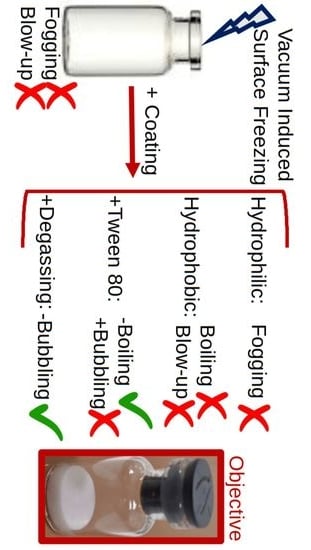
Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Determination of the Nucleation Pressure
2.3. Determination of the Influence of the Degasification Process
2.4. Freeze Drying Experiments
3. Results and Discussion
3.1. Effect of Formulation and Vial Type on the Nucleation Pressure
3.2. Effect of Formulation and Vial Type on Bubbling and Boiling Phenomena
3.3. Effect of Formulation and Vial Type on Nucleation Time and Blow Up
3.4. Effect of Degasification
3.5. Optimized Vacuum Induced Surface Freezing Conditions for Freeze-Drying
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The Ice Nucleation Temperature Determines the Primary Drying Rate of Lyophilization for Samples Frozen on a Temperature-Controlled Shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef]
- Oddone, I.; Barresi, A.A.; Pisano, R. Influence of Controlled Ice Nucleation on the Freeze-Drying of Pharmaceutical Products: The Secondary Drying Step. Int. J. Pharm. 2017, 524, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Arsiccio, A.; Pisano, R. The Ice-Water Interface and Protein Stability: A Review. J. Pharm. Sci. 2020, 109, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Pisano, R. Application of the Quality by Design Approach to the Freezing Step of Freeze-Drying: Building the Design Space. J. Pharm. Sci. 2018, 107, 1586–1596. [Google Scholar] [CrossRef]
- Bald, W.B. On Crystal Size and Cooling Rate. J. Microsc. 1986, 143, 89–102. [Google Scholar] [CrossRef]
- Pisano, R. Alternative Methods of Controlling Nucleation in Freeze Drying. In Lyophilization of Pharmaceuticals and Biologicals: New Technologies and Approaches; Ward, K.R., Matejtschuk, P., Eds.; Springer: New York, NY, USA, 2019; pp. 79–111. [Google Scholar] [CrossRef]
- Geidobler, R.; Winter, G. Controlled Ice Nucleation in the Field of Freeze-Drying: Fundamentals and Technology Review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W. The Freezing Step in Lyophilization: Physico-Chemical Fundamentals, Freezing Methods and Consequences on Process Performance and Quality Attributes of Biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Barresi, A.; De Beer, T.; Oddone, I.; Van Bockstal, P.J.; Pisano, R. Vacuum Induced Surface Freezing as an Effective Method for Improved Inter- and Intra-Vial Product Homogeneity. Eur. J. Pharm. Biopharm. 2018, 128, 210–219. [Google Scholar] [CrossRef]
- Oddone, I.; Pisano, R.; Bullich, R.; Stewart, P. Vacuum-Induced Nucleation as a Method for Freeze-Drying Cycle Optimization. Ind. Eng. Chem. Res. 2014, 53, 18236–18244. [Google Scholar] [CrossRef]
- Oddone, I.; Van Bockstal, P.-J.; De Beer, T.; Pisano, R. Impact of Vacuum-Induced Surface Freezing on Inter- and Intra-Vial Heterogeneity. Eur. J. Pharm. Biopharm. 2016, 103, 167–178. [Google Scholar] [CrossRef]
- Liu, J.; Viverette, T.; Virgin, M.; Anderson, M.; Dalal, P. A Study of the Impact of Freezing on the Lyophilization of a Concentrated Formulation with a High Fill Depth. Pharm. Dev. Technol. 2005, 10, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.; Gieseler, M.; Gieseler, H. Investigation of Two Different Pressure-Based Controlled Ice Nucleation Techniques in Freeze-Drying: The Integral Role of Shelf Temperature After Nucleation in Process Performance and Product Quality. J. Pharm. Sci. 2020, 109, 2746–2756. [Google Scholar] [CrossRef]
- Wenzel, T.; Gieseler, M.; Gieseler, H. Design of Vacuum-Induced Freezing Protocols for High Fill Volume Formulations in Freeze-Drying: A Strategic Approach. J. Pharm. Sci. 2020, 109, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Butt, Y.L.; Mietzner, R.; Schmidt, F.; Luemkemann, J.; Lema Martinez, C. Controlling Ice Nucleation during Lyophilization: Process Optimization of Vacuum-Induced Surface Freezing. Processes 2020, 8, 1263. [Google Scholar] [CrossRef]
- Oddone, I.; Arsiccio, A.; Duru, C.; Malik, K.; Ferguson, J.; Pisano, R.; Matejtschuk, P. Vacuum-Induced Surface Freezing for the Freeze-Drying of the Human Growth Hormone: How Does Nucleation Control Affect Protein Stability? J. Pharm. Sci. 2020, 109, 254–263. [Google Scholar] [CrossRef]
- Arsiccio, A.; Matejtschuk, P.; Ezeajughi, E.; Riches-Duit, A.; Bullen, A.; Malik, K.; Raut, S.; Pisano, R. Impact of Controlled Vacuum Induced Surface Freezing on the Freeze Drying of Human Plasma. Int. J. Pharm. 2020, 582, 119290. [Google Scholar] [CrossRef] [PubMed]
- Sacha, G.A.; Saffell-Clemmer, W.; Abram, K.; Akers, M.J. Practical Fundamentals of Glass, Rubber, and Plastic Sterile Packaging Systems. Pharm. Dev. Technol. 2010, 15, 6–34. [Google Scholar] [CrossRef]
- Schaut, R.A.; Peanasky, J.S.; DeMartino, S.E.; Schiefelbein, S.L. A New Glass Option for Parenteral Packaging. PDA J. Pharm. Sci. Technol. 2014, 68, 527–534. [Google Scholar] [CrossRef]
- Ennis, R.D.; Pritchard, R.; Nakamura, C.; Coulon, M.; Yang, T.; Visor, G.C.; Lee, W.A. Glass Vials for Small Volume Parenterals: Influence of Drug and Manufacturing Processes on Glass Delamination. Pharm. Dev. Technol. 2001, 6, 393–405. [Google Scholar] [CrossRef]
- Ditter, D.; Nieto, A.; Mahler, H.-C.; Roehl, H.; Wahl, M.; Huwyler, J.; Allmendinger, A. Evaluation of Glass Delamination Risk in Pharmaceutical 10 ML/10R Vials. J. Pharm. Sci. 2018, 107, 624–637. [Google Scholar] [CrossRef]
- Cailleteau, C.; Angeli, F.; Devreux, F.; Gin, S.; Jestin, J.; Jollivet, P.; Spalla, O. Insight into Silicate-Glass Corrosion Mechanisms. Nat. Mater. 2008, 7, 978–983. [Google Scholar] [CrossRef]
- Iacocca, R.G.; Allgeier, M. Corrosive Attack of Glass by a Pharmaceutical Compound. J. Mater. Sci. 2007, 42, 801–811. [Google Scholar] [CrossRef]
- Petty, C.; Cunningham, N.L. Insulin Adsorption by Glass Infusion Bottles, Polyvinylchloride Infusion Containers, and Intravenous Tubing. Anesthesiology 1974, 40, 400–404. [Google Scholar] [CrossRef]
- Höger, K.; Mathes, J.; Frieß, W. IgG1 Adsorption to Siliconized Glass Vials—Influence of PH, Ionic Strength, and Nonionic Surfactants. J. Pharm. Sci. 2015, 104, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Grohganz, H.; Rischer, M.; Brandl, M. Adsorption of the Decapeptide Cetrorelix Depends Both on the Composition of Dissolution Medium and the Type of Solid Surface. Eur. J. Pharm. Sci. 2004, 21, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ditter, D.; Mahler, H.-C.; Roehl, H.; Wahl, M.; Huwyler, J.; Nieto, A.; Allmendinger, A. Characterization of Surface Properties of Glass Vials Used as Primary Packaging Material for Parenterals. Eur. J. Pharm. Biopharm. 2018, 125, 58–67. [Google Scholar] [CrossRef]
- Mochel, E.L.; Nordberg, M.E.; Elmer, T.H. Strengthening of Glass Surfaces by Sulfur Trioxide Treatment. J. Am. Ceram. Soc. 1966, 49, 585–589. [Google Scholar] [CrossRef]
- Preston, W.A.; Neil, R.A. Glass and Rubber Closure Effects on the PH of Water l.A Preliminary Investigation. PDA J. Pharm. Sci. Technol. 1984, 38, 11–17. [Google Scholar]
- Iacocca, R.G.; Toltl, N.; Allgeier, M.; Bustard, B.; Dong, X.; Foubert, M.; Hofer, J.; Peoples, S.; Shelbourn, T. Factors Affecting the Chemical Durability of Glass Used in the Pharmaceutical Industry. AAPS PharmSciTech 2010, 11, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Miyajima, M.; Wakiyama, N.; Terada, K. Effects of Phosphate Buffer in Parenteral Drugs on Particle Formation from Glass Vials. Chem. Pharm. Bull. 2013, 61, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Roseman, T.J.; Brown, J.A.; Scothorn, W.W. Glass for Parenteral Products: A Surface View Using the Scanning Electron Microscope. J. Pharm. Sci. 1976, 65, 22–29. [Google Scholar] [CrossRef]
- Huang, M.; Childs, E.; Roffi, K.; Karim, F.; Juneau, J.; Bhatnagar, B.; Tchessalov, S. Investigation of Fogging Behavior in a Lyophilized Drug Product. J. Pharm. Sci. 2019, 108, 1101–1109. [Google Scholar] [CrossRef]
- White, F.; Koberda, M.; Chilamkurti, R. A Systematic Approach for Screening Glass Containers and Elastomeric Closures for Use with Parenteral Solutions. PDA J. Pharm. Sci. Technol. 2008, 62, 157–176. [Google Scholar] [PubMed]
- Ogawa, T.; Miyajima, M.; Nishimoto, N.; Minami, H.; Terada, K. Comparisons of Aluminum and Silica Elution from Various Glass Vials. Chem. Pharm. Bull. 2016, 64, 150–160. [Google Scholar] [CrossRef][Green Version]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of End Point of Primary Drying in Freeze-Drying Process Control. AAPS PharmSciTech 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Henry, C.L.; Craig, V.S.J. Inhibition of Bubble Coalescence by Osmolytes: Sucrose, Other Sugars, and Urea. Langmuir 2009, 25, 11406–11412. [Google Scholar] [CrossRef]
- Langer, C.; Mahler, H.-C.; Koulov, A.; Marti, N.; Grigore, C.; Matter, A.; Chalus, P.; Singh, S.; Lemazurier, T.; Joerg, S.; et al. Method to Predict Glass Vial Fogging in Lyophilized Drug Products. J. Pharm. Sci. 2020, 109, 323–330. [Google Scholar] [CrossRef]
- Abdul-Fattah, A.M.; Oeschger, R.; Roehl, H.; Bauer Dauphin, I.; Worgull, M.; Kallmeyer, G.; Mahler, H.-C. Investigating Factors Leading to Fogging of Glass Vials in Lyophilized Drug Products. Eur. J. Pharm. Biopharm. 2013, 85, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Moino, C.; Bourlés, E.; Pisano, R.; Scutellà, B. In-Line Monitoring of the Freeze-Drying Process by Means of Heat Flux Sensors. Ind. Eng. Chem. Res. 2021, 60, 9637–9645. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Stratta, L.; Fissore, D.; Pisano, R. Investigation of the Freezing Phenomenon in Vials Using an Infrared Camera. Pharmaceutics 2021, 13, 1664. [Google Scholar] [CrossRef]



| Phase | t, min | T, °C | P, μbar |
|---|---|---|---|
| Loading | - | 4 | atm |
| Cooling ramp | 10 | −6.5 | atm |
| Equilibration | see (1) | −6.5 | atm |
| Nucleation | tn | −6.5 | Pn |
| Freezing ramp | 38 | −45 | atm |
| Freezing holding | 60 | −45 | atm |
| Primary drying ramp | 12 | −20 | 100 |
| Primary drying holding | see (2) | −20 | 100 |
| Secondary drying ramp | 120 | 20 | 100 |
| Secondary drying holding | 400 | 20 | 100 |
| S− | ST | S+ | TL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Solution | Tn °C | Pfirst mbar | Plast mbar | Pfirst mbar | Plast mbar | Pfirst mbar | Plast mbar | Pfirst mbar | Plast mbar |
| DW | −5 | 1.3 | 0.7 | 1.2 | 0.9 | 1.2 | 0.8 | 1.3 | 1.2 |
| DW | −10 | 1.3 | 0.8 | 1.3 | 0.9 | 1.2 | 0.9 | 1.2 | 0.9 |
| DW + TW80 | −5 | 1.2 | 0.8 | 1.2 | 0.9 | 1.2 | 0.9 | 1.3 | 0.7 |
| DW + TW80 | −10 | 1.3 | 0.8 | 1.3 | 0.8 | 1.1 | 0.8 | 1.2 | 0.8 |
| Suc | −5 | 1.2 | 0.8 | 1.1 | 0.8 | 1.1 | 0.8 | 1.2 | 0.9 |
| Suc | −10 | 1.2 | 0.8 | 1.1 | 0.8 | 1.0 | 0.8 | 1.1 | 0.8 |
| Suc + TW80 | −5 | 1.2 | 1.0 | 1.2 | 0.9 | 1.2 | 0.8 | 1.1 | 0.7 |
| Suc + TW80 | −10 | 1.2 | 0.9 | 1.3 | 0.9 | 1.1 | 0.9 | 1.2 | 0.8 |
| Man | −5 | 1.2 | 0.7 | 1.2 | 0.6 | 1.1 | 0.6 | 1.1 | 0.8 |
| Man | −10 | 1.0 | 0.7 | 1.3 | 0.7 | 1.2 | 0.8 | 1.2 | 0.8 |
| Man + TW80 | −5 | 1.1 | 0.7 | 1.2 | 0.7 | 1.1 | 0.8 | 0.9 | 0.7 |
| Man + TW80 | −10 | 1.1 | 0.7 | 1.2 | 0.7 | 1.2 | 0.8 | 1.1 | 0.7 |
| S− | ST | S+ | TL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Solution | Tn °C | Bubbling | Boiling | Bubbling | Boiling | Bubbling | Boiling | Bubbling | Boiling |
| DW | −5 | - | - | - | - | - | I/W | - | W/S |
| DW | −10 | - | - | - | - | - | I/W | - | W/S |
| DW + TW80 | −5 | I/M | - | W/M | - | W/M | - | W/S | - |
| DW + TW80 | −10 | I/M | - | W/W | - | W/M | - | W/M | - |
| Suc | −5 | I/M | - | I/W | - | I/M | I/W | I/M | W/S |
| Suc | −10 | I/W | - | I/W | - | I/W | I/W | I/M | W/S |
| Suc + TW80 | −5 | I/M | - | I/M | - | W/M | - | W/S | - |
| Suc + TW80 | −10 | I/M | - | I/W | - | W/M | - | W/S | - |
| Man | −5 | W/M | - | - | - | I/M | I/W | - | W/S |
| Man | −10 | I/W | - | - | - | I/W | I/W | - | W/S |
| Man + TW80 | −5 | W/M | - | I/M | - | W/M | - | W/S | - |
| Man + TW80 | −10 | W/M | - | I/W | - | W/M | - | W/S | - |
| S− | ST | S+ | TL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution | Tn °C | Pn mbar | tn s | c - | Pn mbar | tn s | c - | Pn mbar | tn s | c - | Pn mbar | tn s | c - |
| DW | −5 | 1.1 | 17 | 3 | 1.0 | 12 | 2 | 1.0 | 1 | 2 | 1.2 | 26 * | 1 |
| DW | −10 | 1.1 | 15 | 3 | 1.1 | 4 | 2 | 1.1 | 9 | 3 | 1.0 | 3 * | 1.5 |
| DW + TW80 | −5 | 1.1 | 21 | 3 | 1.0 | 5 | 1.5 | 0.9 | 24 | 1 | 0.9 | 28 | 1.5 |
| DW + TW80 | −10 | 1.0 | 0 | 2 | 1.0 | 3 | 1.5 | 0.9 | 1 | 1.5 | 0.9 | 1 | 1.5 |
| Suc | −5 | 1.0 | 1 | 2 | 0.9 | 1 | 1.5 | 0.9 | 7 * | 1.5 | 1.0 | 31 * | 1.5 |
| Suc | −10 | 1.0 | 2 * | 2 | 0.9 | 9 | 1.5 | 0.9 | 8 * | 2 | 0.9 | 0 * | 1.5 |
| Suc + TW80 | −5 | 1.0 | 0 * | 1.5 | 1.0 | 2 | 1.5 | 0.9 | 1 * | 1.5 | 0.8 | 7 * | 1.5 |
| Suc + TW80 | −10 | 1.0 | 1 * | 1.5 | 1.0 | 2 | 1.5 | 1.0 | 1 * | 2 | 0.9 | 0 * | 1.5 |
| Man | −5 | 0.9 | 4 | 2 | 0.8 | 2 | 1.5 | 1.0 | 24 * | 1.5 | 0.9 | 0 * | 1.5 |
| Man | −10 | 0.8 | 0 | 2 | 0.9 | 58 | 1.5 | 1.0 | 30 * | 2 | 1.0 | 17 * | 2 |
| Man + TW80 | −5 | 0.9 | 28 | 2 | 0.8 | 4 | 1.5 | 0.7 | 1 * | 0.75 | 0.7 | 0 * | 1 |
| Man + TW80 | −10 | 0.9 | 21 | 2 | 0.8 | 1 | 1.5 | 0.9 | 2 * | 1.5 | 0.8 | 3 * | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regis, F.; Arsiccio, A.; Bourlès, E.; Scutellà, B.; Pisano, R. Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing. Pharmaceutics 2021, 13, 1766. https://doi.org/10.3390/pharmaceutics13111766
Regis F, Arsiccio A, Bourlès E, Scutellà B, Pisano R. Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing. Pharmaceutics. 2021; 13(11):1766. https://doi.org/10.3390/pharmaceutics13111766
Chicago/Turabian StyleRegis, Francesco, Andrea Arsiccio, Erwan Bourlès, Bernadette Scutellà, and Roberto Pisano. 2021. "Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing" Pharmaceutics 13, no. 11: 1766. https://doi.org/10.3390/pharmaceutics13111766
APA StyleRegis, F., Arsiccio, A., Bourlès, E., Scutellà, B., & Pisano, R. (2021). Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing. Pharmaceutics, 13(11), 1766. https://doi.org/10.3390/pharmaceutics13111766