP | Plastipak® (Becton-Dickinson)® | PP |
| Extension set | CAIR LGL | PVC | CAIR LGL | PE/PVC |
| Catheter | Turbo-Flo® (Cook Medical) | PUR | Turbo-Flo® (Cook Medical) | PUR |
| Blue FlexTip ® | PowerPICC ® | Turbo-Flo ® | Lifecath ® | |
|---|---|---|---|---|
| pH | 5.0 | 5.0 | 4.9 | 5.1 |
| Zeta potential (mV) | −30.0 | −25.2 | −11.8 | −32.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokhadzé, N.; Chennell, P.; Pereira, B.; Mailhot-Jensen, B.; Sautou, V. Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs. Pharmaceutics 2021, 13, 1709. https://doi.org/10.3390/pharmaceutics13101709
Tokhadzé N, Chennell P, Pereira B, Mailhot-Jensen B, Sautou V. Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs. Pharmaceutics. 2021; 13(10):1709. https://doi.org/10.3390/pharmaceutics13101709
Chicago/Turabian StyleTokhadzé, Nicolas, Philip Chennell, Bruno Pereira, Bénédicte Mailhot-Jensen, and Valérie Sautou. 2021. "Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs" Pharmaceutics 13, no. 10: 1709. https://doi.org/10.3390/pharmaceutics13101709
APA StyleTokhadzé, N., Chennell, P., Pereira, B., Mailhot-Jensen, B., & Sautou, V. (2021). Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs. Pharmaceutics, 13(10), 1709. https://doi.org/10.3390/pharmaceutics13101709







