Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Used Polymer Filaments
2.1.2. Jellifying Agents
2.1.3. Model API’s
2.2. Methods
2.2.1. Design of the Drug Reservoirs and Printing of the Samples
2.2.2. Gel Formation and Sample Manufacturing
2.2.3. Weight Variation and Content Uniformity
2.2.4. Characterization
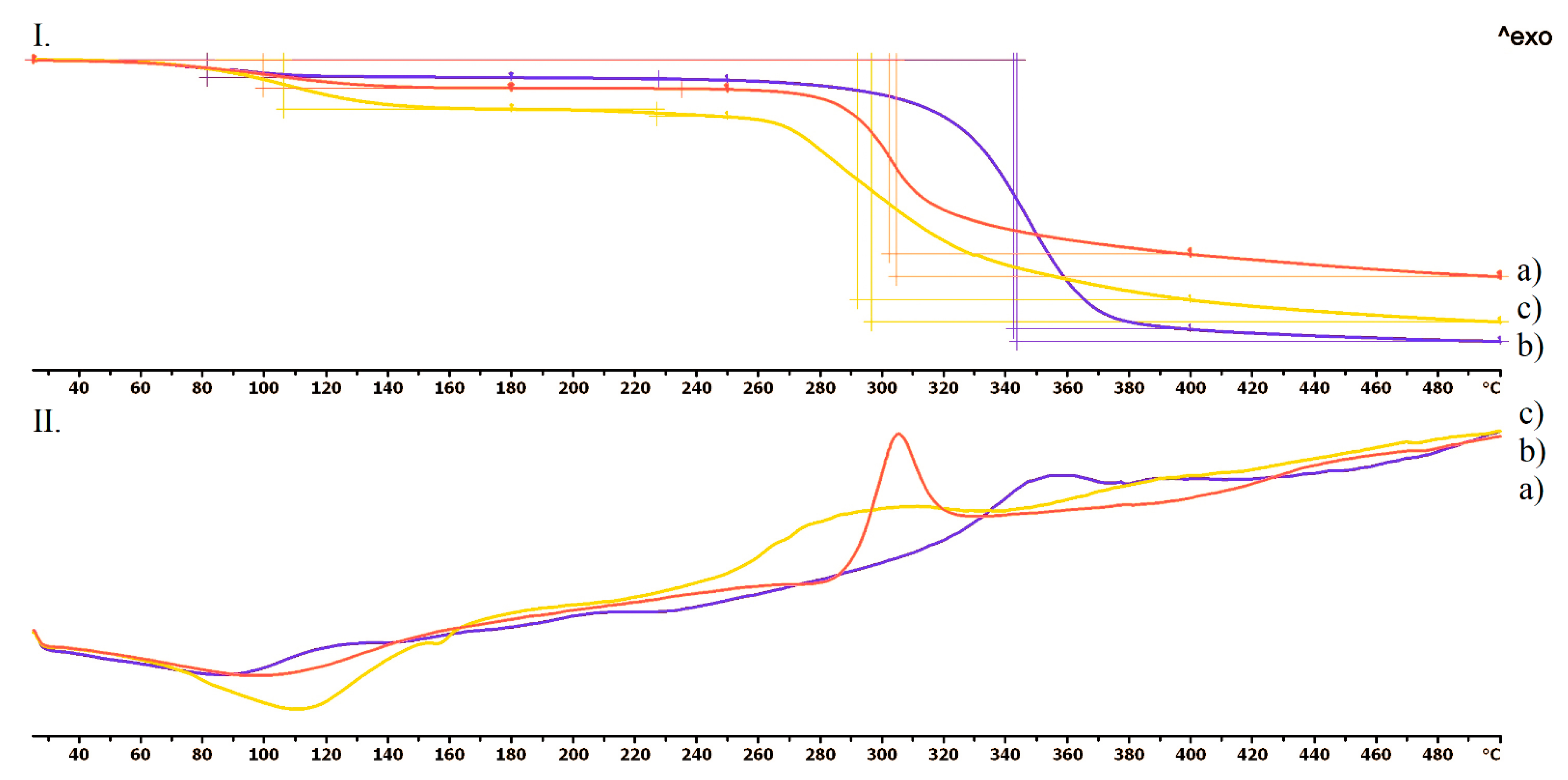
Thermogravimetric (TG) and Heatflow (DSC) Analysis
Contact Angle
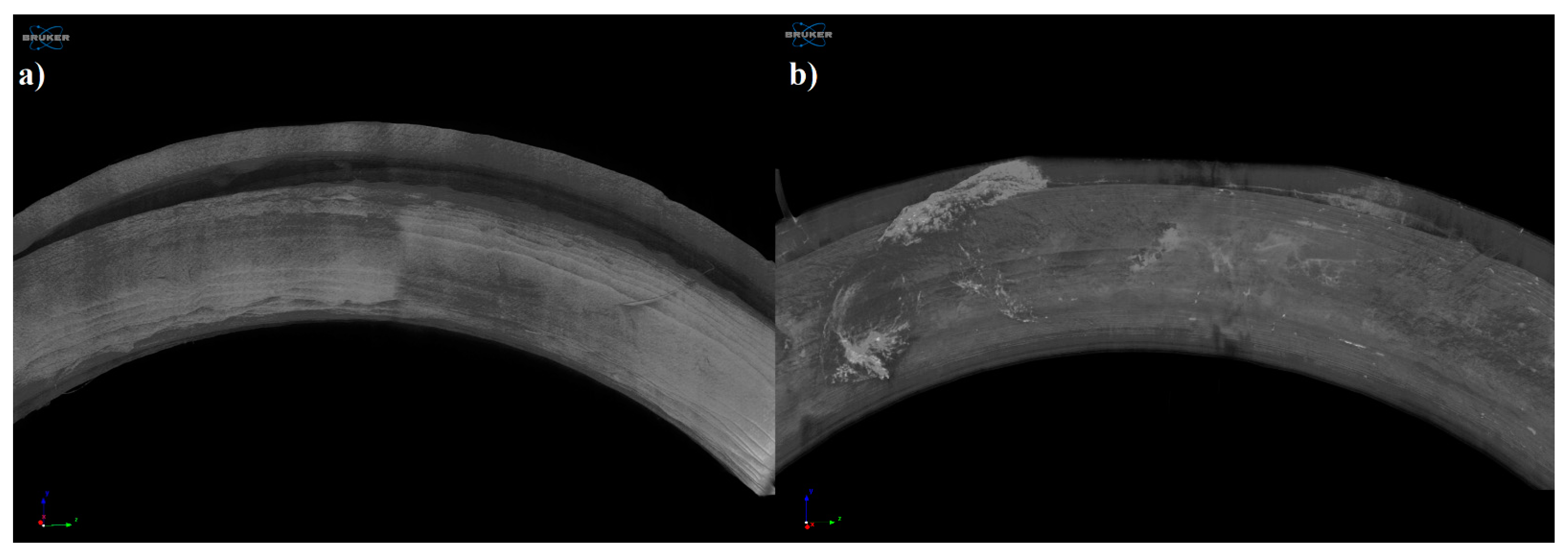
Microcomputed Tomography (MicroCT)
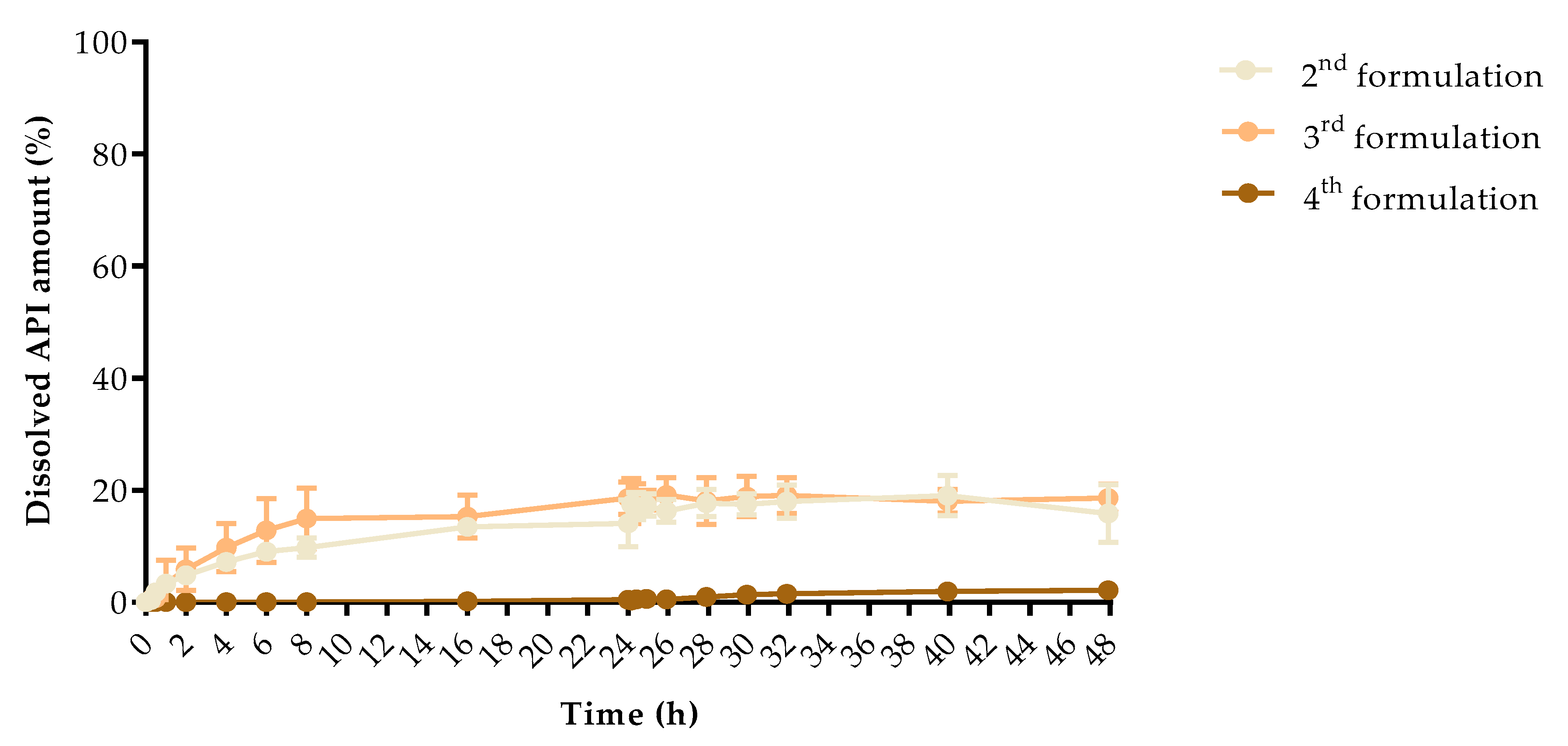
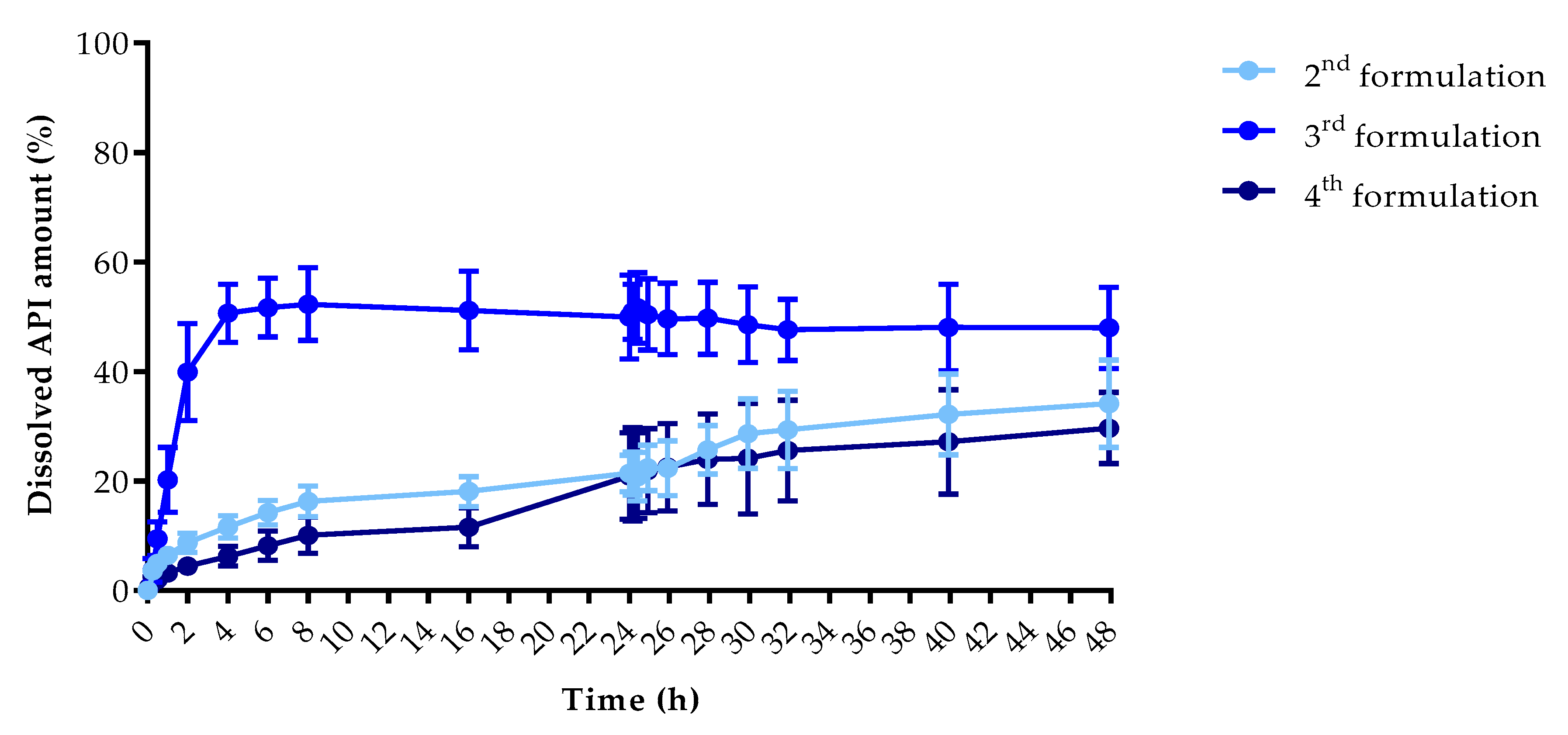
2.2.5. In Vitro Dissolution Test
2.2.6. Biocompatibility Experiments
Cytotoxicity Experiments
- 1.
- Sterilization
- 2.
- Cell Culture
- 3.
- MTT Cell Viability Assay
- 4.
- Microbiological Evaluation
2.2.7. Statistical Analysis
3. Results
3.1. Design of the Drug Reservoirs and Printing of the Samples
3.2. Gel Formation and Sample Manufacturing
3.3. Weight Variation and Content Uniformity
3.4. Characterization
3.4.1. Thermogravimetric (TG) and Heatflow (DSC) Analysis
3.4.2. Contact Angle
3.4.3. Microcomputed Tomography (MicroCT)
3.5. In Vitro Dissolution Test
3.6. Biocompatibility Experiments
3.6.1. Cytotoxicity Experiments
3.6.2. Microbiological Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Sample | Chloramphenicol | Metronidazole | ||||||||||
| 2nd Formulation | 3rd Formulation | 4th Formulation | 2nd Formulation | 3rd Formulation | 4th Formulation | |||||||
| Sampling Time (h) | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.083 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.05 | 0.09 | 0.67 | 0.66 |
| 0.25 | 0.36 | 0.49 | 0.50 | 0.74 | 0.00 | 0.00 | 3.63 | 0.75 | 3.70 | 2.16 | 2.57 | 1.07 |
| 0.5 | 1.77 | 1.01 | 0.76 | 0.84 | 0.00 | 0.00 | 4.98 | 1.10 | 9.48 | 3.07 | 1.99 | 0.33 |
| 1 | 3.36 | 1.01 | 3.31 | 4.15 | 0.00 | 0.00 | 6.42 | 1.00 | 20.20 | 5.90 | 3.18 | 0.47 |
| 2 | 4.79 | 1.36 | 5.89 | 3.77 | 0.00 | 0.00 | 8.76 | 1.74 | 39.89 | 8.87 | 4.47 | 0.72 |
| 4 | 7.21 | 1.23 | 9.75 | 4.33 | 0.00 | 0.00 | 11.63 | 2.02 | 50.64 | 5.32 | 6.25 | 1.82 |
| 6 | 9.01 | 0.75 | 12.81 | 5.71 | 0.00 | 0.00 | 14.24 | 2.24 | 51.68 | 5.37 | 8.18 | 2.65 |
| 8 | 9.76 | 1.70 | 14.93 | 5.42 | 0.00 | 0.00 | 16.31 | 2.79 | 52.31 | 6.64 | 10.11 | 3.33 |
| 16 | 13.44 | 0.92 | 15.32 | 3.83 | 0.16 | 0.32 | 18.08 | 2.69 | 51.15 | 7.16 | 11.54 | 3.55 |
| 24 | 14.11 | 4.15 | 18.60 | 2.85 | 0.43 | 0.58 | 21.40 | 3.35 | 49.99 | 7.66 | 20.92 | 7.86 |
| 24.083 | 17.45 | 1.54 | 18.10 | 4.02 | 0.35 | 0.41 | 21.33 | 3.93 | 50.91 | 5.04 | 21.19 | 8.52 |
| 24.25 | 17.15 | 2.44 | 18.10 | 3.13 | 0.53 | 0.52 | 20.79 | 4.44 | 51.63 | 6.43 | 20.98 | 7.80 |
| 24.5 | 17.36 | 2.02 | 18.22 | 1.77 | 0.61 | 0.54 | 22.42 | 4.15 | 50.42 | 6.49 | 21.90 | 7.66 |
| 26 | 16.31 | 2.03 | 19.17 | 3.06 | 0.53 | 0.57 | 22.34 | 5.01 | 49.63 | 6.55 | 22.51 | 7.98 |
| 28 | 17.69 | 2.42 | 18.08 | 4.19 | 0.98 | 0.79 | 25.71 | 4.43 | 49.73 | 6.56 | 23.98 | 8.26 |
| 30 | 17.49 | 1.86 | 18.91 | 3.59 | 1.40 | 0.95 | 28.65 | 6.32 | 48.55 | 6.87 | 24.13 | 10.07 |
| 32 | 17.94 | 2.96 | 19.06 | 3.20 | 1.52 | 0.68 | 29.34 | 7.08 | 47.61 | 5.57 | 25.57 | 9.22 |
| 40 | 19.03 | 3.60 | 18.04 | 2.14 | 1.96 | 0.64 | 32.15 | 7.38 | 48.05 | 7.92 | 27.16 | 9.51 |
| 48 | 15.86 | 5.10 | 18.57 | 2.51 | 2.14 | 0.92 | 34.12 | 7.97 | 47.98 | 7.42 | 29.67 | 6.50 |
Appendix B
| Pairwise Comparison of Dissolution Profiles | |||
| Sample 1 vs. | Sample 2 | f1 | f2 (%) |
| Chloramphenicol 2nd formulation | Chloramphenicol 3rd formulation | 13.32 | 76.07 |
| Chloramphenicol 2nd formulation | Chloramphenicol 4th formulation | 2156.98 | 39.86 |
| Chloramphenicol 2nd formulation | Metronidazole 2nd formulation | 35.74 | 50.57 |
| Chloramphenicol 2nd formulation | Metronidazole 3rd formulation | 71.56 | 19.96 |
| Chloramphenicol 2nd formulation | Metronidazole 4th formulation | 24.55 | 61.43 |
| Chloramphenicol 3rd formulation | Chloramphenicol 4th formulation | 2434.11 | 37.36 |
| Chloramphenicol 3rd formulation | Metronidazole 2nd formulation | 27.84 | 53.95 |
| Chloramphenicol 3rd formulation | Metronidazole 3rd formulation | 68.07 | 21.20 |
| Chloramphenicol 3rd formulation | Metronidazole 4th formulation | 24.88 | 63.33 |
| Chloramphenicol 4th formulation | Metronidazole 2nd formulation | 97.15 | 30.20 |
| Chloramphenicol 4th formulation | Metronidazole 3rd formulation | 98.74 | 13.00 |
| Chloramphenicol 4th formulation | Metronidazole 4th formulation | 96.53 | 33.80 |
| Metronidazole 2nd formulation | Metronidazole 3rd formulation | 55.75 | 24.62 |
| Metronidazole 2nd formulation | Metronidazole 4th formulation | 22.79 | 62.45 |
| Metronidazole 3rd formulation | Metronidazole 4th formulation | 176.28 | 21.99 |
References
- Duncan, G.W. Medicated Devices and Methods. U.S. Patent US3545439A, 8 December 1970. [Google Scholar]
- Bashi, Y.H.D.; Murphy, D.J.; McCoy, C.F.; Boyd, P.; Brown, L.; Kihara, M.; Martin, F.; McMullen, N.; Kleinbeck, K.; Dangi, B.; et al. Silicone elastomer formulations for improved performance of a multipurpose vaginal ring releasing dapivirine and levonorgestrel. Int. J. Pharm. X 2021, 3, 100091. [Google Scholar]
- Brache, V.; Payán, L.J.; Faundes, A. Current status of contraceptive vaginal rings. Contraception 2013, 87, 264–272. [Google Scholar] [CrossRef]
- Liu, A.Y.; Islas, C.D.; Gundacker, H.; Neradilek, B.; Hoesley, C.; van der Straten, A.; Hendrix, C.W.; Beamer, M.; Jacobson, C.E.; McClure, T.; et al. Phase 1 pharmacokinetics and safety study of extended duration dapivirine vaginal rings in the United States. J. Int. AIDS Soc. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Welsh, N.R.; Malcolm, R.K.; Devlin, B.; Boyd, P. Dapivirine-releasing vaginal rings produced by plastic freeforming additive manufacturing. Int. J. Pharm. 2019, 572, 118725. [Google Scholar] [CrossRef]
- Sandeep, B.; Kannan, T.T.M.; Chandradass, J.; Ganesan, M.; Rajan, A.J. Materials Today: Proceedings Scope of 3D printing in manufacturing industries—A review. Mater. Today Proc. 2021, 45, 6941–6945. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. Eur. J. Pharm. Sci. 2016, 90, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Ricci, M.; Rossi, C. Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels. Colloids Surf. B Biointerfaces 2008, 66, 141–145. [Google Scholar] [CrossRef]
- Pelle, P.; Pete, I.; Végh, G.; Illei, G. Preparation and clinical use of combined broad spectrum vaginal suppositories. Orv. Hetil. 1980, 17, 1015–1017. [Google Scholar]
- Wu, G.; Kong, W.; Gao, Y.; Kong, Y.; Dai, Z.; Dan, H.; Shang, Y.; Wang, S.; Yin, F.; Yue, Q.; et al. Removal of chloramphenicol by sulfide-modified nanoscale zero-valent iron activated persulfate: Performance, salt resistance, and reaction mechanisms. Chemosphere 2021, 286, 131876. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, S.; Rao, B.M.; Prasad, K.P.; Raman, P.; Prasad, M. Antibiotic resistance of culturable heterotrophic bacteria isolated from shrimp (Penaeus vannamei) aquaculture ponds. Mar. Pollut. Bull. 2021, 172, 112887. [Google Scholar]
- Farah, K.; Syed, M.F.H.; Madiha, M.; Rabia, N.; Sana, G.; Iyad, N.M.; Fouzia, H. Comparative analysis of biopharmaceutic classification system (BCS) based biowaiver protocols to validate equivalence of a multisource product. Afr. J. Pharm. Pharmacol. 2020, 14, 212–220. [Google Scholar] [CrossRef]
- Magoulas, G.E.; Kostopoulou, O.N.; Garnelis, T.; Athanassopoulos, C.M.; Kournoutou, G.G.; Leotsinidis, M.; Dinos, G.P.; Papaioannou, D.; Kalpaxis, D.L. Synthesis and antimicrobial activity of chloramphenicol-polyamine conjugates. Bioorganic Med. Chem. 2015, 23, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Spec-, S.J.; Acta, A. Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield treatment. Pharm. Anal. Acta 2016, 6, 395. [Google Scholar]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Tentor, F.; Siccardi, G.; Sacco, P.; Demarchi, D.; Marsich, E.; Almdal, K.; Bose Goswami, S.; Boisen, A. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery. J. Mater. Sci. Mater. Med. 2020, 31, 25. [Google Scholar] [CrossRef]
- Sobel, R.; Sobel, J.D. Metronidazole for the treatment of vaginal infections. Expert Opin. Pharmacother. 2015, 16, 1109–1115. [Google Scholar] [CrossRef]
- Naveed, S.; Qamar, F. Simple UV Spectrophotometric Assay of Metronidazole. OALib 2014, 01, 1–4. [Google Scholar] [CrossRef]
- Tan, F.; Zhu, Y.; Ma, Z.; Al-Rubeai, M. Recent advances in the implant-based drug delivery in otorhinolaryngology. Acta Biomater. 2020, 108, 46–55. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Coppola, B.; Cappetti, N.; Di Maio, L.; Scarfato, P.; Incarnato, L. 3D printing of PLA/clay nanocomposites: Influence of printing temperature on printed samples properties. Materials 2018, 11, 1947. [Google Scholar] [CrossRef]
- Arany, P.; Papp, I.; Zichar, M.; Csontos, M.; Elek, J.; Regdon, G.; Budai, I.; Béres, M.; Gesztelyi, R.; Fehér, P.; et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules 2020, 25, 5889. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech 2006, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.M.; Elkafrawy, M.A.; Yehia, R.M.; Attia, D.A. Clinical comparative study of optimized metronidazole loaded lipid nanocarrier vaginal emulgel for management of bacterial vaginosis and its recurrence. Drug Deliv. 2021, 28, 814–825. [Google Scholar] [CrossRef]
- Tietz, K.; Klein, S. In vitro methods for evaluating drug release of vaginal ring formulations—A critical review. Pharmaceutics 2019, 11, 538. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Adriana, C.; Morales-dorantes, V.; Ayala-herrera, J.L.; Castillo-aguill, M.; Soto-barreras, U.; Ver, C. Antibiotic Resistance Decreases the Efficacy of Endodontic Filling Pastes for Root Canal Treatment in Children’s Teeth. Children 2021, 8, 692. [Google Scholar] [CrossRef]
- Zichar, M.; Papp, I. Interaction Between 3D Printing and Geometry Studies. In Proceedings of the ICGG 2018—The 18th International Conference on Geometry and Graphics, Milan, Italy, 3–7 August 2018; Cocchiarella, L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1177–1190. [Google Scholar]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges with Unique Opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Regdon, G.; Hegyesi, D.; Pintye-Hódi, K. Thermal study of ethyl cellulose coating films used for modified release (MR) dosage forms. J. Therm. Anal. Calorim. 2012, 108, 347–352. [Google Scholar] [CrossRef]
- Vasvári, G.; Haimhoffer, Á.; Horváth, L.; Budai, I.; Trencsényi, G.; Béresová, M.; Dobó-Nagy, C.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Development and Characterisation of Gastroretentive Solid Dosage Form Based on Melt Foaming. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Fetherston, S.M.; Geer, L.; Veazey, R.S.; Goldman, L.; Murphy, D.J.; Ketas, T.J.; Klasse, P.J.; Blois, S.; Colla, P.L.; Moore, J.P.; et al. Partial protection against multiple rt-shiv162p3 vaginal challenge of rhesus macaques by a silicone elastomer vaginal ring releasing the nnrti mc1220. J. Antimicrob. Chemother. 2013, 68, 2013. [Google Scholar] [CrossRef][Green Version]
- Seyednejad, H.; Gawlitta, D.; Kuiper, R.V.; De Bruin, A.; Van Nostrum, C.F.; Vermonden, T.; Dhert, W.J.A.; Hennink, W.E. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef]
- Srinivasan, P.; Moss, J.A.; Gunawardana, M.; Churchman, S.A.; Yang, F.; Dinh, C.T.; Mitchell, J.M.; Zhang, J.; Fanter, R.; Miller, C.S.; et al. Topical delivery of tenofovir disoproxil fumarate and emtricitabine from pod-intravaginal rings protects macaques from multiple SHIV exposures. PLoS ONE 2016, 11, e0157061. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Róka, E.; Fenyvesi, F.; Póka, R.; Kozma, B.; Bácskay, I. The enhanced inhibitory effect of different antitumor agents in self-microemulsifying drug delivery systems on human cervical cancer HeLa cells. Molecules 2015, 20, 13226–13239. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics 2019, 11, 277. [Google Scholar] [CrossRef]
- Mohseni, M.; Hutmacher, D.W.; Castro, N.J. Independent evaluation of medical-grade bioresorbable filaments for fused deposition modelling/fused filament fabrication of tissue engineered constructs. Polymers 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.P.; Barile, S.; Grasso, A.; Saviano, M. Envisioning smart and sustainable healthcare: 3D Printing technologies for personalized medication. Futures 2018, 103, 35–50. [Google Scholar] [CrossRef]
- Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar] [CrossRef]
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596, 6. [Google Scholar] [CrossRef] [PubMed]
- Grimling, B.; Karolewicz, B.; Nawrot, U.; Włodarczyk, K.; Górniak, A. Physicochemical and antifungal properties of clotrimazole in combination with high-molecular weight chitosan as a multifunctional excipient. Mar. Drugs 2020, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef]
- Balla, E.D.; Bikiaris, N.D.; Nanaki, S.G.; Papoulia, C.; Chrissafis, K.; Klonos, P.A.; Kyritsis, A.; Kostoglou, M.; Zamboulis, A.; Papageorgiou, G.Z. Chloramphenicol loaded sponges based on PVA/nanocellulose nanocomposites for topical wound delivery. J. Compos. Sci. 2021, 5, 208. [Google Scholar] [CrossRef]
- de Souza, N.A.; De Souza, F.S.; Basílio, I.D.; Medeiros, A.C.; Oliveira, E.J.; Santos, A.F.; Macwdo, R.O.; Macędo, R.O. Thermal Stability of Metronidazole Drug and Tablets. J. Therm. Anal. Calorim. 2003, 72, 535–538. [Google Scholar] [CrossRef]
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Paulo Costa, J.M.S.L. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Karl Malcolm, R.; Fetherston, S.M.; McCoy, C.F.; Boyd, P.; Major, I. Vaginal rings for delivery of HIV microbicides. Int. J. Women’s Health 2012, 4, 595–605. [Google Scholar] [CrossRef][Green Version]
- ISO. ISO 10093-10:2010, 3rd ed.; International Standard Office: Geneva, Switzerland, 2010. [Google Scholar]
- Pizzoferrato, A.; Ciapetti, G.; Stea, S.; Cenni, E.; Arciola, C.R.; Granchi, D.; Savarino, L. Cell culture methods for testing biocompatibility. Clin. Mater. 1994, 15, 173–190. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef]
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of Chitosan Gels to Disrupt Bacterial Biofilms and Their Applications in the Treatment of Bacterial Vaginosis. J. Pharm. Sci. 2013, 99, 2386–2398. [Google Scholar] [CrossRef]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-based nanomedicine to fight genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed]









| Properties | Method | PLA | PLA Gypsum | PLA Foam | TPU |
|---|---|---|---|---|---|
| Specific gravity (g/cm3) | D792 | 1.24 | 1.25 | 1.00 | 1.2 |
| Heat distortion temperature at 0.45 MPa (°C) | D790 | 55 | 55 | 55 | 106 |
| Glass Trans. temperature (°C) | D3418 | 55–60 | 55–60 | 55–60 | 150–230 |
| Tensile strength (MPa) | ISO 527 | 60 | 54 | 53 | No break |
| Tensile modulus (MPa) | ISO 527 | 3800 | 3200 | 6040 | No break |
| Notched Izod impact (kJ/m2) | ISO 180 | 16 | 14 | 12 | No break |
| Properties | Method | PLA | PLA Gypsum | PLA Foam | TPU |
|---|---|---|---|---|---|
| Tensile strength (MPa) | ISO 527 | 31.6 | 25.0 | 20.0 | No break |
| Tensile modulus (GPa) | ISO 527 | 1.8 | 1.4 | 5.1 | No break |
| Notched Izod impact (kJ/m2) | ISO 180 | 2.6 | 2.9 | 2.2 | No break |
| Filament Type | PLA | PLA Gypsum | PLA Foam | TPU |
|---|---|---|---|---|
| Filament Diameter (mm) | 1.75 | 1.75 | 1.75 | 1.75 |
| Extruder Nozzle Diameter (µm) | 400 | 400 | 400 | 400 |
| Infill Percentage (%) | 0 | 0 | 0 | 0 |
| Extrusion Temperature (°C) | 215 | 215 | 215 | 233 |
| Bed Temperature (°C) | 60 | 60 | 60 | 65 |
| Layer Thickness (µm) | 100 | 100 | 100 | 100 |
| Sample | Weight | Content Uniformity | |||
|---|---|---|---|---|---|
| Average (g) | ±SD | Average (g) | ±SD | ||
| 2nd formulation | Chloramphenicol | 2.83 | 0.42 | 1.51 | 0.20 |
| 3rd formulation | 2.82 | 0.38 | 1.49 | 0.14 | |
| 4th formulation | 2.86 | 0.24 | 1.52 | 0.23 | |
| 2nd formulation | Metronidazole | 2.81 | 0.41 | 1.52 | 0.01 |
| 3rd formulation | 2.84 | 0.12 | 1.48 | 0.25 | |
| 4th formulation | 2.84 | 0.36 | 1.50 | 0.11 | |
| Sample | 2 h | 8 h | 48 h | ||||
|---|---|---|---|---|---|---|---|
| Dissolved API Amount (%) | ±SD | Dissolved API Amount (%) | ±SD | Dissolved API Amount (%) | ±SD | ||
| Chloramphenicol | 2nd formulation | 4.79 | 1.36 | 9.76 | 1.70 | 15.86 | 5.10 |
| 3rd formulation | 5.89 | 3.77 | 14.93 | 5.42 | 18.57 | 2.51 | |
| 4th formulation | 0.00 | 0.00 | 0.00 | 0.00 | 2.14 | 0.92 | |
| Metronidazole | 2nd formulation | 8.76 | 1.74 | 16.31 | 2.79 | 34.12 | 7.97 |
| 3rd formulation | 39.89 | 8.87 | 52.31 | 6.64 | 47.98 | 7.42 | |
| 4th formulation | 4.47 | 0.72 | 10.11 | 3.33 | 29.67 | 6.50 | |
| Sample | Zero-Order Kinetics | First-Order Kinetics | Zero-Order Kinetics | First-Order Kinetics | Zero-Order Kinetics | First-Order Kinetics | |
|---|---|---|---|---|---|---|---|
| 0–48 h | 0–48 h | 0–8 h | 0–8 h | 8–48 h | 8–48 h | ||
| Chloramphenicol | 2nd formulation | 0.808 | 0.806 | 0.922 | 0.925 | 0.177 | 0.176 |
| 3rd formulation | 0.730 | 0.726 | 0.967 | 0.971 | 0.215 | 0.214 | |
| 4th formulation | 0.765 | 0.763 | 0.000 | 0.000 | 0.870 | 0.871 | |
| Metronidazole | 2nd formulation | 0.921 | 0.944 | 0.891 | 0.978 | 0.902 | 0.910 |
| 3rd formulation | 0.410 | 0.361 | 0.796 | 0.818 | 0.635 | 0.632 | |
| 4th formulation | 0.896 | 0.896 | 0.950 | 0.973 | 0.465 | 0.473 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arany, P.; Papp, I.; Zichar, M.; Regdon, G., Jr.; Béres, M.; Szalóki, M.; Kovács, R.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; et al. Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics 2021, 13, 1714. https://doi.org/10.3390/pharmaceutics13101714
Arany P, Papp I, Zichar M, Regdon G Jr., Béres M, Szalóki M, Kovács R, Fehér P, Ujhelyi Z, Vecsernyés M, et al. Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics. 2021; 13(10):1714. https://doi.org/10.3390/pharmaceutics13101714
Chicago/Turabian StyleArany, Petra, Ildikó Papp, Marianna Zichar, Géza Regdon, Jr., Mónika Béres, Melinda Szalóki, Renátó Kovács, Pálma Fehér, Zoltán Ujhelyi, Miklós Vecsernyés, and et al. 2021. "Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing" Pharmaceutics 13, no. 10: 1714. https://doi.org/10.3390/pharmaceutics13101714
APA StyleArany, P., Papp, I., Zichar, M., Regdon, G., Jr., Béres, M., Szalóki, M., Kovács, R., Fehér, P., Ujhelyi, Z., Vecsernyés, M., & Bácskay, I. (2021). Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics, 13(10), 1714. https://doi.org/10.3390/pharmaceutics13101714













