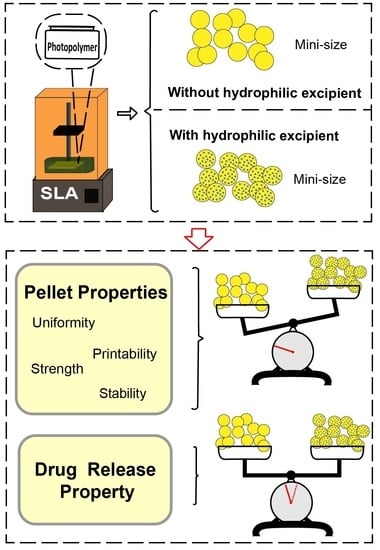
Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photopolymer Solution
2.3. Printing Pellets
2.4. Printability Evaluation
2.5. Characterization of Printed Pellets
2.5.1. Physical Properties/Evaluation
2.5.2. Tensile Strength
2.5.3. X-ray Powder Diffraction (XRD)
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Drug Content
2.6. Dissolution Tests of Printed Pellets
Drug Release Kinetic Profile
2.7. Stability Study of Printed Pellets
2.8. Statistical Analysis
3. Results and Discussion
3.1. Printability Evaluation
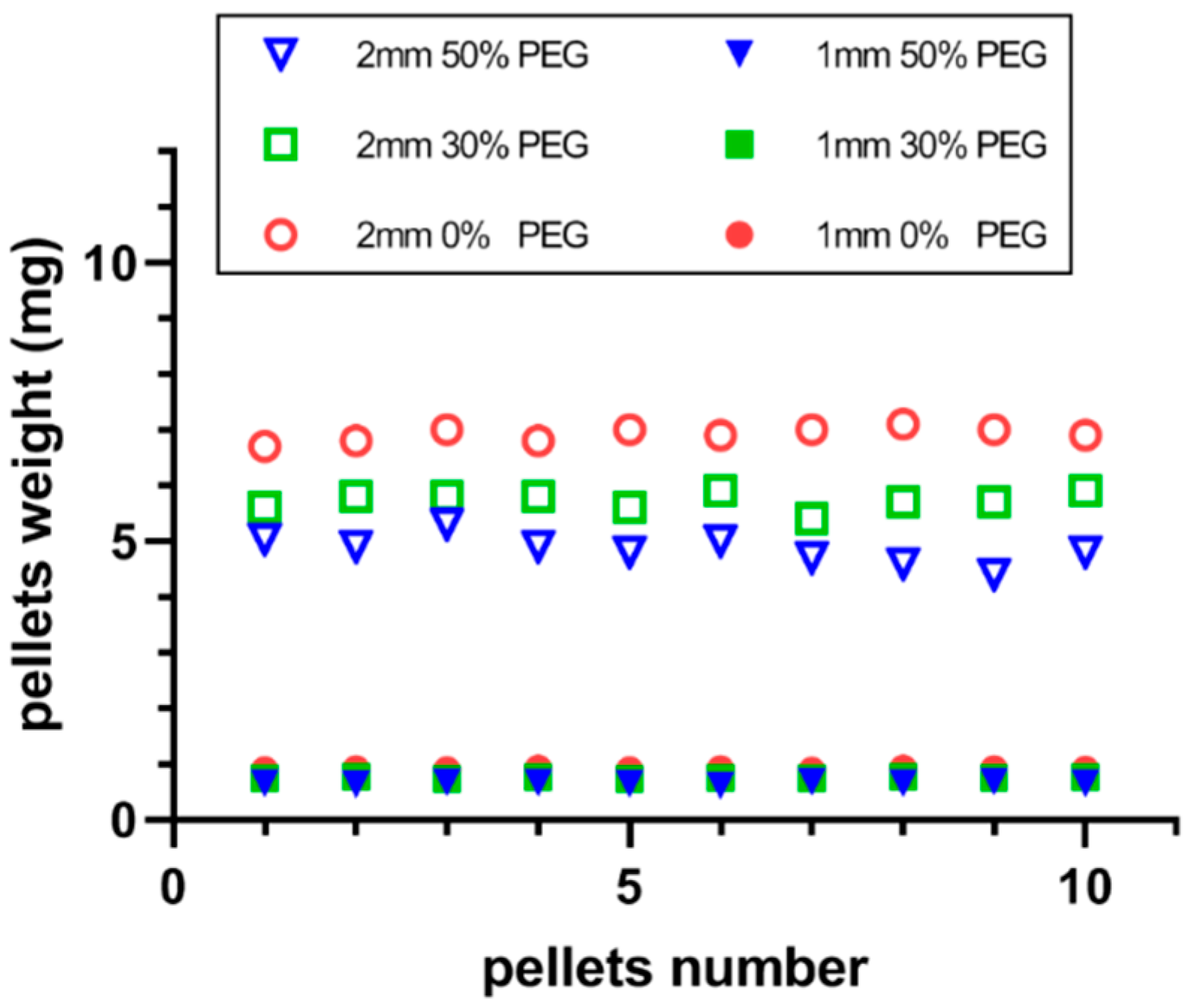
3.2. Physical Propertiesof the Printed Pellets
3.3. Physical Form of the Applied Drug
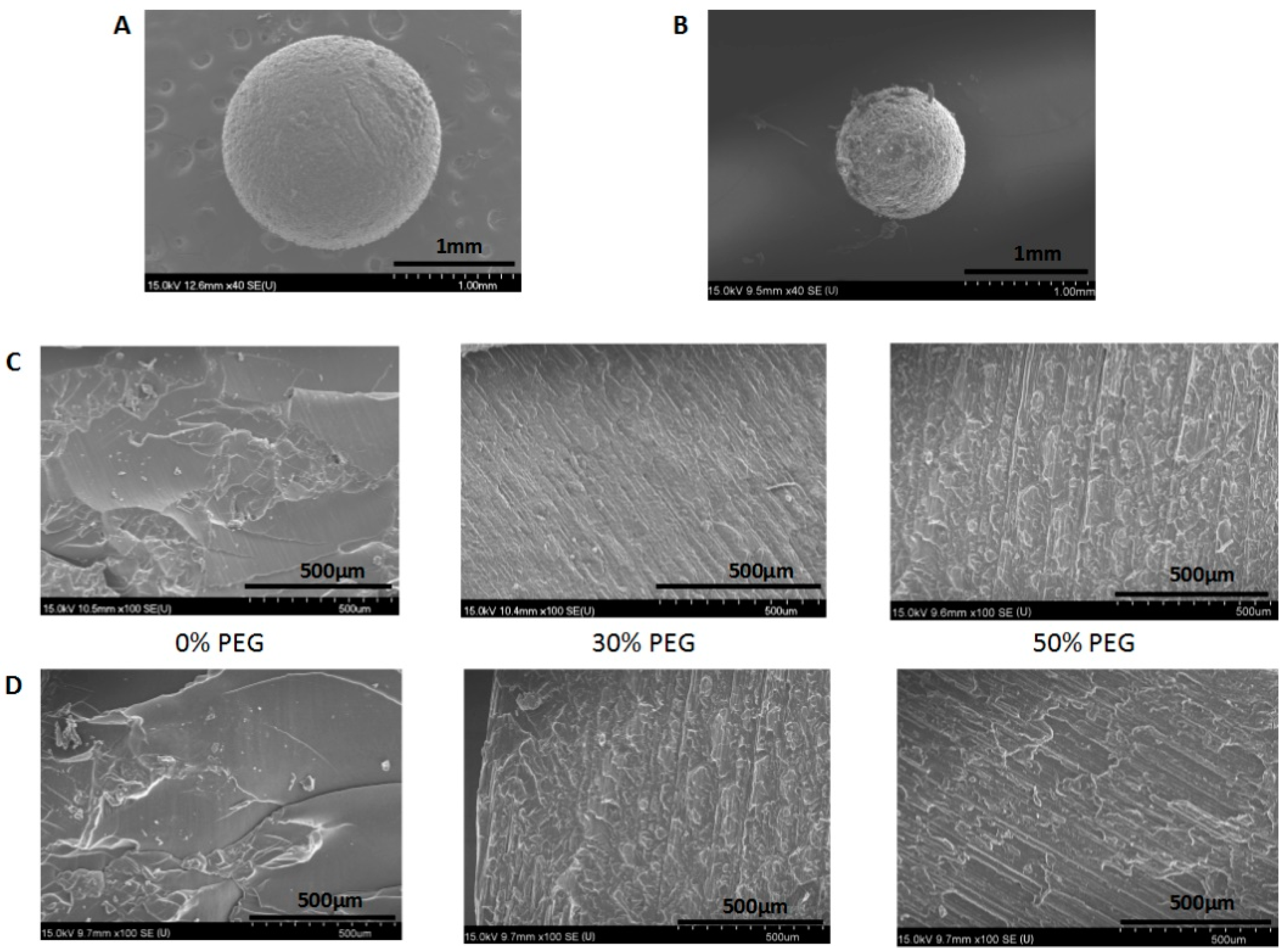
3.4. Morphology of the Printed Pellets
3.5. Drug Content and Uniformity
3.6. Dissolution Analysis
3.7. Drug Release Kinetics
3.8. Stability of the Printed Pellets
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.P.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.D.P.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharmaceut. 2020, 589, 119820. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Feng, P.; Wu, P.; Gao, C.D.; Yang, Y.W.; Guo, W.; Yang, W.J.; Shuai, C.J. A multimaterial scaffold with tunable properties: Toward bone tissue repair. Adv. Sci. 2018, 5, 1700817. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.A.; Kim, J.; Lee, J. Fabrication and characterization of bioresorbable drug-coated porous scaffolds for vascular tissue engineering. Materials 2019, 12, 1438. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.C.; Xu, Y.Y.; Dong, Y.C.; Shan, W.G.; Shen, J. Fabrication and evaluation of dental fillers using customized molds via 3D printing technology. Int. J. Pharmaceut. 2019, 562, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Worthington, K.S.; Tucker, B.A.; Salem, A.K. Controlled drug delivery from 3D printed two-photon polymerized poly (ethylene glycol) dimethacrylate devices. Int. J. Pharmaceut. 2018, 552, 217–224. [Google Scholar] [CrossRef]
- Abdel-Salam, F.S.; Elkheshen, S.A.; Mahmoud, A.A.; Basalious, E.B.; Amer, M.S.; Mostafa, A.A.; Elkasabgy, N.A. In-situ forming chitosan implant-loaded with raloxifene hydrochloride and bioactive glass nanoparticles for treatment of bone injuries: Formulation and biological evaluation in animal model. Int. J. Pharmaceut. 2020, 580, 119213. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharmaceut. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharmaceut. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Tec. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydogdu, A.; Cikrikci, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.Y.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printed pellets (miniprintlets): A novel, multi-drug, controlled release platform technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of intragastric floating, controlled release 3D printed theophylline tablets using hot-melt extrusion and fused deposition modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharmaceut. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.H.; Li, H.C.; Ou, Z.M.; Yang, G.S. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.Y.; Lin, X.; Xie, L.X.; Ivone, R.; Shen, J.; Yang, G.S. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int. J. Pharmaceut. 2020, 583, 119360. [Google Scholar] [CrossRef]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. Int. J. Pharmaceut. 2021, 598, 120360. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.H.; Xu, X.C.; Yang, G.S. Strategies and mechanisms to improve the printability of pharmaceutical polymers eudragit (R) EPO and soluplus (R). Int. J. Pharmaceut. 2021, 599, 120410. [Google Scholar] [CrossRef]
- ASTM International. Standard guidelines for design for additive manufacturing. In Section 3: Terminology; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly stretchable and UV curable elastomers for digital light processing based 3D printing. Adv. Mater. 2017, 29, 1606000. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Su, P.C.; Wei, J. Curing characteristics of shape memory polymers in 3D projection and laser stereolithography. Virtual. Phys. Prototy. 2017, 12, 77–84. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 102, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive manufacturing of personalized pharmaceutical dosage forms via stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef]
- Madzarevic, M.; Ibric, S. Evaluation of exposure time and visible light irradiation in LCD 3D printing of ibuprofen extended release tablets. Eur. J. Pharm. Sci. 2021, 158, 105688. [Google Scholar] [CrossRef]
- Lin, X.; Fu, H.; Hou, Z.H.; Si, Y.L.; Shan, W.G.; Yang, Y. Three-dimensional printing of gastro-floating tablets using polyethylene glycol diacrylate-based photocurable printing material. Int. J. Pharmaceut. 2021, 603, 120674. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharmaceut. 2017, 532, 313–317. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.X.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.; Devine, D.M.; Major, I. Effect of stereolithography 3D printing on the properties of PEGDMA hydrogels. Polymers-Basel 2020, 12, 2015. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharmaceut. 2020, 577, 119066. [Google Scholar] [CrossRef]
- Krkobabic, M.; Medarevic, D.; Cvijic, S.; Grujic, B.; Ibric, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharmaceut. 2019, 572, 118790. [Google Scholar] [CrossRef]
- Costa, P.; Manuel, J.; Lobo, S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. Aaps J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.J.; Lin, X.; Yang, Q.L.; Yang, G.S. Printability of external and internal structures based on digital light processing 3D printing technique. Pharmaceutics 2020, 12, 207. [Google Scholar] [CrossRef]
- Additives, E.P.O.F.; Food, N.S.A.T. Scientific opinion on the re-evaluation Tartrazine (E 102). EFSA J. 2009, 7, 1331. [Google Scholar]
- USP. U.S. Pharmacopoeia-National Formulary; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2015. [Google Scholar]
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Krkobabiv, M.; Medarevic, D.; Pesic, N.; Vasiljevic, D.; Ivkovic, B.; Ibric, S. Digital light processing (DLP) 3D printing of atomoxetine hydrochloride tablets using photoreactive suspensions. Pharmaceutics 2020, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Parojcic, J.; Duric, Z.; Jovanovic, M.; Ibric, S. An investigation into the factors influencing drug release from hydrophilic matrix tablets based on novel carbomer polymers. Drug Deliv. 2004, 11, 59–65. [Google Scholar] [CrossRef][Green Version]








| Formulations | PEGDA600 (w/w, %) | PEG400 (w/w, %) | Ibuprofen (w/w, %) | TPO (w/w, %) |
|---|---|---|---|---|
| Formulation 1 | 89.5 | 0 | 10 | 0.5 |
| Formulation 2 | 59.5 | 30 | 10 | 0.5 |
| Formulation 3 | 39.5 | 50 | 10 | 0.5 |
| Formulation 4 | 19.5 | 70 | 10 | 0.5 |
| Tartrazine Ratio | Model | 0.00% 1 | 0.02% | 0.04% | 0.08% | 0.10% | 0.12% |
|---|---|---|---|---|---|---|---|
| Long D(mm) | 2 | 4.00 ± 0.18 | 3.15 ± 0.10 | 2.95 ± 0.08 | 2.49 ± 0.05 | 2.21 ± 0.03 | 2.24 ± 0.06 |
| Short D(mm) | 2 | 2.10 ± 0.11 | 2.09 ± 0.09 | 2.09 ± 0.06 | 2.08 ± 0.10 | 2.03 ± 0.03 | 1.98 ± 0.04 |
| Roundness | 0 | 1.90 | 1.06 | 0.86 | 0.51 | 0.18 | 0.26 |
| Picture |  |  |  |  |  |  |  |
| Formulations | Drug Content in Photopolymer Solution (%) | Drug Content in 1 mm Pellets (%) | Drug Content in 2 mm Pellets (%) |
|---|---|---|---|
| Formulation 1 (0% PEG) | 98.0% ± 0.2 | 91.2% ± 1.6 | 89.1% ± 0.9 |
| Formulation 2 (30% PEG) | 97.5% ± 0.3 | 89.3% ± 1.2 | 87.6% ± 1.0 |
| Formulation 3 (50% PEG) | 97.1% ± 0.3 | 87.4% ± 0.9 | 86.3% ± 0.6 |
| Formulations | Zero Order | First Order | Higuchi | Peppas | Crowell | n Value |
|---|---|---|---|---|---|---|
| 1 mm F1 | 0.292 | 0.990 | 0.587 | 0.937 | 0.656 | - |
| 1 mm F2 | 0.289 | 0.992 | 0.583 | 0.924 | 0.657 | - |
| 1 mm F3 | 0.207 | 0.995 | 0.485 | 0.947 | 0.526 | - |
| 2 mm F1 | 0.446 | 0.963 | 0.719 | 0.958 | 0.656 | - |
| 2 mm F2 | 0.451 | 0.952 | 0.721 | 0.958 | 0.703 | - |
| 2 mm F3 | 0.375 | 0.983 | 0.655 | 0.957 | 0.684 | - |
| 3 mm F1 | 0.746 | 0.951 | 0.952 | 0.982 | 0.871 | 0.36 |
| 3 mm F2 | 0.750 | 0.962 | 0.950 | 0.976 | 0.918 | 0.37 |
| 3 mm F3 | 0.654 | 0.966 | 0.900 | 0.967 | 0.928 | 0.31 |
| 6 mm F1 | 0.860 | 0.931 | 0.990 | 0.998 | 0.894 | 0.41 |
| 6 mm F2 | 0.807 | 0.946 | 0.974 | 0.992 | 0.853 | 0.38 |
| 6 mm F3 | 0.816 | 0.953 | 0.975 | 0.988 | 0.895 | 0.40 |
| Size | Formulation | f2 (Similarity Factors) |
|---|---|---|
| 1 mm | F1 | 79.07 |
| F2 | 78.82 | |
| F3 | 62.14 | |
| 2 mm | F1 | 82.22 |
| F2 | 79.25 | |
| F3 | 68.08 | |
| 3 mm | F1 | 78.59 |
| F2 | 80.87 | |
| F3 | 74.07 | |
| 6 mm | F1 | 76.75 |
| F2 | 82.36 | |
| F3 | 81.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, Q.; Qiang, W.; Li, H.; Zhong, W.; Pan, S.; Yang, G. Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets. Pharmaceutics 2021, 13, 1717. https://doi.org/10.3390/pharmaceutics13101717
Xu L, Yang Q, Qiang W, Li H, Zhong W, Pan S, Yang G. Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets. Pharmaceutics. 2021; 13(10):1717. https://doi.org/10.3390/pharmaceutics13101717
Chicago/Turabian StyleXu, Lei, Qingliang Yang, Wei Qiang, Huijie Li, Weizhen Zhong, Siying Pan, and Gensheng Yang. 2021. "Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets" Pharmaceutics 13, no. 10: 1717. https://doi.org/10.3390/pharmaceutics13101717
APA StyleXu, L., Yang, Q., Qiang, W., Li, H., Zhong, W., Pan, S., & Yang, G. (2021). Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets. Pharmaceutics, 13(10), 1717. https://doi.org/10.3390/pharmaceutics13101717