Telmisartan Loaded Nanofibers Enhance Re-Endothelialization and Inhibit Neointimal Hyperplasia
Abstract
:1. Introduction
2. Materials and Method
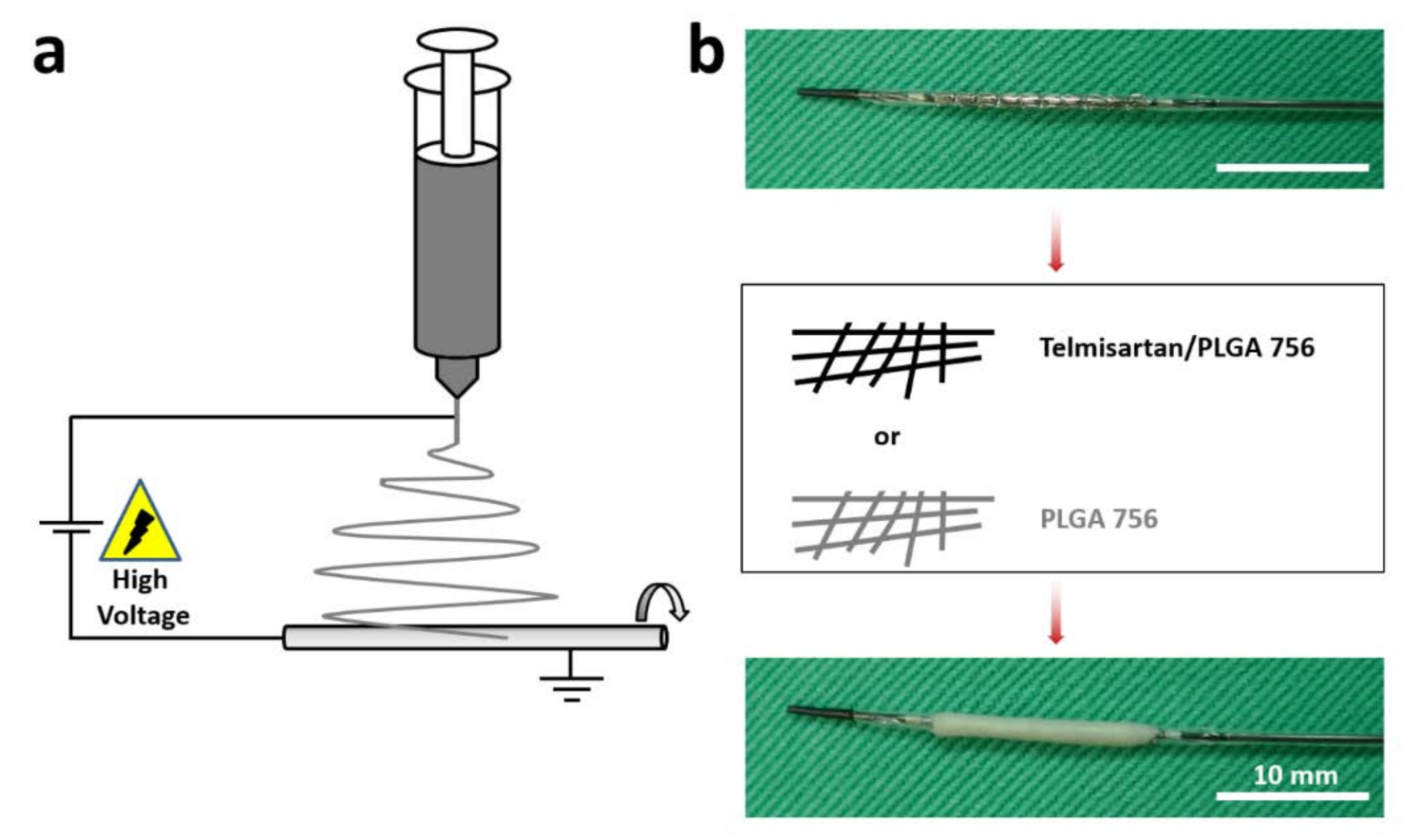
2.1. Fabrication of Telmisartan-Loaded Nanofibrous Tubes
2.2. Porosity
2.3. Scanning Electron Microscopy (SEM) Observation
2.4. Mechanical Properties of Materials
2.5. Wetting Angles
2.6. Water Uptake Capacity
2.7. In Vitro Release
2.8. Cell Cultures for Migration Assay
2.9. In Vivo Study
2.10. Microscopic Observation
2.11. Statistics and Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, P.C.; Myler, R.K.; Stertzer, S.; Fallon, J.T. Morphology after Transluminal Angioplasty in Human Beings. N. Engl. J. Med. 1981, 305, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Fajar, J.K.; Heriansyah, T.; Rohman, M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J. 2018, 70, S406–S418. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell. Signal. 2020, 66, 109485. [Google Scholar] [CrossRef] [PubMed]
- Scott-Burden, T.; Vanhoutte, P.M. The endothelium as a regulator of vascular smooth muscle proliferation. Circulation 1993, 87. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Singer, A.H.; Tsao, P.S.; Drexler, H.; Kosek, J.; Cooke, J.P. Dietary arginine prevents atherogenesis in the coronary artery of the hypercholesterolemic rabbit. J. Am. Coll. Cardiol. 1994, 23, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Hamon, M.; Vallet, B.; Bauters, C.; Wernert, N.; McFadden, E.P.; Lablanche, J.M.; Dupuis, B.; Bertrand, M.E. Long-term oral administration of L-arginine reduces intimal thickening and enhances neoendothelium-dependent acetylcholine-induced relaxation after arterial injury. Circulation 1994, 90, 1357–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Frank, P.; Woodman, S.E.; Park, D.S.; Lisanti, M.P. Caveolin, Caveolae, and Endothelial Cell Function. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1161–1168. [Google Scholar] [CrossRef]
- Kufner, S.; Joner, M.; Thannheimer, A.; Hoppmann, P.; Ibrahim, T.; Mayer, K.; Cassese, S.; Laugwitz, K.-L.; Schunkert, H.; Kastrati, A. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: Results from the ISAR-TEST 4 randomized trial. Circulation 2019, 139, 325–333. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan Attenuates Colon Inflammation, Oxidative Perturbations and Apoptosis in a Rat Model of Experimental Inflammatory Bowel Disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef] [Green Version]
- Sil, P. Therapeutic Insights against oxidative stress induced diabetic nephropathy: A review. J. Autoimmune Disord. 2015, 1. [Google Scholar] [CrossRef]
- Chen, T.; Xing, J.; Liu, Y. Effects of telmisartan on vascular endothelial function, inflammation and insulin resistance in patients with coronary heart disease and diabetes mellitus. Exp. Ther. Med. 2018, 15, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Tan, Q.; Sun, B. Telmisartan ameliorates vascular endothelial dysfunction in coronary slow flow phenomenon (CSFP). Cell Biochem. Funct. 2018, 36, 18–26. [Google Scholar] [CrossRef]
- Pellegrin, M.; Szostak, J.; Bouzourène, K.; Aubert, J.-F.; Berthelot, A.; Nussberger, J.; Laurant, P.; Mazzolai, L. Running exercise and angiotensin II type I receptor blocker telmisartan are equally effective in preventing angiotensin II-mediated vulnerable atherosclerotic lesions. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 159–168. [Google Scholar] [CrossRef]
- Klinghammer, L.; Urschel, K.; Cicha, I.; Lewczuk, P.; Raaz-Schrauder, R.; Achenbach, S.; Garlichs, C.D. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis—Influence on IP-10, TNF-α and MCP-1. Cytokine 2013, 62, 290–296. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Pleva, L.; Kukla, P.; Hlinomaz, O. Treatment of coronary in-stent restenosis: A systematic review. J. Geriatr. Cardiol. 2018, 15, 173–184. [Google Scholar] [CrossRef]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chang, S.-H.; Wen, M.-S.; Lin, Y.-H.; Liu, S.-J.; Wang, C.-J.; Hsu, M.-Y.; Hung, K.-C.; Yeh, Y.-H.; Chen, W.-J.; et al. Acceleration of re-endothelialization and inhibition of neointimal formation using hybrid biodegradable nanofibrous rosuvastatin-loaded stents. Biomaterials 2014, 35, 4417–4427. [Google Scholar] [CrossRef]
- Lee, C.-H.; Liu, K.-S.; Cheng, C.-W.; Chan, E.-C.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Fu, X.; Juang, J.-H.; Hsieh, I.-C.; et al. Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hsieh, M.-J.; Chang, S.-H.; Hung, K.-C.; Wang, C.-J.; Hsu, M.-Y.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.-S.; Liu, S.-J. Nanofibrous vildagliptin-eluting stents enhance re-endothelialization and reduce neointimal formation in diabetes: In Vitro and in vivo. Int. J. Nanomed. 2019, 14, 7503. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Hashmi, M.; Kharaghani, D.; Khan, M.Q.; Saito, Y.; Yamamoto, T.; Lee, J.; Kim, I.S. Antibacterial properties of in situ and surface functionalized impregnation of silver sulfadiazine in polyacrylonitrile nanofiber mats. Int. J. Nanomed. 2019, 14, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K., II; Cha, J.H.; Kim, I.S. Reusability comparison of melt-blown vs. Nanofiber face mask filters for use in the coronavirus pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Electrospun Momordica charantia incorporated polyvinyl alcohol (PVA) nanofibers for antibacterial applications. Mater. Today Commun. 2020, 24, 101161. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Kharaghani, D.; Gitigard, P.; Ohtani, H.; Kim, K.O.; Ullah, S.; Saito, Y.; Khan, M.Q.; Kim, I.S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019, 9, 12640. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, G.; Granada, J.F.; Virmani, R.; Alviar, C.L.; Tellez, A.; Kaluza, G.L.; Guilhermier, M.Y.; Parker, S.; Rowland, S.M.; Kolodgie, F.D.; et al. Anti-CD34 Antibodies Immobilized on the Surface of Sirolimus-Eluting Stents Enhance Stent Endothelialization. JACC Cardiovasc. Interv. 2010, 3, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.C.; Pershadsingh, H.A.; Ho, C.I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M.A.; Kurtz, T.W. Identification of Telmisartan as a Unique Angiotensin II Receptor Antagonist with Selective PPARγ–Modulating Activity. Hypertension 2004, 43, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmieder, R.E.; Delles, C.; Mimran, A.; Fauvel, J.P.; Ruilope, L.M. Impact of Telmisartan versus Ramipril on Renal Endothelial Function in Patients with Hypertension and Type 2 Diabetes. Diabetes Care 2007, 30, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Knorr, M.; Hausding, M.; Kröller-Schuhmacher, S.; Steven, S.; Oelze, M.; Heeren, T.; Scholz, A.; Gori, T.; Wenzel, P.; Schulz, E. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: Beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2223–2231. [Google Scholar] [CrossRef] [Green Version]
- Nakaya, K.; Ayaori, M.; Hisada, T.; Sawada, S.; Tanaka, N.; Iwamoto, N.; Ogura, M.; Yakushiji, E.; Kusuhara, M.; Nakamura, H.; et al. Telmisartan Enhances Cholesterol Efflux from THP-1 Macrophages by Activating PPARγ. J. Atheroscler. Thromb. 2007, 14, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.; Yarin, A.; Reneker, D. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Houchin, M.L.; Topp, E.M. Physical properties of PLGA films during polymer degradation. J. Appl. Polym. Sci. 2009, 114, 2848–2854. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Moffa, M.; Polini, A.; Sciancalepore, A.G.; Persano, L.; Mele, E.; Passione, L.G.; Potente, G.; Pisignano, D. Microvascular endothelial cell spreading and proliferation on nanofibrous scaffolds by polymer blends with enhanced wettability. Soft Matter 2013, 9, 5529–5539. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved Cellular Infiltration in Electrospun Fiber via Engineered Porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.-F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Siegel, S.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef]
- Rigatelli, G.; Palena, M.; Cardaioli, P.; Dell’Avvocata, F.; Giordan, M.; Vassilev, D.; Manzi, M. Prolonged high-pressure balloon angioplasty of femoropopliteal lesions: Impact on stent implantation rate and mid-term outcome. J. Geriatr. Cardiol. 2014, 11, 126–130. [Google Scholar] [CrossRef]
- Sobrino, T.; Hurtado, O.; Moro, M.A.; Rodríguez-Yáñez, M.; Castellanos, M.D.M.; Brea, D.; Moldes, O.; Blanco, M.; Arenillas, J.F.; Leira, R.; et al. The Increase of Circulating Endothelial Progenitor Cells after Acute Ischemic Stroke Is Associated with Good Outcome. Stroke 2007, 38, 2759–2764. [Google Scholar] [CrossRef] [Green Version]
- Shantsila, E.; Watson, T.; Lip, G.Y. Endothelial Progenitor Cells in Cardiovascular Disorders. J. Am. Coll. Cardiol. 2007, 49, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, T.; Hano, T.; Nishio, I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J. Hypertens. 2005, 23, 97–104. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Lee, P.S.S.; Poh, K.K. Endothelial progenitor cells in cardiovascular diseases. World J. Stem Cells 2014, 6, 355–366. [Google Scholar] [CrossRef]
- Pelliccia, F.; Pasceri, V.; Cianfrocca, C.; Vitale, C.; Speciale, G.; Gaudio, C.; Rosano, G.M.; Mercuro, G. Angiotensin II receptor antagonism with telmisartan increases number of endothelial progenitor cells in normotensive patients with coronary artery disease: A randomized, double-blind, placebo-controlled study. Atherosclerosis 2010, 210, 510–515. [Google Scholar] [CrossRef]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef]
- L’Abbate, A.; Neglia, D.; Vecoli, C.; Novelli, M.; Ottaviano, V.; Baldi, S.; Barsacchi, R.; Paolicchi, A.; Masiello, P.; Drummond, G.S.; et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3532–H3541. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Liu, K.-S.; Roth, J.G.; Hung, K.-C.; Liu, Y.-W.; Wang, S.-H.; Kuo, C.-C.; Liu, S.-J. Telmisartan Loaded Nanofibers Enhance Re-Endothelialization and Inhibit Neointimal Hyperplasia. Pharmaceutics 2021, 13, 1756. https://doi.org/10.3390/pharmaceutics13111756
Lee C-H, Liu K-S, Roth JG, Hung K-C, Liu Y-W, Wang S-H, Kuo C-C, Liu S-J. Telmisartan Loaded Nanofibers Enhance Re-Endothelialization and Inhibit Neointimal Hyperplasia. Pharmaceutics. 2021; 13(11):1756. https://doi.org/10.3390/pharmaceutics13111756
Chicago/Turabian StyleLee, Chen-Hung, Kuo-Sheng Liu, Julien George Roth, Kuo-Chun Hung, Yen-Wei Liu, Shin-Huei Wang, Chi-Ching Kuo, and Shih-Jung Liu. 2021. "Telmisartan Loaded Nanofibers Enhance Re-Endothelialization and Inhibit Neointimal Hyperplasia" Pharmaceutics 13, no. 11: 1756. https://doi.org/10.3390/pharmaceutics13111756
APA StyleLee, C. -H., Liu, K. -S., Roth, J. G., Hung, K. -C., Liu, Y. -W., Wang, S. -H., Kuo, C. -C., & Liu, S. -J. (2021). Telmisartan Loaded Nanofibers Enhance Re-Endothelialization and Inhibit Neointimal Hyperplasia. Pharmaceutics, 13(11), 1756. https://doi.org/10.3390/pharmaceutics13111756







