In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Isolation and Culture of FSK-MSCs
2.3. Characterization of FSK-MSCs
2.4. In Vitro Differentiation Assay
2.4.1. Osteogenic Differentiation
2.4.2. Adipogenic Differentiation
2.4.3. Chondrogenic Differentiation
2.5. Inflammatory Priming of FSK-MSCs
2.6. Immunomodulation and Coculture Assays
2.7. Human Th17 Characterization
2.8. IL-17A Expression
2.9. RORγt Expression
2.10. IL-23 Receptor Expression
2.11. Th17 Cytokine Pathway
2.12. Experimental Conditions
- MSCs at two different cell ratios were used: 1/80 (low MSCs) and 1/5 (high MSCs).
- MSCs were or were not primed with inflammation.
- MSCs were or were not cultured with activated T cells.
- T cells activated or not were used as controls.
2.13. Flow Cytometry
2.14. Statistics
3. Results
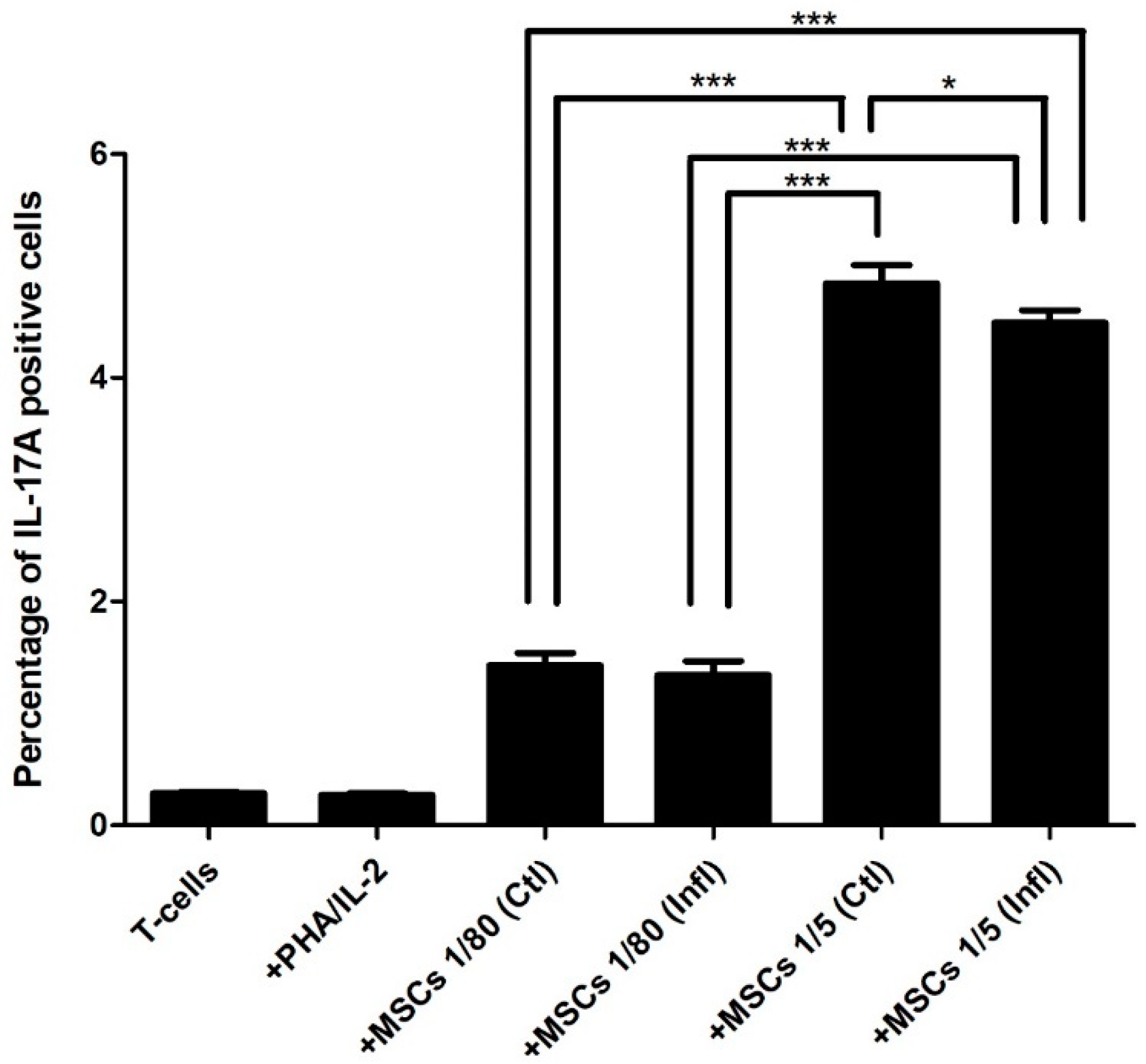
3.1. Th17 Proportion in Coculture with FSK-MSCs
3.2. Th17-Associated ROR-γt Expression during Coculture with FSK-MSCs
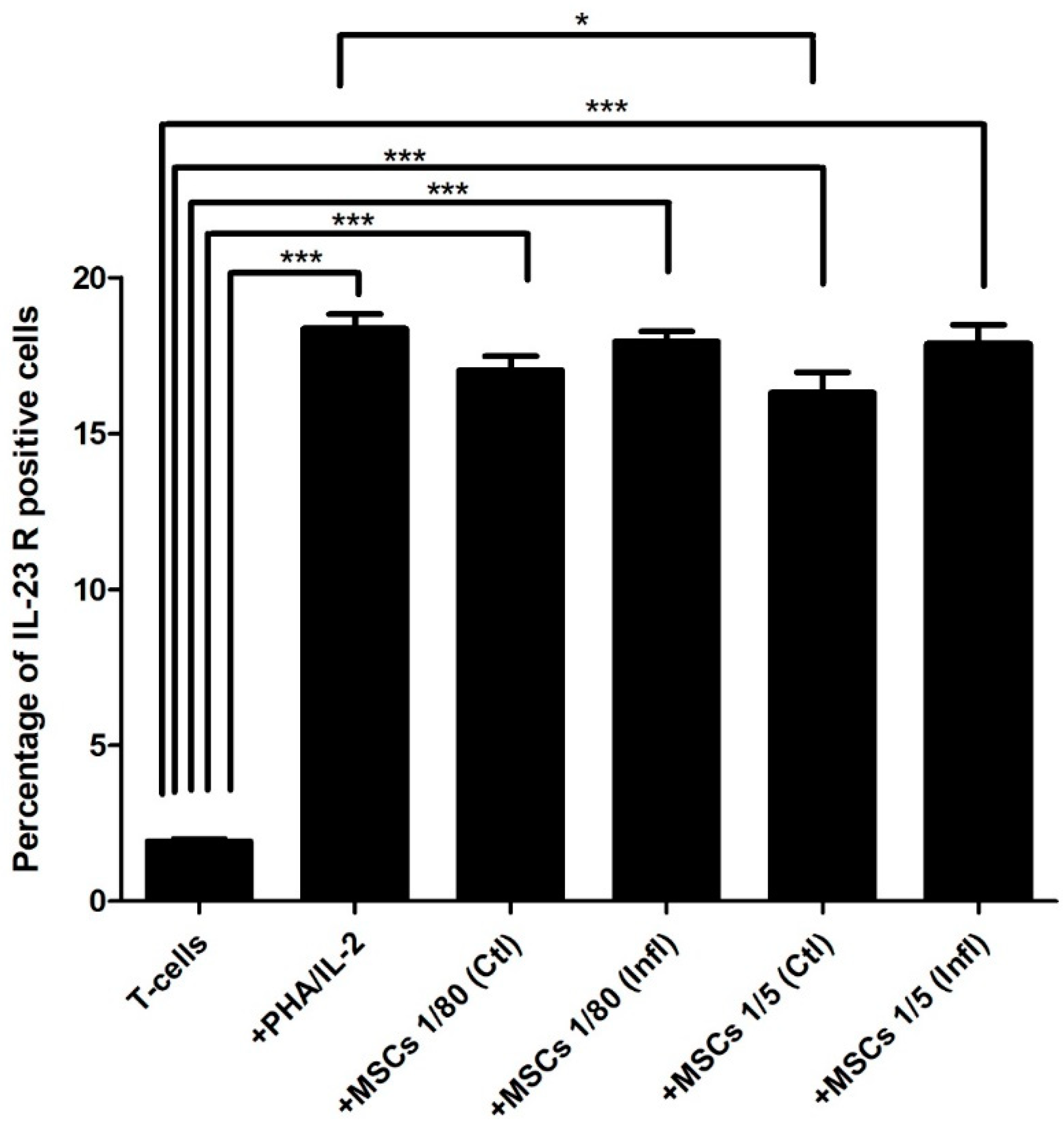
3.3. Th17-Associated IL-23 Receptor Expression during Coculture with FSK-MSCs
3.4. Th17 Associated Cytokine Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merimi, M.; Lewalle, P.; Meuleman, N.; Agha, D.; El-Kehdy, H.; Bouhtit, F.; Ayoub, S.; Burny, A.; Fahmi, H.; Lagneaux, L.; et al. Mesenchymal Stem/stromal cell therapeutic features: The bridge between the bench and the clinic. J. Clin. Med. 2021, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal stromal cell-based therapy: New perspectives and challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rameshwar, P. Microenvironment at tissue injury, a key focus for efficient stem cell therapy: A discussion of mesenchymal stem cells. World J. Stem Cells 2009, 1, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.C.; Christy, B.A.; Montgomery, R.K.; Delavan, C.P.; Jensen, K.J.; Lovelace, S.E.; Cantu, C.; Salgado, C.L.; Cap, A.P.; Bynum, J.A. Interactions of human mesenchymal stromal cells with peripheral blood mononuclear cells in a Mitogenic proliferation assay. J. Immunol. Methods 2021, 492, 113000. [Google Scholar] [CrossRef]
- Taşlı, P.; Somuncu, O.; Turan, R.D.; Kocabas, F.; Sahin, F. Immunomodulatory Properties of Human Newborn Foreskin Stem Cells. 2015. Available online: https://pubmed.ncbi.nlm.nih.gov/20712449/ (accessed on 27 June 2021).
- Merimi, M.; Buyl, K.; Daassi, D.; Rodrigues, R.; Melki, R.; Lewalle, P.; Vanhaecke, T.; Fahmi, H.; Rogiers, V.; Lagneaux, L.; et al. Transcriptional profile of cytokines, regulatory mediators and TLR in mesenchymal stromal cells after inflammatory signaling and cell-passaging. Int. J. Mol. Sci. 2021, 22, 7309. [Google Scholar] [CrossRef]
- Farkhad, N.K.; Mahmoudi, A.; Mahdipour, E. How similar are human mesenchymal stem cells derived from different origins? a review of comparative studies. Curr. Stem Cell Res. Ther. 2021, 16, 980–993. [Google Scholar] [CrossRef]
- Najar, M.; Lagneaux, L. Foreskin as a source of immunotherapeutic mesenchymal stromal cells. Immunotherapy 2017, 9, 207–217. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; André, T.; Fayyad-Kazan, H.; Pieters, K.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells from the foreskin: Tissue isolation, cell characterization and immunobiological properties. Cytotherapy 2016, 18, 320–335. [Google Scholar] [CrossRef]
- Leung, S.; Liu, X.; Fang, L.; Chen, X.; Guo, T.; Zhang, J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell. Mol. Immunol. 2010, 7, 182–189. [Google Scholar] [CrossRef]
- Najar, M.; Crompot, E.; Van Grunsven, L.A.; Dollé, L.; Lagneaux, L. Foreskin-derived mesenchymal stromal cells with aldehyde dehydrogenase activity: Isolation and gene profiling. BMC Cell Biol. 2018, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Jebbawi, F.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Characterization and functionality of the CD200–CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol. Lett. 2012, 146, 50–56. [Google Scholar] [CrossRef]
- De Kock, J.; Rodrigues, R.M.; Branson, S.; Verhoye, L.; Colemonts-Vroninks, H.; Rombaut, M.; Boeckmans, J.; Neuckermans, J.; Lequeue, S.; Buyl, K.; et al. Inflammation alters the secretome and immunomodulatory properties of human skin-derived precursor cells. Cells 2020, 9, 914. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Pievani, A.; Scagliotti, V.; Russo, F.; Azario, I.; Rambaldi, B.; Sacchetti, B.; Marzorati, S.; Erba, E.; Giudici, G.; Riminucci, M.; et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy 2014, 16, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.S.; Choi, Y.; Kim, H.-S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najar, M.; Raicevic, G.; Kazan, H.F.; De Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: The expression and impact of inflammatory priming. Stem Cell Rev. Rep. 2012, 8, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, E.; McKeel, D.T.; Gerlach, J.C. Characterization of human mesenchymal stem cells from different tissues and their membrane encasement for prospective transplantation therapies. BioMed Res. Int. 2019, 2019, 6376271. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Xie, Y.; Song, B.; Kong, S.; Sun, X. Different culture method changing CD105 expression in amniotic fluid MSCs without affecting differentiation ability or immune function. J. Cell. Mol. Med. 2020, 24, 4212–4222. [Google Scholar] [CrossRef] [Green Version]
- Roobrouck, V.D.; Vanuytsel, K.; Verfaillie, C.M. Concise review: Culture mediated changes in fate and/or potency of stem cells. Stem Cells 2011, 29, 583–589. [Google Scholar] [CrossRef]
- Haack-Sørensen, M.; Hansen, S.K.; Hansen, L.; Gaster, M.; Hyttel, P.; Ekblond, A.; Kastrup, J. Mesenchymal stromal cell phenotype is not influenced by confluence during culture expansion. Stem Cell Rev. Rep. 2013, 9, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; See, C.W.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najar, M.; Rouas, R.; Raicevic, G.; Boufker, H.I.; Lewalle, P.; Meuleman, N.; Bron, D.; Toungouz, M.; Martiat, P.; Lagneaux, L. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: The importance of low cell ratio and role of interleukin. Cytotherapy 2009, 11, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Lee, O.J.; Luk, F.; Korevaar, S.S.; Koch, T.G.; Baan, C.; Merino, A.; Hoogduijn, M.J. The Importance of dosing, timing, and (in)activation of adipose tissue-derived mesenchymal stromal cells on their immunomodulatory effects. Stem Cells Dev. 2020, 29, 38–48. [Google Scholar] [CrossRef]
- Mckinnirey, F.; Herbert, B.; Vesey, G.; McCracken, S. Immune modulation via adipose derived Mesenchymal Stem cells is driven by donor sex in vitro. Sci. Rep. 2021, 11, 12454. [Google Scholar] [CrossRef]
- Bianconi, E.; Casadei, R.; Frabetti, F.; Ventura, C.; Facchin, F.; Canaider, S. Sex-specific transcriptome differences in human adipose mesenchymal stem cells. Genes 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Skubis, A.; Gola, J.; Sikora, B.; Hybiak, J.; Paul-Samojedny, M.; Mazurek, U.; Łos, M.J. Impact of antibiotics on the proliferation and differentiation of human adipose-derived mesenchymal stem cells. Int. J. Mol. Sci. 2017, 18, 2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khasawneh, R.R.; Al Sharie, A.H.; Rub, E.A.-E.; Serhan, A.O.; Obeidat, H.N. Addressing the impact of different fetal bovine serum percentages on mesenchymal stem cells biological performance. Mol. Biol. Rep. 2019, 46, 4437–4441. [Google Scholar] [CrossRef]
- Tonarova, P.; Lochovska, K.; Pytlik, R.; Kalbacova, M.H. The impact of various culture conditions on human mesenchymal stromal cells metabolism. Stem Cells Int. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Hoang, V.T.; Trinh, Q.-M.; Phuong, D.T.M.; Bui, H.T.H.; Hang, L.M.; Ngan, N.T.H.; Anh, N.T.T.; Nhi, P.Y.; Nhung, T.T.H.; Lien, H.T.; et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy 2021, 23, 88–99. [Google Scholar] [CrossRef]
- Sanz-Nogués, C.; O’Brien, T. Current Good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater. Biosyst. 2021, 2, 100018. [Google Scholar] [CrossRef]
- Lechanteur, C.; Briquet, A.; Bettonville, V.; Baudoux, E.; Beguin, Y. MSC Manufacturing for academic clinical trials: From a clinical-grade to a full GMP-compliant process. Cells 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Godthardt, K.; Heifer, C.; Jüngerkes, F.; Bosio, A.; Knöbel, S. Efficient GMP compliant expansion of mesenchymal stromal cells (MSCs) from umbilical cord, bone marrow and adipose tissue using a closed cultivation system. Cytotherapy 2019, 21, S90–S91. [Google Scholar] [CrossRef]
- Wu, X.; Ma, Z.; Wu, D. Derivation of clinical-grade mesenchymal stromal cells from umbilical cord under chemically defined culture condition—Platform for future clinical application. Cytotherapy 2020, 22, 377–387. [Google Scholar] [CrossRef]
- Cugno, C.; Alkhualifi, M.; Al-Sulaiti, A.; Guerrouahen, B.; Al-Khawaga, S.; Calderone, Z. Foreskin DerivedMesenchymal stromal cell FSKMSC: Setting the ground for the clinical grade production of MSC at Sidra’s GMP facility. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 19–20 March 2018; Volume 2018. Issue 2. [Google Scholar]
- Nikolits, I.; Nebel, S.; Egger, D.; Kreß, S.; Kasper, C. Towards physiologic culture approaches to improve standard cultivation of mesenchymal stem cells. Cells 2021, 10, 886. [Google Scholar] [CrossRef]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 2008, 26, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.; Ferrara, J.L. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003, 17, 187–194. [Google Scholar] [CrossRef]
- Michael, S.; Achilleos, C.; Panayiotou, T.; Strati, K. Inflammation shapes stem cells and stemness during infection and beyond. Front. Cell Dev. Biol. 2016, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crop, M.J.; Baan, C.C.; Korevaar, S.S.; Ijzermans, J.N.M.; Pescatori, M.; Stubbs, A.P.; Van Ijcken, W.F.J.; Dahlke, M.H.; Eggenhofer, E.; Weimar, W.; et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin. Exp. Immunol. 2010, 162, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Jakob, M.; Ludwig, A.-K.; Giebel, B.; Lang, S.; Brandau, S. Interferon-γ and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010, 19, 693–706. [Google Scholar] [CrossRef]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-inflammatory cytokines, IFNγ and TNFα, influence immune properties of human bone marrow and Wharton Jelly Mesenchymal stem cells differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef]
- Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef] [Green Version]
- Daxecker, H.; Raab, M.; Markovic, S.; Karimi, A.; Griesmacher, A.; Mueller, M.M. Endothelial adhesion molecule expression in an in vitro model of inflammation. Clin. Chim. Acta 2002, 325, 171–175. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Popp, F.; Verbeek, R.; Masoodi, M.; Nicolaou, A.; Baan, C.; Dahlke, M.-H. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int. Immunopharmacol. 2010, 10, 1496–1500. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Wankowicz-Kalinska, A.; Cai, Q.; Wesa, A.; Hilkens, C.M.; Kapsenberg, M.L.; Kirkwood, J.M.; Storkus, W.J.; Kalinski, P. α-Type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004, 64, 5934–5937. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Goeddel, D.V. TNF-R1 Signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [Green Version]
- Last-Barney, K.; Homon, A.C.; Faanes, R.B.; Merluzzi, V.J. Synergistic and overlapping activities of tumor necrosis factor-alpha and IL. J. Immunol. 1988, 141, 527–530. [Google Scholar]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Boufker, H.I.; Stamatopoulos, B.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Modulated expression of adhesion molecules and galectin-1: Role during mesenchymal stromal cell immunoregulatory functions. Exp. Hematol. 2010, 38, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Buyl, K.; Merimi, M.; Rodrigues, R.; Agha, D.M.; Melki, R.; Vanhaecke, T.; Bron, D.; Lewalle, P.; Meuleman, N.; Fahmi, H.; et al. The Impact of cell-expansion and inflammation on the immune-biology of human adipose tissue-derived mesenchymal stromal cells. J. Clin. Med. 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raicevic, G.; Najar, M.; Stamatopoulos, B.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell. Immunol. 2011, 270, 207–216. [Google Scholar] [CrossRef] [PubMed]
- El-Kehdy, H.; Sargiacomo, C.; Fayyad-Kazan, M.; Fayyad-Kazan, H.; Lombard, C.; Lagneaux, L.; Sokal, E.; Najar, M.; Najimi, M. Immunoprofiling of adult-derived human liver stem/progenitor cells: Impact of hepatogenic differentiation and inflammation. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Raicevic, G.; Najar, M.; Pieters, K.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Inflammation and toll-like receptor ligation differentially affect the osteogenic potential of human mesenchymal stromal cells depending on their tissue origin. Tissue Eng. Part A 2012, 18, 1410–1418. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 1–24. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Kim, N.; Cho, S.-G. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int. J. Hematol. 2016, 103, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhang, H.; Hu, K.; Lv, G.; Fu, Y.; Ayana, D.A.; Zhao, P.; Jiang, Y. The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- El-Kehdy, H.; Najar, M.; De Kock, J.; Agha, D.M.; Rogiers, V.; Merimi, M.; Lagneaux, L.; Sokal, E.M.; Najimi, M. Inflammation Differentially modulates the biological features of adult derived human liver stem/progenitor cells. Cells 2020, 9, 1640. [Google Scholar] [CrossRef]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.; Lombard, C.A.; Fahmi, H. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm. Res. 2019, 68, 203–213. [Google Scholar] [CrossRef]
- Najar, M.; Lombard, C.A.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.; Lagneaux, L.; Fahmi, H. Th17 immune response to adipose tissue-derived mesenchymal stromal cells. J. Cell. Physiol. 2019, 234, 21145–21152. [Google Scholar] [CrossRef] [Green Version]
- André, T.; Najar, M.; Stamatopoulos, B.; Pieters, K.; Pradier, O.; Bron, D.; Meuleman, N.; Lagneaux, L. Immune impairments in multiple myeloma bone marrow mesenchymal stromal cells. Cancer Immunol. Immunother. 2015, 64, 213–224. [Google Scholar] [CrossRef]
- Darlington, P.J.; Boivin, M.-N.; Renoux, C.; François, M.; Galipeau, J.; Freedman, M.S.; Atkins, H.L.; Cohen, J.A.; Solchaga, L.; Bar-Or, A. Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: Implication for multiple sclerosis. Ann. Neurol. 2010, 68, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Zeng, K.; Zeng, F.; Wei, J.; Tan, G. Allogeneic adipose-derived stem cells suppress Th17 lymphocytes in patients with active lupus in vitro. Acta Biochim. Biophys. Sin. 2011, 43, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zachar, L.; Bačenková, D.; Rosocha, J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016, 9, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Chen, H.; Min, X.-H.; Wang, Q.-Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.-M.; An, G.; Sha, W.; et al. re-activation of mesenchymal stem cells with TNF-alpha, IL-1beta and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef] [Green Version]
- De Cássia Noronha, N.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Iwakura, Y. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Martinez, G.; Nurieva, R.I.; Yang, X.O.; Dong, C. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 2008, 1143, 188–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hu, J.; Liao, L.; Sun, Z.; Han, Q.; Song, Z.; Zhao, R.C. Flk-1+mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin. Clin. Exp. Immunol. 2010, 159, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Alturaihi, H.; Hassan, G.S.; Al-Zoobi, L.; Salti, S.; Darif, Y.; Yacoub, D.; El Akoum, S.; Oudghiri, M.; Merhi, Y.; Mourad, W. Interaction of CD154 with different receptors and its role in bidirectional signals. Eur. J. Immunol. 2015, 45, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Willrich, M.A.; Murray, D.L.; Snyder, M.R. Tumor necrosis factor inhibitors: Clinical utility in autoimmune diseases. Transl. Res. 2015, 165, 270–282. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297, Erratum in 2019, 41, 299. [Google Scholar] [CrossRef]
- Salami, F.; Tavassoli, A.; Mehrzad, J.; Parham, A. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology 2018, 223, 786–791. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Zhu, A.; Zeng, K.; Zhang, X.; Gong, L.; Peng, Y.; Lai, K.; Qu, S. Suppression of interleukin 17 contributes to the immunomodulatory effects of adipose-derived stem cells in a murine model of systemic lupus erythematosus. Immunol. Res. 2016, 64, 1157–1167. [Google Scholar] [CrossRef]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Ortega, L.O.; Feliu, A.; Frutos, M.G.-D.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, A.; Rezk, A.; Boivin, M.-N.; Darlington, P.; Nyirenda, M.; Li, R.; Jalili, F.; Winer, R.; Artsy, E.A.; Uccelli, A.; et al. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl. Med. 2016, 5, 1506–1514. [Google Scholar] [CrossRef] [Green Version]
- Taghavi-Farahabadi, M.; Mahmoudi, M.; Rezaei, N.; Hashemi, S.M. Wharton’s jelly mesenchymal stem cells exosomes and conditioned media increased neutrophil lifespan and phagocytosis capacity. Immunol. Investig. 2020, 1–16. [Google Scholar] [CrossRef]
- Pawankar, R.; Hayashi, M.; Yamanishi, S.; Igarashi, T. The paradigm of cytokine networks in allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.; Rigo, V.; Ferrini, S. IL-21: A pleiotropic cytokine with potential applications in oncology. J. Immunol. Res. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pène, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal Stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermajer, N.; Popp, F.C.; Soeder, Y.; Haarer, J.; Geissler, E.K.; Schlitt, H.J.; Dahlke, M.H. Conversion of Th17 into IL-17aneg regulatory T Cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell–supported minimized immunosuppressive therapy. J. Immunol. 2014, 193, 4988–4999. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Han, C.; Xian, S.; Zhuang, F.; Ding, F.; Zhang, W.; Liu, Y. Placental mesenchymal stromal cells (PMSCs) and PMSC-derived extracellular vesicles (PMSC-EVs) attenuated renal fibrosis in rats with unilateral ureteral obstruction (UUO) by regulating CD4+ T cell polarization. Stem Cells Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Nemeth, K. Mesenchymal stem cell therapy for immune-modulation: The donor, the recipient, and the drugs in-between. Exp. Dermatol. 2014, 23, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-T.; Ting, C.-H.; Yen, M.-L.; Liu, K.-J.; Sytwu, H.-K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef] [Green Version]







| Human Primary Antibody | Species | Dilution | Source | Isotype Control |
|---|---|---|---|---|
| anti-CD73-APC | mouse | 1/20 | BD Biosciences | APC mouse IgG1 |
| anti-CD90-PE | mouse | 1/20 | R&D Systems | PE mouse IgG2A |
| anti-CD105-FITC | mouse | 1/20 | BioLegend | FITC mouse IgG1 |
| anti-CD34-PC5 | mouse | 1/20 | BD Biosciences | PC5 mouse IgG1 |
| anti-CD14-PE | mouse | 1/21 | BD Biosciences | PE mouse IgG2a |
| anti-D19-PE | mouse | 1/22 | BD Biosciences | PE mouse IgG1 |
| anti-CD45-PC7 | mouse | 1/20 | BD Biosciences | PC7 mouse IgG2a |
| anti-HLA-DR-PerCP | mouse | 1/20 | BD Biosciences | PerCP mouse IgG2a |
| Markers | HLA-Dr | CD14 | CD19 | CD73 | CD105 | CD90 | CD34 |
|---|---|---|---|---|---|---|---|
| FSK-MSCs | |||||||
| 1 | 0.6 | 1 | 0.9 | 99 | 95 | 97 | 1 |
| 2 | 3 | 0.5 | 1 | 97 | 85 | 96 | 4 |
| 3 | 1 | 0.8 | 0.5 | 98 | 82 | 95 | 2 |
| 4 | 0.5 | 1.3 | 1.8 | 94 | 90 | 98 | 3 |
| 5 | 1.5 | 3 | 1 | 96 | 89 | 95 | 2 |
| 6 | 2 | 1 | 2 | 93 | 97 | 99 | 3 |
| 7 | 0.7 | 2 | 3 | 100 | 92 | 96 | 5 |
| X | 1.32857143 | 1.37142857 | 1.45714286 | 96.7142857 | 90 | 96.5714286 | 2.85714286 |
| SEM | 0.34483556 | 0.32419445 | 0.3250327 | 0.96890428 | 2 | 0.57142857 | 0.5084323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najar, M.; Merimi, M.; Faour, W.H.; Lombard, C.A.; Moussa Agha, D.; Ouhaddi, Y.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics 2021, 13, 1736. https://doi.org/10.3390/pharmaceutics13101736
Najar M, Merimi M, Faour WH, Lombard CA, Moussa Agha D, Ouhaddi Y, Sokal EM, Lagneaux L, Fahmi H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics. 2021; 13(10):1736. https://doi.org/10.3390/pharmaceutics13101736
Chicago/Turabian StyleNajar, Mehdi, Makram Merimi, Wissam H. Faour, Catherine A. Lombard, Douâa Moussa Agha, Yassine Ouhaddi, Etienne M. Sokal, Laurence Lagneaux, and Hassan Fahmi. 2021. "In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway" Pharmaceutics 13, no. 10: 1736. https://doi.org/10.3390/pharmaceutics13101736
APA StyleNajar, M., Merimi, M., Faour, W. H., Lombard, C. A., Moussa Agha, D., Ouhaddi, Y., Sokal, E. M., Lagneaux, L., & Fahmi, H. (2021). In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics, 13(10), 1736. https://doi.org/10.3390/pharmaceutics13101736








