Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Oil Extraction
2.3. Development of Curcuma Oil Based SNEDDS
2.4. Preparation of Pastes
2.5. Rheological Characterization
2.6. 3D-Printing of Tablets
2.7. Characterization of the 3DP Tablets
2.8. Solid-State Physicochemical Characterization
2.9. Scanning Electron Microscope (SEM)
2.10. Pharmacokinetics Study
2.11. Statistical Analysis
3. Results and Discussion
3.1. Mixture Experimental Screening Design
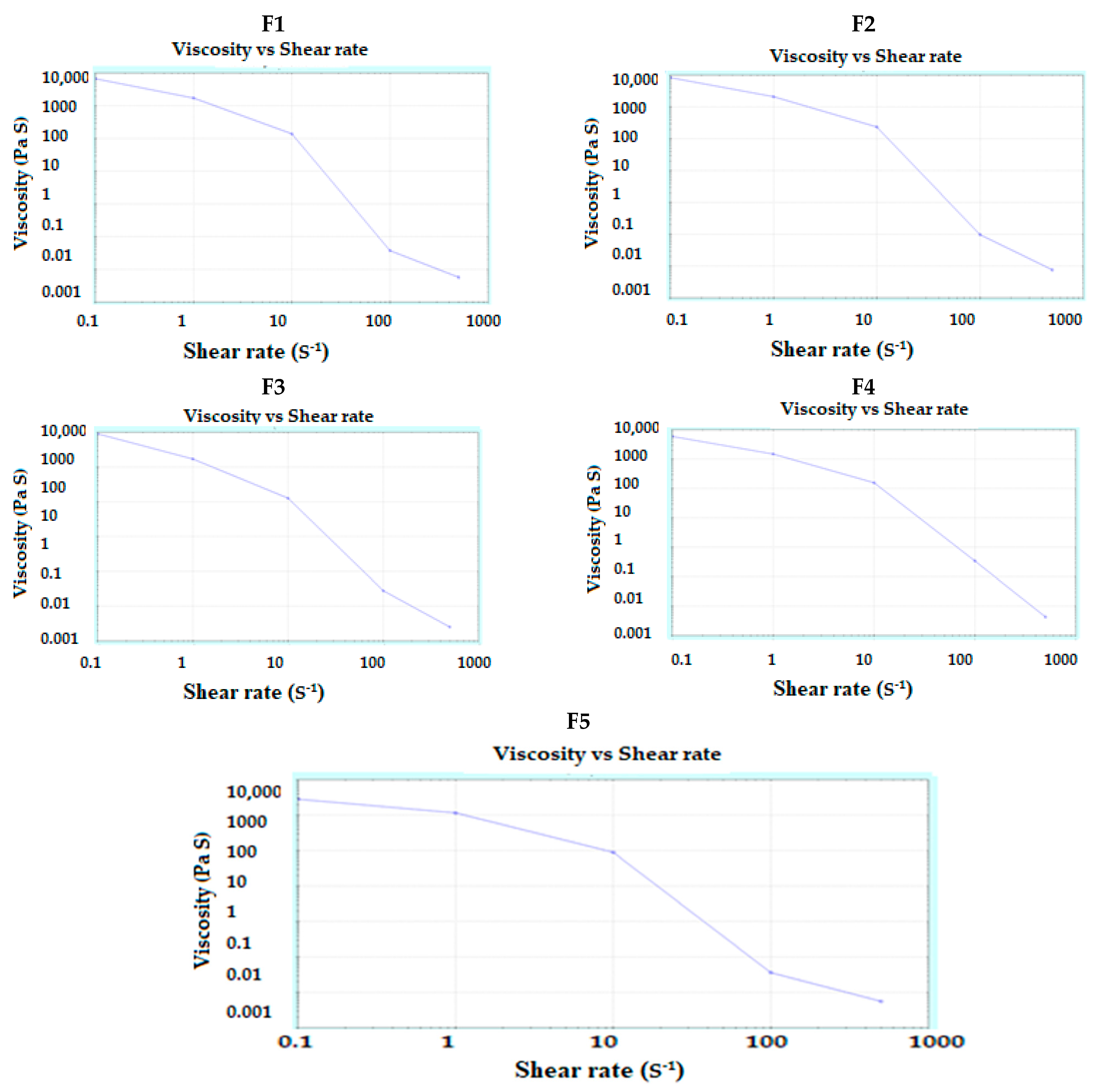
3.2. Characterization of Pastes
3.3. Characterization of 3DP Tablets
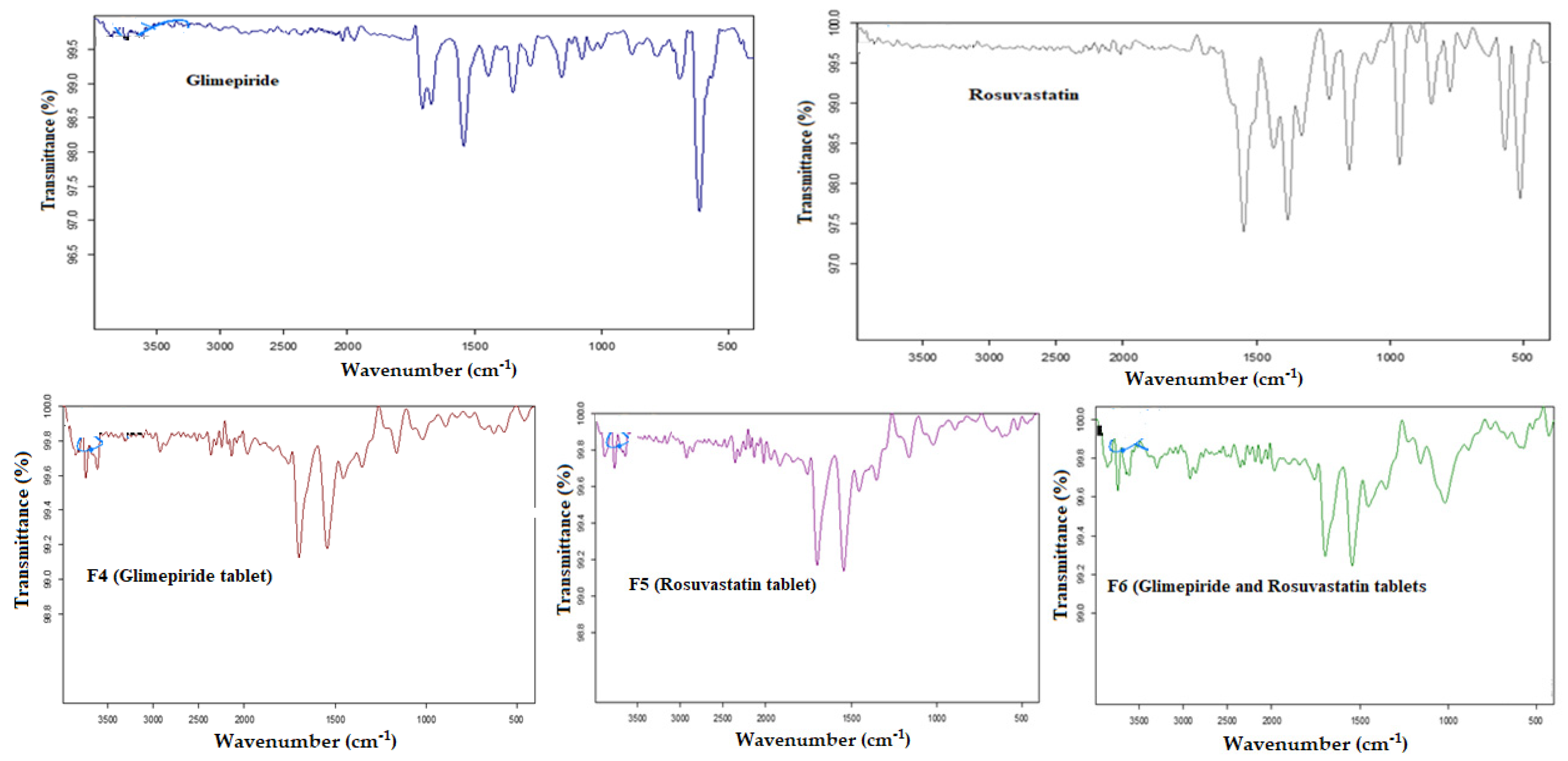
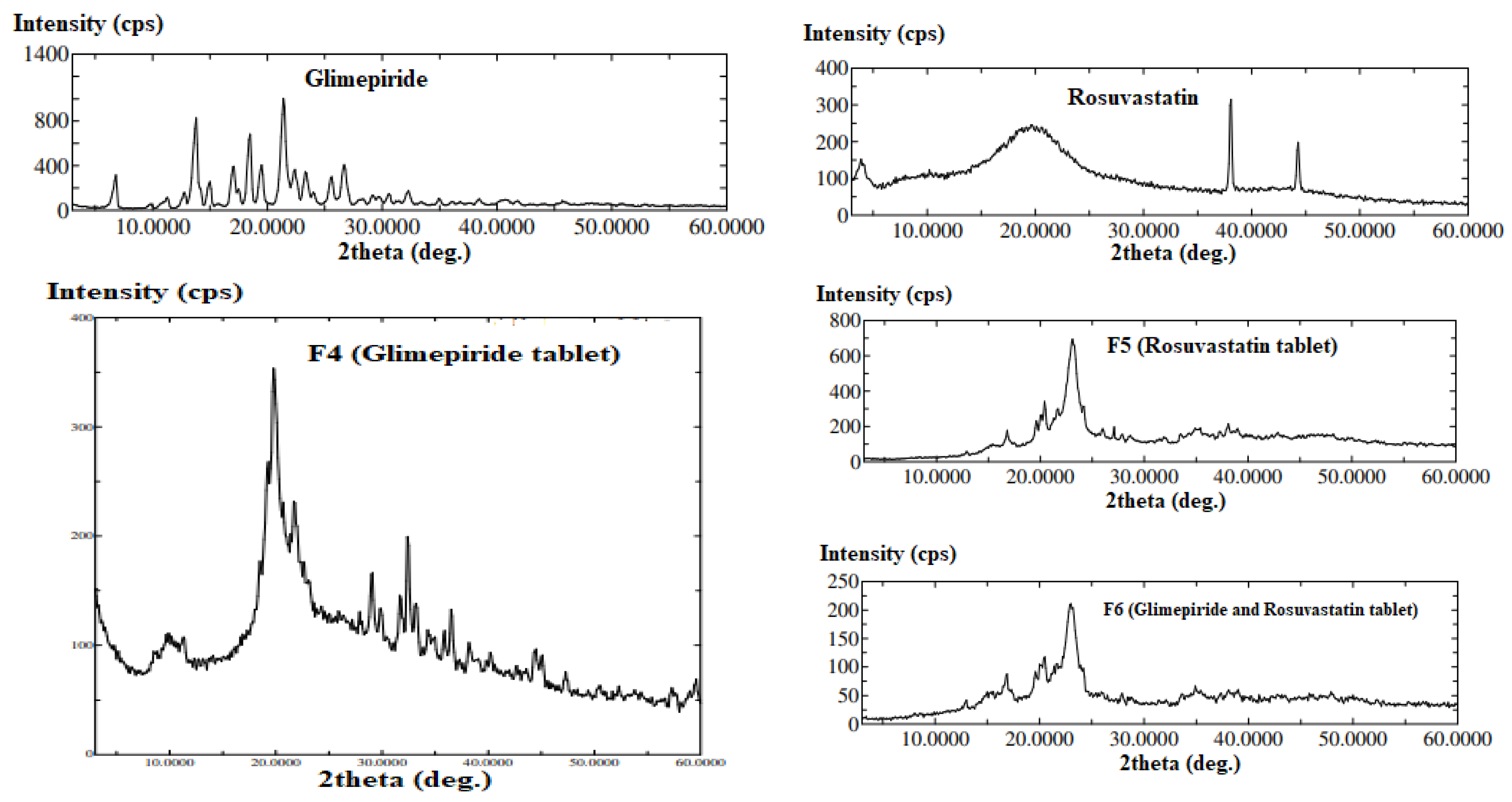
3.4. Solid-State Physicochemical Characterization
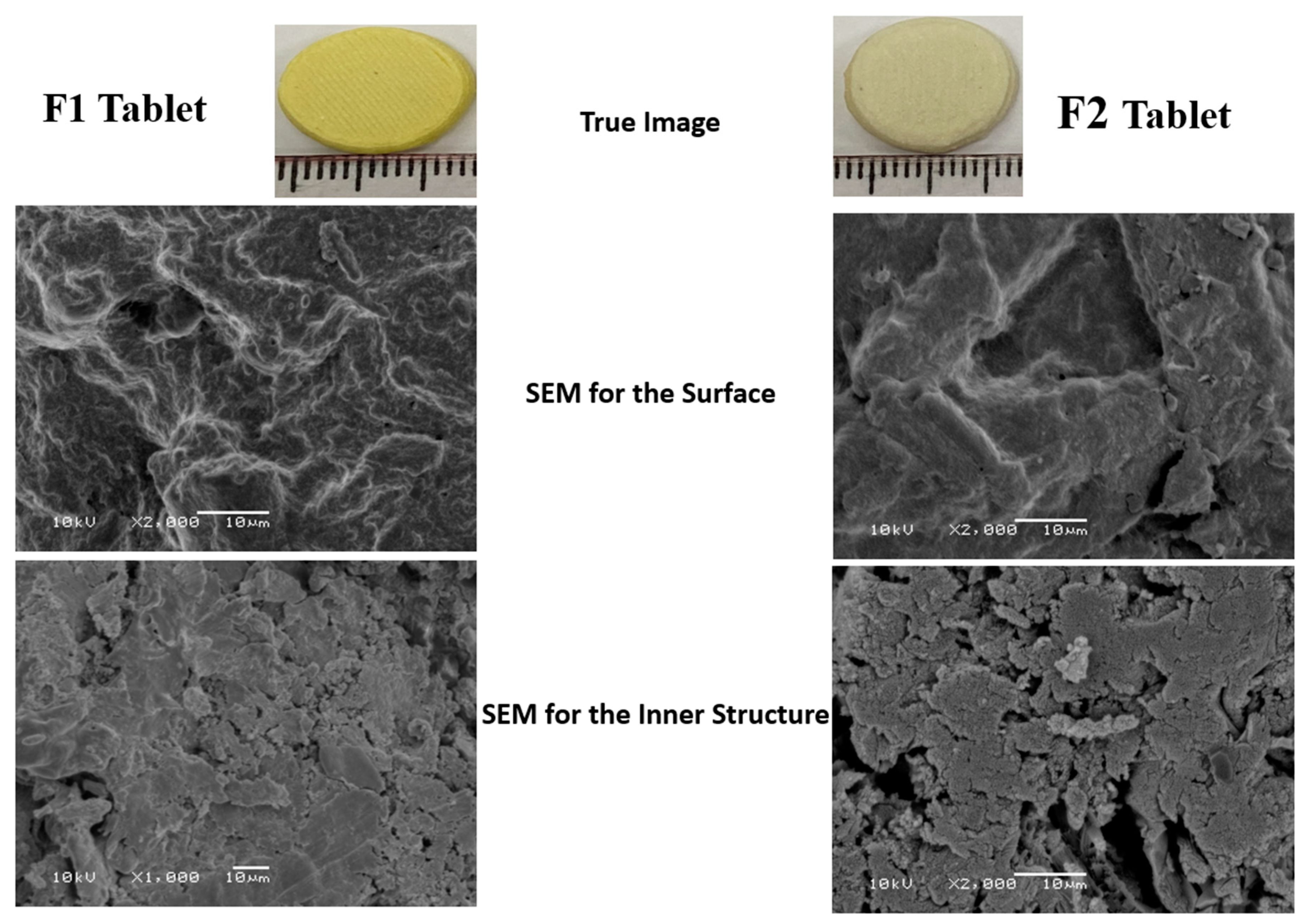
3.5. Scanning Electron Microscope (SEM)
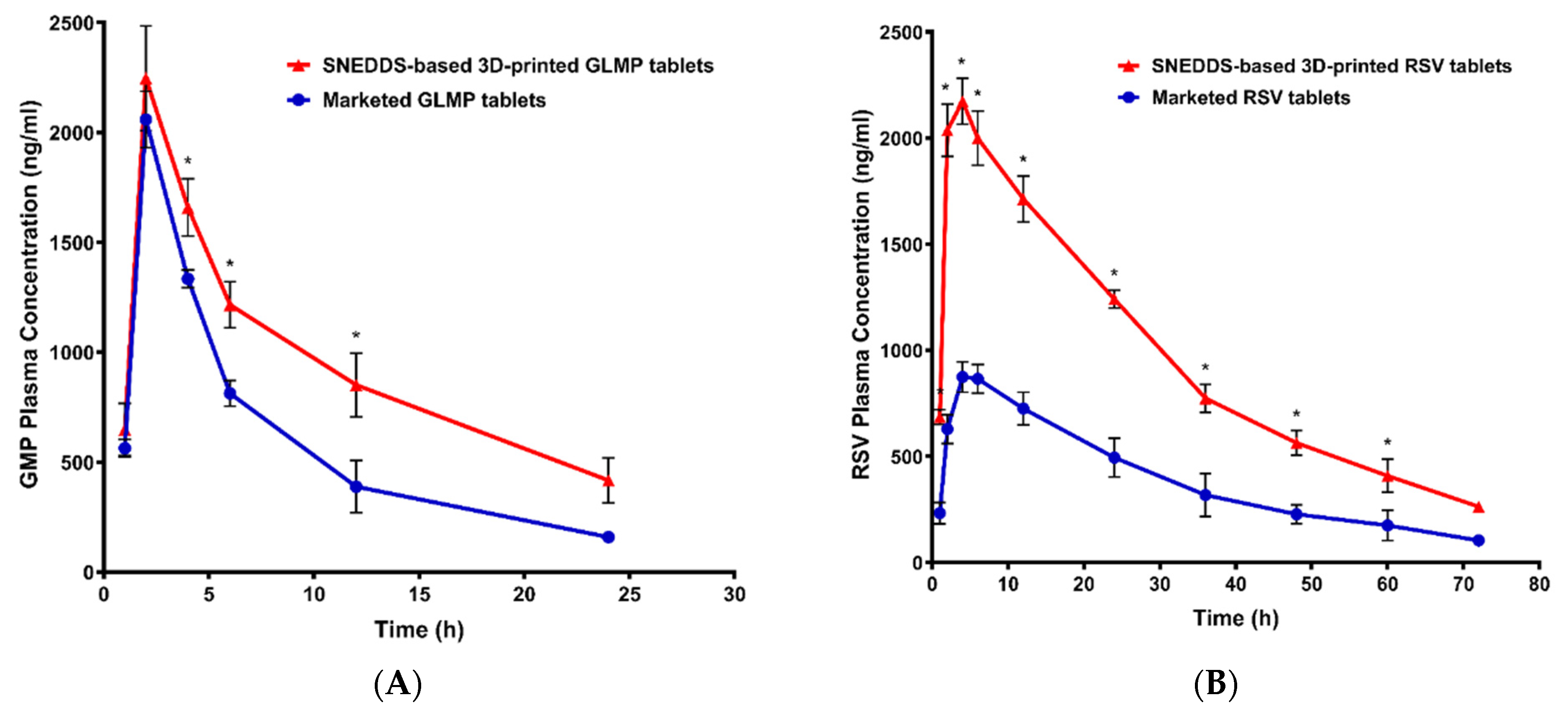
3.6. Pharmacokinetics Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litman, T. Personalized medicine-concepts, technologies, and applications in inflammatory skin diseases. APMIS 2019, 127, 386–424. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tagami, T.; Ozeki, T. Fabrication of 3D-Printed Fish-Gelatin-Based Polymer Hydrogel Patches for Local Delivery of PEGylated Liposomal Doxorubicin. Mar. Drugs 2020, 18, 325. [Google Scholar] [CrossRef]
- EBishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Aguilar-De-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.; Alexander, M.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Nabavi, S.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sánchez, E.; Alinezhad, H. Curcumin: A Natural Product for Diabetes and its Complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharmacol. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef]
- Oliveira, S.; Monteiro-Alfredo, T.; Silva, S.; Matafome, P. Curcumin derivatives for Type 2 Diabetes management and prevention of complications. Arch. Pharmacal Res. 2020, 43, 567–581. [Google Scholar] [CrossRef]
- Kuttan, R.; Liju, V.B.; Jeena, K. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [CrossRef]
- Arshami, J.; Pilevar, M.; Azghadi, M.A.; Raji, A.R. Hypolipidemic and antioxidative effects of curcumin on blood parameters, humoral immunity, and jejunum histology in Hy-line hens. Avicenna J. Phytomed. 2013, 3, 178–185. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf. B Biointerfaces 2011, 86, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Chopra, S.; Dhar, D.; Arora, S.; Khar, R.K. Self-emulsifying drug delivery systems: An approach to enhance oral bioavailability. Drug Discov. Today 2010, 15, 958–965. [Google Scholar] [CrossRef]
- Dake, A.W.; Sora, N.D. Diabetic Dyslipidemia Review: An Update on Current Concepts and Management Guidelines of Diabetic Dyslipidemia. Am. J. Med Sci. 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Kim, C.O.; Oh, E.S.; Kim, H.; Park, M.S. Pharmacokinetic interactions between glimepiride and rosuvastatin in healthy Korean subjects: Does the SLCO1B1 or CYP2C9 genetic polymorphism affect these drug interactions? Drug Des. Dev. Ther. 2017, 11, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, T.; Hirose, T.; Asakawa, H.; Itoh, Y.; Kamado, K.; Tokunaga, K.; Tomita, K.; Masuda, H.; Watanabe, N.; Namba, M. Efficacy of glimepiride in type 2 diabetic patients treated with glibenclamide. Diabetes Res. Clin. Pract. 2004, 66, S129–S132. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A. Study the pharmacokinetics, pharmacodynamics and hepatoprotective activity of rosuvastatin from drug loaded lyophilized orodispersible tablets containing transfersomes nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102489. [Google Scholar] [CrossRef]
- Butt, S.; Hasan, S.M.F.; Hassan, M.M.; Alkharfy, K.M.; Neau, S.H. Directly compressed rosuvastatin calcium tablets that offer hydrotropic and micellar solubilization for improved dissolution rate and extent of drug release. Saudi Pharm. J. 2019, 27, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Lan, K.; Zhang, R.; Li, X.; Jiang, Q. Pharmacokinetic Properties of Rosuvastatin After Single-Dose, Oral Administration in Chinese Volunteers: A Randomized, Open-Label, Three-Way Crossover Study. Clin. Ther. 2007, 29, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Dohare, P.; Varma, S.; Ray, A.; Sharma, U.; Jaganathanan, N.R.; Ray, M. Curcuma Oil: Reduces Early Accumulation of Oxidative Product and is Anti-apoptogenic in Transient Focal Ischemia in Rat Brain. Neurochem. Res. 2007, 33, 1672–1682. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Badr-Eldin, S.M.; Tawfik, M.K.; Ahmed, T.A.; El-Say, K.M.; Badr, J.M. Design and Optimization of Self-Nanoemulsifying Delivery System to Enhance Quercetin Hepatoprotective Activity in Paracetamol-Induced Hepatotoxicity. J. Pharm. Sci. 2014, 103, 602–612. [Google Scholar] [CrossRef]
- El-Say, K.; Ahmed, T.A.; Badr-Eldin, S.M.; Fahmy, U.A.; Aldawsari, H.; Ahmed, O.A.A. Enhanced permeation parameters of optimized nanostructured simvastatin transdermal films: Ex vivo and in vivo evaluation. Pharm. Dev. Technol. 2014, 20, 1–8. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; El-Halawany, A.M.; Ahmed, T.A.; Mohamed, G.A.; Malebari, A.M.; Hassan, N.A. Self-Nanoemulsifying Drug Delivery System Loaded with Psiadia punctulata Major Metabolites for Hypertensive Emergencies: Effect on Hemodynamics and Cardiac Conductance. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Sheng, J.J. Polymer Flooding (Chapter 5). In Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Oxford, UK, 2011; pp. 101–206. [Google Scholar]
- USP 41-NF 36. The United States Pharmacopeia National Formulary; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018. [Google Scholar]
- Ahmed, T.A.; Suhail, M.A.A.; Hosny, K.M.; Allah, F.A. Clinical pharmacokinetic study for the effect of glimepiride matrix tablets developed by quality by design concept. Drug Dev. Ind. Pharm. 2017, 44, 66–81. [Google Scholar] [CrossRef]
- El-Say, K.M.; A Ahmed, T.; A A Ahmed, O.; Elimam, H. Enhancing the Hypolipidemic Effect of Simvastatin in Poloxamer-Induced Hyperlipidemic Rats via Liquisolid Approach: Pharmacokinetic and Pharmacodynamic Evaluation. AAPS PharmSciTech 2020, 21. [Google Scholar] [CrossRef]
- Afroz, A.; Haque, T.; Talukder, M.U.; Islam, S.A. Spectrophotometric Estimation of Rosuvastatin Calcium and Glimepiride in Tablet Dosage Form. Asian J. Pharm. 2011, 1, 74–78. [Google Scholar]
- Ahmed, T.; El-Say, K.; Abd-Allah, F.; Omar, A.; El-Araby, M.; Muhammad, Y.; Pagare, P.; Zhang, Y.; Mohmmad, K.; Abdulmalik, O.; et al. Improving the Solubility and Oral Bioavailability of a Novel Aromatic Aldehyde Antisickling Agent (PP10) for the Treatment of Sickle Cell Disease. Pharmaceutics 2021, 13, 1148. [Google Scholar] [CrossRef]
- Ahmed, O.; Ahmed, T.; Abdel-Naim, A.; Khedr, A.; Banjar, Z.; Afouna, M. Enhancement of In Vitro Skin Transport and In Vivo Hypoglycemic Efficacy of Glimepiride Transdermal Patches. Trop. J. Pharm. Res. 2014, 13, 1207. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Elimam, H.; Alrifai, A.O.; Nadhrah, H.M.; Masoudi, L.Y.; Sairafi, W.O.; El-Say, K. Rosuvastatin lyophilized tablets loaded with flexible chitosomes for improved drug bioavailability, anti-hyperlipidemic and anti-oxidant activity. Int. J. Pharm. 2020, 558, 1. [Google Scholar] [CrossRef]
- Mahajan, T.C.; Deshmukh, S.B.; Badgujar, V.L.; Chhajed, P.N.; Patil, J.A. RP-HPLC Method Development and Validation for estimation of Metformin, Voglibose, Glimepiride in Bulk and Combined Tablet Dosage Forms. Int. J. Chem. Pharm. Sci. 2016, 4, 697–702. Available online: www.pharmaresearchlibrary.com/ijcps (accessed on 7 September 2021).
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability analysis of drug loaded pastes for 3D printing of modified release tablets. Int. J. Pharm. 2018, 554, 292–301. [Google Scholar] [CrossRef]
- Rahman, L.; Rowe, P.; Cheyne, A.; Wilson, D. Ram Extrusion of Potato Starch Dough Through Multi-Holed Dies. Food Bioprod. Process. 2002, 80, 12–19. [Google Scholar] [CrossRef]
- Zhang, M.; Rough, S.; Ward, R.; Seiler, C.; Wilson, D. A comparison of ram extrusion by single-holed and multi-holed dies for extrusion–spheronisation of microcrystalline-based pastes. Int. J. Pharm. 2011, 416, 210–222. [Google Scholar] [CrossRef]
- Torres, O.; Yamada, A.; Rigby, N.M.; Hanawa, T.; Kawano, Y.; Sarkar, A. Gellan gum: A new member in the dysphagia thickener family. Biotribology 2019, 17, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kapure, V.J.; Pande, V.V.; Deshmukh, P.K. Dissolution Enhancement of Rosuvastatin Calcium by Liquisolid Compact Technique. J. Pharm. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, N.M.E.-M.; Badawi, A.; Abdullah, M.E.; Abdelmalak, N.S. Dissolution enhancement of leflunomide incorporating self emulsifying drug delivery systems and liquisolid concepts. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 53–62. [Google Scholar] [CrossRef]
- Roy, A.; Roy, K.; Roy, S.; Deb, J.; Ghosh, A.; Ali, K.A. Response Surface Optimization of Sustained Release Metformin-Hydrochloride Matrix Tablets: Influence of Some Hydrophillic Polymers on the Release. ISRN Pharm. 2012, 2012, 364261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Say, K.M.; Ahmed, T.A.; Ahmed, O.; Hosny, K.M.; Allah, F.A. Self-Nanoemulsifying Lyophilized Tablets for Flash Oral Transmucosal Delivery of Vitamin K: Development and Clinical Evaluation. J. Pharm. Sci. 2017, 106, 2447–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, H.; Salama, H.; Ghorab, M.; Mahmoud, A. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. Int. J. Pharm. 2006, 309, 129–138. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Pan, T.; Cui, Y.; Li, X.; Gao, J.; Shen, S. Improved oral bioavailability of poorly water-soluble glimepiride by utilizing microemulsion technique. Int. J. Nanomed. 2016, 11, 3777–3788. [Google Scholar] [CrossRef] [Green Version]

| Run | X1 (%) | X2 (%) | X3 (%) | Droplet Size (nm) | PDI | |
|---|---|---|---|---|---|---|
| Observed | Fitted | |||||
| 1 | 15.0 | 30.0 | 55.0 | 105.0 | 103.44 | 0.303 ± 0.018 |
| 2 | 15.0 | 10.0 | 75.0 | 93.0 | 92.31 | 0.398 ± 0.020 |
| 3 | 25.0 | 20.0 | 55.0 | 359.0 | 355.01 | 0.554 ± 0.033 |
| 4 | 25.0 | 10.0 | 65.0 | 313.0 | 306.84 | 0.667 ± 0.008 |
| 5 | 17.5 | 23.75 | 58.75 | 157.0 | 156.95 | 0.320 ± 0.020 |
| 6 | 17.5 | 13.75 | 68.75 | 144.0 | 140.92 | 0.323 ± 0.022 |
| 7 | 22.5 | 18.75 | 58.75 | 279.0 | 272.67 | 0.454 ± 0.009 |
| 8 | 22.5 | 13.75 | 63.75 | 251.0 | 254.19 | 0.554 ± 0.019 |
| 9 | 15.0 | 20.0 | 65.0 | 123.0 | 126.03 | 0.234 ± 0.030 |
| 10 | 20.0 | 25.0 | 55.0 | 233.0 | 238.56 | 0.421 ± 0.021 |
| 11 | 20.0 | 10.0 | 70.0 | 196.0 | 200.69 | 0.428 ± 0.012 |
| 12 | 25.0 | 15.0 | 60.0 | 295.0 | 305.94 | 0.664 ± 0.088 |
| 13 | 20.0 | 17.5 | 62.5 | 205.0 | 199.45 | 0.330 ± 0.021 |
| F1 | F2 | F3 | F4 | F5 | F6 | |
|---|---|---|---|---|---|---|
| Drug | - | GLMP | RSV | GLMP | RSV | GLMP and RSV |
| Vehicle | SNEDDS in DW | DW | DW | SNEDDS in DW | SNEDDS in DW | SNEDDS in DW |
| Viscosity (Pa. s) | 6550 ± 278 | 8604 ± 295 | 9185 ± 153 | 6648 ± 135 | 6684 ± 451 | - |
| Weight before drying (mg) | 1003.33 ±1.32 | 965.46 ± 46.55 | 983.75 ± 4.29 | 1053.53 ± 5.84 | 1017.14 ± 61.69 | 2095.11 ± 167.56 |
| Weight after drying (mg) | 532.58 ± 23.7 | 490.53 ±27.76 | 498.88 ± 5.32 | 541.68 ± 61.02 | 559.39 ± 41.45 | 1067.54 ± 77.32 |
| Thickness (mm) | 3.03 5± 0.038 | 2.647 ± 0.160 | 2.826 ± 0.228 | 3.239 ± 0.178 | 2.876 ± 0.128 | 5.797 ± 0.156 |
| Diameter (mm) | 14.656 ± 0.148 | 13.289 ± 0.485 | 15.201 ± 0.228 | 14.855 ± 0.319 | 14.082 ± 0.249 | 14.342 ± 0.109 |
| Friability (%) | 0.068 | 0.159 | 0.199 | 0.117 | 0.043 | 0.089 |
| Drug content (mg) | - | 3.98 ± 0.201 | 9.76 ± 0.359 | 3.918 ± 0.219 | 9.691 ± 0.594 | 4.052 ± 0.087 and 9.711 ± 0.097 |
| Parameter | Unit | SNEDDS-Based 3D-Printed GLMP Tablets | Marketed GLMP Tablets | SNEDDS-Based 3D-Printed RSV Tablets | Marketed RSV Tablets | ||||
|---|---|---|---|---|---|---|---|---|---|
| AVERAGE | STDEV | AVERAGE | STDEV | AVERAGE | STDEV | AVERAGE | STDEV | ||
| K | 1/h | 0.060412 | 0.012131 | 0.097177 | 0.015336 | 0.030944 | 0.001221 | 0.033122 | 0.004295 |
| t½ | h | 11.75933 | 2.135468 | 7.247718 | 1.095357 | 22.42318 | 0.875258 | 21.18031 | 2.931922 |
| Tmax | h | 2 | 0 | 2 | 0 | 4.666667 | 1.154701 | 4.666667 | 1.154701 |
| Cmax | ng/mL | 2246.667 | 238.5211 | 2058.667 | 128.8112 | 2191 | 82.48636 | 888 | 54.06478 |
| AUC 0-t | ng/mL × h | 22365.33 | 2683.317 | 14021.67 | 1539.859 | 68940.67 | 4257.624 | 28120 | 3200.072 |
| AUC 0-inf_obs | ng/mL × h | 29643.17 | 5503.715 | 15688.86 | 1717.492 | 77473.63 | 4696.094 | 31334.14 | 3829.658 |
| AUC 0-t/0-inf_obs | 0.761104 | 0.057117 | 0.893852 | 0.014435 | 0.700672 | 0.002253 | 0.898177 | 0.01551 | |
| AUMC 0-inf_obs | ng/mL × h2 | 505737.5 | 169476.8 | 159519.7 | 24539.36 | 2572924 | 198656.9 | 1021568 | 217442.1 |
| MRT 0-inf_obs | h | 16.73354 | 2.834548 | 10.13952 | 0.700672 | 33.18806 | 0.655388 | 32.36328 | 3.440672 |
| VD | (mg)/(ng/mL) | 0.005731 | 0.000401 | 0.006671 | 0.000822 | 0.008361 | 0.000289 | 0.019567 | 0.002129 |
| Cl | (mg)/(ng/mL)/h | 0.000345 | 6.45 × 10−5 | 0.000643 | 7.04 × 10−5 | 0.000259 | 1.62 × 10−5 | 0.000645 | 8.48 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.A.; Felimban, R.I.; Tayeb, H.H.; Rizg, W.Y.; Alnadwi, F.H.; Alotaibi, H.A.; Alhakamy, N.A.; Abd-Allah, F.I.; Mohamed, G.A.; Zidan, A.S.; et al. Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine. Pharmaceutics 2021, 13, 1733. https://doi.org/10.3390/pharmaceutics13101733
Ahmed TA, Felimban RI, Tayeb HH, Rizg WY, Alnadwi FH, Alotaibi HA, Alhakamy NA, Abd-Allah FI, Mohamed GA, Zidan AS, et al. Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine. Pharmaceutics. 2021; 13(10):1733. https://doi.org/10.3390/pharmaceutics13101733
Chicago/Turabian StyleAhmed, Tarek A., Raed I. Felimban, Hossam H. Tayeb, Waleed Y. Rizg, Fuad H. Alnadwi, Hanadi A. Alotaibi, Nabil A. Alhakamy, Fathy I. Abd-Allah, Gamal A. Mohamed, Ahmed S. Zidan, and et al. 2021. "Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine" Pharmaceutics 13, no. 10: 1733. https://doi.org/10.3390/pharmaceutics13101733
APA StyleAhmed, T. A., Felimban, R. I., Tayeb, H. H., Rizg, W. Y., Alnadwi, F. H., Alotaibi, H. A., Alhakamy, N. A., Abd-Allah, F. I., Mohamed, G. A., Zidan, A. S., & El-Say, K. M. (2021). Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine. Pharmaceutics, 13(10), 1733. https://doi.org/10.3390/pharmaceutics13101733








