Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing
Abstract
:1. Introduction
2. Traditional ECM Scaffolds Derived from Body Membranes for Skin Wound Healing
2.1. Acellular Dermal Matrix (ADM)
2.2. Small Intestinal Submucosa (SIS)
2.3. Acellular Amniotic Membrane (AAM)
2.4. Other ECM Membranes
3. Limitations of Traditional ECM Membranes
4. Scaffold Modification for Advanced ECM-Based Membranes
4.1. Crosslinking
4.1.1. Chemical Crosslinking
| Materials | Crosslinking Methods | Physical and Chemical Properties | Biological Results | Ref. |
|---|---|---|---|---|
| GP-AAM | GP crosslinking | Lower swelling ratio | Improved anti-collagenase degradation ability | [73] |
| Quercetin-ADM | Quercetin crosslinking | Improved mechanical strength and stiffness; Reorientation of the amino groups | / | [72] |
| PC-SIS | PC crosslinking | Improved max load and elastic modulus; Improved anti-calcification property | Improved anti-collagenase degradation ability; Facilitated cells organization, enhanced ECM deposition, and promoted functional gene expression | [65] |
| OHTCC-ADM | OHTCC crosslinking | Improved thermal stability; Improved tensile strength | Improved anti-collagenase degradation ability; Improved antibacterial activity; Preserved good cytocompatibility | [75] |
| EHTCC- ADM | EHTCC crosslinking | Improved mechanical properties; Improved thermal stability; Improved hydrophilicity | Excellent cellular compatibility and wound healing capacity | [76] |
| Riboflavin/UV-AAM | UV crosslinking with riboflavin as photosensitizer | Improved young’s modulus and ultimate tensile strength; Decreased water content | Improved anti-collagenase degradation ability; Preserved good cytocompatibility | [77] |
4.1.2. Physical Crosslinking
4.2. Combining ECM with Other Biomaterials
4.3. Loading ECM with Therapeutic Agents
4.3.1. Loading with Antibacterial Agents
4.3.2. Loading with Anti-Inflammatory Agents
4.3.3. Loading with Antioxidant Agents
4.3.4. Loading with Other Therapeutic Agents
5. Fabrication Technologies for Advanced Membranous ECM-Based Scaffolds
5.1. Electrospinning
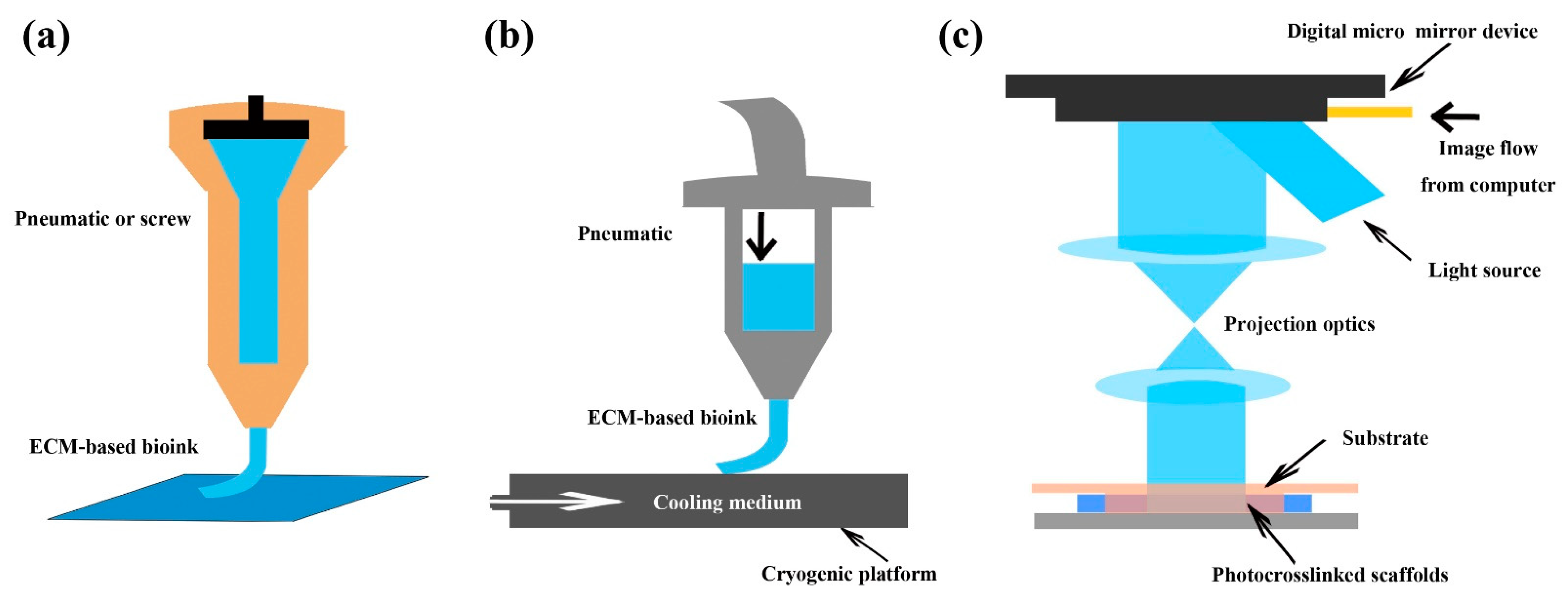
5.2. Three-Dimensional (3D) Bioprinting
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jarbrink, K.; Ni, G.; Sonnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inci, I.; Norouz Dizaji, A.; Ozel, C.; Morali, U.; Dogan Guzel, F.; Avci, H. Decellularized inner body membranes for tissue engineering: A review. J. Biomater. Sci. Polym. Ed. 2020, 31, 1287–1368. [Google Scholar] [CrossRef]
- Parveen, S.; Singh, S.P.; Panicker, M.M.; Gupta, P.K. Amniotic membrane as novel scaffold for human iPSC-derived cardiomyogenesis. In Vitro Cell Dev. Biol. Anim. 2019, 55, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Huang, Y.; Li, K.; Fan, Y.; Xie, H.; Li, X. Small intestinal submucosa: Superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mater. Chem. B 2019, 7, 5038–5055. [Google Scholar] [CrossRef]
- Vernon, R.B.; Gooden, M.D.; Lara, S.L.; Wight, T.N. Native fibrillar collagen membranes of micron-scale and submicron thicknesses for cell support and perfusion. Biomaterials 2005, 26, 1109–1117. [Google Scholar] [CrossRef]
- Cui, W.; Khan, K.M.; Ma, X.; Chen, G.; Desai, C.S. Human amniotic epithelial cells and human amniotic membrane as a vehicle for islet cell transplantation. Transplant. Proc. 2020, 52, 982–986. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 1–19. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.Q.; Cao, J.; Gong, M.; Zhang, Y.; Chen, X.; Tian, M.X.; Xie, H.Q. Accelerating effects of genipin-crosslinked small intestinal submucosa for defected gastric mucosa repair. J. Mater. Chem. B 2017, 5, 7059–7071. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber—Extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [Green Version]
- Thao, N.T.T.; Lee, S.; Shin, G.R.; Kang, Y.; Choi, S.; Kim, M.S. Preparation of electrospun small intestinal submucosa/poly(caprolactone-co-lactide-co-glycolide) nanofiber sheet as a potential drug carrier. Pharmaceutics 2021, 13, 253. [Google Scholar] [CrossRef]
- Da, L.; Gong, M.; Chen, A.; Zhang, Y.; Huang, Y.; Guo, Z.; Li, S.; Li-Ling, J.; Zhang, L.; Xie, H. Composite elastomeric polyurethane scaffolds incorporating small intestinal submucosa for soft tissue engineering. Acta Biomater. 2017, 59, 45–57. [Google Scholar] [CrossRef]
- Fan, M.R.; Gong, M.; Da, L.C.; Bai, L.; Li, X.Q.; Chen, K.F.; Li-Ling, J.; Yang, Z.M.; Xie, H.Q. Tissue engineered esophagus scaffold constructed with porcine small intestinal submucosa and synthetic polymers. Biomed. Mater. 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.; Akhavan-Tavakoli, M.; Khanjani, S.; Zare, S.; Edalatkhah, H.; Arasteh, S.; Mehrabani, D.; Zarnani, A.H.; Kazemnejad, S.; Shirazi, R. Bilayer amniotic membrane/nano-fibrous fibroin scaffold promotes differentiation capability of menstrual blood stem cells into keratinocyte-like cells. Mol. Biotechnol. 2018, 60, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Rezakhani, L.; khodaei, M.; Alizadeh, A. Characterization of the decellularized ovine pericardium for skin tissue engineering. J. Shahrekord Univ. Med Sci. 2020, 22, 173–180. [Google Scholar] [CrossRef]
- Brantley, J.N.; Verla, T.D. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv. Wound Care 2015, 4, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Shakouri-Motlagh, A.; Khanabdali, R.; Heath, D.E.; Kalionis, B. The application of decellularized human term fetal membranes in tissue engineering and regenerative medicine (TERM). Placenta 2017, 59, 124–130. [Google Scholar] [CrossRef]
- Frazao, L.P.; Vieira de Castro, J.; Nogueira-Silva, C.; Neves, N.M. Decellularized human chorion membrane as a novel biomaterial for tissue regeneration. Biomolecules 2020, 10, 1208. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized scaffolds for skin repair and regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- AL-Bayati, A.H.F.; Hameed, F.M. Effect of acellular bovine pericardium and dermal matrixes on cutaneous wounds healing in male rabbits: Histopathological evaluation. J. Entomol. Zool. Stud. 2018, 6, 1976–1986. [Google Scholar]
- Da, L.C.; Huang, Y.Z.; Xie, H.Q. Progress in development of bioderived materials for dermal wound healing. Regen. Biomater. 2017, 4, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Tsung-Hsuan, W.; Bertasi, G.; Elfahar, M. The use of dermacell® in fingertip injury. J. Clin. Case Rep. Images 2019, 1, 14–22. [Google Scholar] [CrossRef]
- Ariganello, M.B.; Simionescu, D.T.; Labow, R.S.; Lee, J.M. Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials 2011, 32, 439–449. [Google Scholar] [CrossRef]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. Part A 2017, 105, 138–147. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Q.Y.; Huang, L.P.; Huang, K.; Xie, H.Q. Decellularized scaffold and its elicited immune response towards the host: The underlying mechanism and means of immunomodulatory modification. Biomater. Sci. 2021, 9, 4803–4820. [Google Scholar] [CrossRef]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J. A porcine acellular dermal matrix induces human fibroblasts to secrete hyaluronic acid by activating JAK2/STAT3 signalling. RSC Adv. 2020, 10, 18959–18969. [Google Scholar] [CrossRef]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Cazzell, S.; Moyer, P.M.; Samsell, B.; Dorsch, K.; McLean, J.; Moore, M.A. A prospective, multicenter, single-arm clinical trial for treatment of complex diabetic foot ulcers with deep exposure using acellular dermal matrix. Adv. Skin Wound Care 2019, 32, 409–415. [Google Scholar] [CrossRef]
- Lewis, R.E.; Towery, E.A.; Bhat, S.G.; Ward, A.J.; Heidel, R.E.; Bielak, K.M.; Simpson, H.E.; McLoughlin, J.M.; Lewis, J.M. Human acellular dermal matrix is a viable alternative to autologous skin graft in patients with cutaneous malignancy. Am. Surg. 2019, 85, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.J.; Khan, F.; Belch, J.J.; Mitchell, M.R.; Leese, G.P. Blood flow changes in diabetic foot ulcers treated with dermal replacement therapy. J. Foot Ankle Surg. 2002, 41, 233–237. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic diabetic wounds and their treatment with skin substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Alvaro-Afonso, F.J.; Garcia-Alvarez, Y.; Lazaro-Martinez, J.L.; Kakagia, D.; Papanas, N. Advances in dermoepidermal skin substitutes for diabetic foot ulcers. Curr. Vasc. Pharmacol. 2020, 18, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Najafi, A.; Karami, M.Y. Thin split thickness skin grafting on human acellular dermal matrix scaffold for the treatment of deep burn wounds. Int. J. Organ. Transplant. Med. 2021, 12, 44–51. [Google Scholar]
- Reyzelman, A.; Crews, R.T.; Moore, J.C.; Moore, L.; Mukker, J.S.; Offutt, S.; Tallis, A.T.W.B.; Vayser, D.; Winters, C.; Armstrong, D.G. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: A prospective, randomised, multicentre study. Int. Wound J. 2009, 6, 196–208. [Google Scholar] [CrossRef]
- Reyzelman, A.M.; Bazarov, I. Human acellular dermal wound matrix for treatment of DFU: Literature review and analysis. J. Wound Care 2015, 24, 128–134. [Google Scholar] [CrossRef]
- Asodiya, F.A.; Kumar, V.; Vora, S.D.; Singh, V.K.; Fefar, D.T.; Gajera, H.P. Preparation, characterization, and xenotransplantation of the caprine acellular dermal matrix. Xenotransplantation 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, L.; Mamat, M.; Chen, Z.; Liu, L.; Wang, Z. Xenogenic (porcine) acellular dermal matrix is useful for the wound healing of severely damaged extremities. Exp. Ther. Med. 2014, 7, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Park, J.H.; Jeong, H.G.; Wee, S.Y. The utility of novel fish-skin derived acellular dermal matrix (Kerecis) as a wound dressing material. J. Wound Manag. Res. 2021, 17, 39–47. [Google Scholar] [CrossRef]
- Sitje, T.S.; Grøndahl, E.C.; Sørensen, J.A. Clinical innovation: Fish-derived wound product for cutaneous wounds. Wounds Int. 2018, 9, 44–50. [Google Scholar]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elcin, A.E.; Elcin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Magden, G.K.; Vural, C.; Bayrak, B.Y.; Ozdogan, C.Y.; Kenar, H. Composite sponges from sheep decellularized small intestinal submucosa for treatment of diabetic wounds. J. Biomater. Appl. 2021, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andree, B.; Bar, A.; Haverich, A.; Hilfiker, A. Small intestinal submucosa segments as matrix for tissue engineering: Review. Tissue Eng. Part B Rev. 2013, 19, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Lucich, E.A.; Rendon, J.L.; Valerio, I.L. Advances in addressing full-thickness skin defects: A review of dermal and epidermal substitutes. Regen. Med. 2018, 13, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; McCabe, G.P.; Stewart-Akers, A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 2008, 14, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ramsay, S.; Ermis, R.; Carson, D. In vitro and in vivo studies on matrix metalloproteinases interacting with small intestine submucosa wound matrix. Int. Wound J. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef]
- Lazaro, J.L.; Lzzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated levels of matrix metalloproteinases and chronic wound healing_an updated review of clinical evidence. J. Wound Care 2016, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Brown-Etris, M.; Milne, C.T.; Hodde, J.P. An extracellular matrix graft (Oasis® wound matrix) for treating fullthickness pressure ulcers: A randomized clinical trial. J. Tissue Viability 2019, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hodde, J.P.; Hiles, M.C.; Metzger, D.W. Characterization of the local wound environment following treatment of chronic leg ulcers with SIS wound matrix. J. Tissue Viability 2020, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.C. Human acellular amniotic membrane implantation for lower third nasal reconstruction: A promising therapy to promote wound healing. Burn. Trauma 2018, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef]
- Dadkhah Tehrani, F.; Firouzeh, A.; Shabani, I.; Shabani, A. A review on modifications of amniotic membrane for biomedical applications. Front. Bioeng. Biotechnol. 2020, 8, 1–25. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, L.; Yan, J. Amniotic membrane for treating skin graft donor sites: A systematic review and meta-analysis. Burns 2020, 46, 621–629. [Google Scholar] [CrossRef]
- Su, Y.N.; Zhao, D.Y.; Li, Y.H.; Yu, T.Q.; Sun, H.; Wu, X.Y.; Zhou, X.Q.; Li, J. Human amniotic membrane allograft, a novel treatment for chronic diabetic foot ulcers: A systematic review and meta-analysis of randomised controlled trials. Int. Wound J. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Negron, L.; Lun, S.; May, B.C. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int. Wound J. 2014, 11, 392–397. [Google Scholar] [CrossRef]
- Arrizabalaga, J.H.; Nollert, M.U. Human amniotic membrane: A versatile scaffold for tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 2226–2236. [Google Scholar] [CrossRef]
- Ramirez-Acuna, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Borgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human amniotic membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 941–954. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Jiang, Y.L.; Hu, J.G.; Zhao, L.M.; Chen, Q.Z.; Liang, Y.; Zhang, Y.; Lei, X.X.; Wang, R.; Lei, Y.; et al. Procyanidins-crosslinked small intestine submucosa: A bladder patch promotes smooth muscle regeneration and bladder function restoration in a rabbit model. Bioact. Mater. 2021, 6, 1827–1838. [Google Scholar] [CrossRef]
- Boekema, B.K.; Vlig, M.; Olde Damink, L.; Middelkoop, E.; Eummelen, L.; Buhren, A.V.; Ulrich, M.M. Effect of pore size and cross-linking of a novel collagen-elastin dermal substitute on wound healing. J. Mater. Sci. Mater. Med. 2014, 25, 423–433. [Google Scholar] [CrossRef]
- Glynn, J.J.; Polsin, E.G.; Hinds, M.T. Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa. Acta Biomater. 2015, 14, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wu, B.; Li, C.; Mu, C.; Li, H.; Lin, W. Collagen cryogel cross-linked by naturally derived dialdehyde carboxymethyl cellulose. Carbohydr. Polym. 2015, 129, 17–24. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, X.; Wu, Q.; Li, Y.; Zhou, Y.; Li, L.; Bu, H. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhai, W.; Wu, C.; Ma, B.; Zhang, J.; Zhang, H.; Zhu, Z.; Chang, J. Procyanidins-crosslinked aortic elastin scaffolds with distinctive anti-calcification and biological properties. Acta Biomater. 2015, 16, 81–93. [Google Scholar] [CrossRef]
- Greco, K.V.; Francis, L.; Huang, H.; Ploeg, R.; Boccaccini, A.R.; Ansari, T. Is quercetin an alternative natural crosslinking agent to genipin for long-term dermal scaffolds implantation? J. Tissue Eng. Regen. Med. 2018, 12, e1716–e1724. [Google Scholar] [CrossRef]
- Gobinathan, S.; Zainol, S.S.; Azizi, S.F.; Iman, N.M.; Muniandy, R.; Hasmad, H.N.; Yusof, M.R.B.; Husain, S.; Abd Aziz, H.; Lokanathan, Y. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Dan, N.; Huang, Y.; Bai, Z.; Yang, C.; Dan, W.; Cong, L. Functional chemical modification of a porcine acellular dermal matrix with a modified naturally derived polysaccharide crosslinker. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Dan, N.; Dan, W.; Li, Z. Highly stable collagen scaffolds crosslinked with an epoxidized natural polysaccharide for wound healing. Int. J. Biol. Macromol. 2021, 182, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, J.H.; Nollert, M.U. Riboflavin-UVA crosslinking of amniotic membranes and its influence on the culture of adipose-derived stem cells. J. Mech. Behav. Biomed. Mater. 2020, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ziaei, M.; Barsam, A.; Shamie, N.; Vroman, D.; Kim, T.; Donnenfeld, E.D.; Holland, E.J.; Kanellopoulos, J.; Mah, F.S.; Randleman, J.B.; et al. Reshaping procedures for the surgical management of corneal ectasia. J. Cataract. Refract. Surg. 2015, 41, 842–872. [Google Scholar] [CrossRef]
- Zhang, C.; Du, T.; Mu, G.; Wang, J.; Gao, X.; Long, F.; Wang, Q. Evaluation and ultrastructural changes of amniotic membrane fragility after UVA/riboflavin cross-linking and its effects on biodegradation. Medicine 2020, 99, 1–6. [Google Scholar] [CrossRef]
- Pereira, A.T.; Schneider, K.H.; Henriques, P.C.; Grasl, C.; Melo, S.F.; Fernandes, I.P.; Kiss, H.; Martins, M.C.L.; Bergmeister, H.; Goncalves, I.C. Graphene oxide coating improves the mechanical and biological properties of decellularized umbilical cord arteries. ACS Appl. Mater. Interfaces 2021, 13, 32662–32672. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.W.; Lee, Y.M.; Lee, H.B.; Khang, G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine submucosa impregnated poly(D,L-lactide-co-glycolide) for tissue-engineered bone. J. Biomater. Sci. Polym. Ed. 2004, 15, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, D.; Hsu, C.C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Krishnan, L.K.; Nair, R.P.; Kalliyana Krishnan, V. Reinforcement of amniotic membrane with fibrin coated poly-[Lactide-co-Glycolide-co-Caprolactone] terpolymer containing silver nanoparticles for potential wound healing applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 810–819. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yan, Y.; Ji, Q.; Dai, Y.; Jin, S.; Liu, Y.; Chen, J.; Teng, L. A sponge-like double-layer wound dressing with chitosan and decellularized bovine amniotic membrane for promoting diabetic wound healing. Polymers 2020, 12, 535. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei-Parsa, M.J.; Ghanbari, H.; Alipoor, B.; Tavakoli, A.; Najafabadi, M.R.H.; Faridi-Majidi, R. Nanofiber-acellular dermal matrix as a bilayer scaffold containing mesenchymal stem cell for healing of full-thickness skin wounds. Cell. Tissue Res. 2019, 375, 1–13. [Google Scholar] [CrossRef]
- Kshersagar, J.; Kshirsagar, R.; Desai, S.; Bohara, R.; Joshi, M. Decellularized amnion scaffold with activated PRP: A new paradigm dressing material for burn wound healing. Cell Tissue Bank 2018, 19, 1–15. [Google Scholar] [CrossRef]
- Kim, M.J.; Ji, Y.B.; Seo, J.Y.; Park, S.H.; Kim, J.H.; Min, B.H.; Kim, M.S. Substance P-loaded electrospun small intestinal submucosa/poly(epsilon-caprolactone)-ran-poly(l-lactide) sheet to facilitate wound healing through MSC recruitment. J. Mater. Chem. B 2019, 7, 7599–7611. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Singh, L.; Roy, P.; Mishra, N.C. Silk fibroin protein modified acellular dermal matrix for tissue repairing and regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsu, W.S.; Chung, W.Y.; Ko, T.H.; Lin, J.H. Silver-based wound dressings reduce bacterial burden and promote wound healing. Int. Wound J. 2016, 13, 505–511. [Google Scholar] [CrossRef]
- Robinson, J.; Sulzer, J.K.; Motz, B.; Baker, E.H.; Martinie, J.B.; Vrochides, D.; Iannitti, D.A. Long-term clinical outcomes of an antibiotic-coated non-cross-linked porcine acellular dermal graft for abdominal wall reconstruction for high-risk and contaminated wounds. Am. Surg. 2021, 31348211023392. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.H.; Moon, J.H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef]
- Soltan Dallal, M.M.; Nikkhahi, F.; Molaei, S.; Hosseini, K.; Kalafi, Z.; Khoshzaban, A.; Lari, A.R.; Kheirkhah, A.; Sharifi Yazdi, M.K. Effect of amniotic membrane combined with ciprofloxacin in curing the primary stages of pseudomonal keratitis. J. Med. Bacteriol. 2012, 1, 31–37. [Google Scholar]
- Sohail, M.R.; Esquer Garrigos, Z.; Elayi, C.S.; Xiang, K.; Catanzaro, J.N. Preclinical evaluation of efficacy and pharmacokinetics of gentamicin containing extracellular-matrix envelope. Pacing Clin. Electrophysiol. 2020, 43, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Goller, S.; Turner, N.J. The antimicrobial effectiveness and cytotoxicity of the antibiotic-loaded chitosan: ECM scaffolds. Appl. Sci. 2020, 10, 3446. [Google Scholar] [CrossRef]
- Zieger, M.A.; Ochoa, M.; Rahimi, R.; Campana, G.; Tholpady, S.; Ziaie, B.; Sood, R. Skin regeneration using dermal substrates that contain autologous cells and silver nanoparticles to promote antibacterial activity: In vitro studies. Mil. Med. 2017, 182, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, J.; Chai, Y.; Zhang, J.; Hu, Z.; Zhou, H. Nano-silver modified porcine small intestinal submucosa for the treatment of infected partial-thickness burn wounds. Burns 2019, 45, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Krishnakumar, R.; Rekha, R.; Vaseeharan, B.; Saraswathi, K.; Raj, M.; Hanna, R.E.B.; Brennan, G.P.; Dayanithi, G.; Vijayakumar, S. Bio-fabrication of human amniotic membrane Zinc oxide nanoparticles and the wet/dry HAM dressing membrane for wound healing. Front. Bioeng. Biotechnol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Kasetty, G.; Kalle, M.; Morgelin, M.; Brune, J.C.; Schmidtchen, A. Anti-endotoxic and antibacterial effects of a dermal substitute coated with host defense peptides. Biomaterials 2015, 53, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Pesaraklou, A.; Mahdavi-Shahri, N.; Hassanzadeh, H.; Ghasemi, M.; Kazemi, M.; Mousavi, N.S.; Matin, M.M. Use of cerium oxide nanoparticles: A good candidate to improve skin tissue engineering. Biomed. Mater. 2019, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.K.; Ghosh, S.K.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater. Chem. B 2020, 8, 9277–9294. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ma, H.; Wu, Z.; Zeng, H.; Li, Z.; Wang, Y.; Liu, G.; Xu, B.; Lin, Y.; Zhang, P.; et al. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater. Sci. Eng. C 2014, 44, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Purohit, S.D.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Mishra, N.C. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 1–10. [Google Scholar] [CrossRef]
- Dhasmana, A.; Singh, L.; Roy, P.; Chandra Mishra, N. Honey incorporated antibacterial acellular dermal matrix for full-thickness wound healing. Ann. Biotechnol. 2018, 1, 8–10. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.L.; Yoo, J.W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 1–11. [Google Scholar] [CrossRef]
- Thanganadar Appapalam, S.; Paul, B.; Arockiasamy, S.; Panchamoorthy, R. Phytofabricated silver nanoparticles: Discovery of antibacterial targets against diabetic foot ulcer derived resistant bacterial isolates. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small 2019, 15, e1902232. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Andre-Levigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuenod, B. Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int. J. Mol. Sci. 2017, 18, 2149. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Jin, Q.; Zhao, H.; Zhu, W.; Liu, Z.; Chen, Q. Reactive oxygen species scavenging sutures for enhanced wound sealing and repair. Small Struct. 2021, 2, 2100002. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B 2017, 5, 6579–6592. [Google Scholar] [CrossRef] [PubMed]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Liu, C.; Fu, J.S.; Xiong, Q.H.; Liu, S.Q.; Xiong, L. Application of acellular artificial dermis carrying nanospheres containing platelet derived growth factor-BB for repairing skin defects of nude mice. J. Shanghai Jiaotong Univ. (Med. Sci.) 2014, 34, 1454–1458. [Google Scholar]
- Siddika, A.; Arifuzzaman, M.; Hossain, L.; Adnan, M.H.; Diba, F.; Hasan, M.Z.; Asaduzzaman, S.M.; Uddin, M.J. Assortment of human amniotic membrane and curcumin: A potential therapeutic strategy for burn wound healing. Curr. Drug Ther. 2021, 16, 3–10. [Google Scholar] [CrossRef]
- Wang, L.; Gong, J.; Dan, Y.; Huang, Y.; Dan, N.; Dan, W. Preparation and characterization of antibacterial porcine acellular dermal matrices with high performance. ACS Omega 2020, 5, 20238–20249. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhuo, R.; Yu, A.; Huang, S. A novel curcumin-loaded composite dressing facilitates wound healing due to its natural antioxidant effect. Drug Des. Devel. Ther. 2019, 13, 3269–3280. [Google Scholar] [CrossRef] [Green Version]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lei, I.M.; Davoodi, P.; Huleihel, L.; Huang, Y.Y.S. Solution formulation and rheology for fabricating extracellular matrix-derived fibers using low-voltage electrospinning patterning. ACS Biomater. Sci. Eng. 2019, 5, 3676–3684. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Morsi, Y.; Zhu, T.; Ahmad, A.; Xie, X.; Yu, F.; Mo, X. Electrospinning: An emerging technology to construct polymer-based nanofibrous scaffolds for diabetic wound healing. Front. Mater. Sci. 2021, 15, 10–35. [Google Scholar] [CrossRef]
- Du, P.; Casavitri, C.; Suhaeri, M.; Wang, P.Y.; Lee, J.H.; Koh, W.G.; Park, K. A fibrous hybrid patch couples cell-derived matrix and poly(l-lactide-co-caprolactone) for endothelial cells delivery and skin wound repair. ACS Biomater. Sci. Eng. 2019, 5, 900–910. [Google Scholar] [CrossRef]
- Masaeli, E.; Karamali, F.; Loghmani, S.; Eslaminejad, M.B.; Nasr-Esfahani, M.H. Bio-engineered electrospun nanofibrous membranes using cartilage extracellular matrix particles. J. Mater. Chem. B 2017, 5, 765–776. [Google Scholar] [CrossRef]
- Grant, R.; Hallett, J.; Forbes, S.; Hay, D.; Callanan, A. Blended electrospinning with human liver extracellular matrix for engineering new hepatic microenvironments. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Samadikuchaksaraei, A.; Seifalian, A.M.; Urbanska, A.M.; Ghanbarian, H.; Hardy, J.G.; Omrani, M.D.; Mozafari, M.; Reis, R.L.; Kundu, S.C. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed. Mater. 2018, 13, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef]
- Fernandez-Perez, J.; Kador, K.E.; Lynch, A.P.; Ahearne, M. Characterization of extracellular matrix modified poly(epsilon-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 1–9. [Google Scholar] [CrossRef]
- Sobreiro-Almeida, R.; Fonseca, D.R.; Neves, N.M. Extracellular matrix electrospun membranes for mimicking natural renal filtration barriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Choi, M.; Sultana, T.; Park, M.; Lee, B.T. Fibroblast cell derived extracellular matrix containing electrospun scaffold as a hybrid biomaterial to promote in vitro endothelial cell expansion and functionalization. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 1–8. [Google Scholar] [CrossRef]
- Wakuda, Y.; Nishimoto, S.; Suye, S.I.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Politi, S.; Carotenuto, F.; Rinaldi, A.; Di Nardo, P.; Manzari, V.; Albertini, M.C.; Araneo, R.; Ramakrishna, S.; Teodori, L. Smart ECM-based electrospun biomaterials for skeletal muscle regeneration. Nanomaterials 2020, 10, 1781. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-based medical devices for the treatment of chronic skin lesions: From research to the clinic. Pharmaceutics 2020, 12, 815. [Google Scholar] [CrossRef]
- Iacob, A.T.; Dragan, M.; Ionescu, O.M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupascu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive electrospun scaffolds for wound healing applications: A comprehensive review. Polym. Test. 2021, 93, 1–22. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun biomaterials in the treatment and prevention of scars in skin wound healing. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, Y.; Kim, S.H. Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng. Part A 2018, 24, 830–848. [Google Scholar] [CrossRef]
- Jeon, O.; Bin Lee, Y.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef]
- Santschi, M.; Vernengo, A.; Eglin, D.; D'Este, M.; Wuertz-Kozak, K. Decellularized matrix as a building block in bioprinting and electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Ding, H.; Chang, R.C. Simulating image-guided in situ bioprinting of a skin graft onto a phantom burn wound bed. Addit. Manuf. 2018, 22, 708–719. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Gao, G.; Cho, D.W. Tissue-specific decellularized extracellular matrix bioinks for musculoskeletal tissue regeneration and modeling using 3D bioprinting technology. Int. J. Mol. Sci. 2021, 22, 7837. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, 1–18. [Google Scholar] [CrossRef]
- Won, J.Y.; Lee, M.H.; Kim, M.J.; Min, K.H.; Ahn, G.; Han, J.S.; Jin, S.; Yun, W.S.; Shim, J.H. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cells Nanomed. Biotechnol. 2019, 47, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.A.; Florez, S.L.; Talero, V.A.; Briceño, J.C.; Muñoz-Camargo, C.; Cruz, J.C. Formulation and characterization of a SIS-based photocrosslinkable bioink. Polymers 2019, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Jeong, W.; Lee, S.M.; Kim, J.B.; Jin, S.; Kang, H.W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020, 12, 1–35. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital light processing based three-dimensional printing for medical applications. Int. J. Bioprint. 2020, 6, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ullah, I.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 1–44. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Jin, R.; Cui, Y.; Chen, H.; Zhang, Z.; Weng, T.; Xia, S.; Yu, M.; Zhang, W.; Shao, J.; Yang, M.; et al. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 2021, 131, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S.; et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 1–16. [Google Scholar] [CrossRef]
- Ventura, R.D.; Padalhin, A.R.; Park, C.M.; Lee, B.T. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.L.; Castells-Sala, C.; Lopez-Chicon, P.; Nieto-Nicolau, N.; Aiti, A.; Farinas, O.; Casaroli-Marano, R.P.; Porta, O.; Vilarrodona, A. Fast protocol for the processing of split-thickness skin into decellularized human dermal matrix. Tissue Cell 2021, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, F.; Hu, E.; Yu, K.; Li, J.; Bao, R.; Dai, F.; Lan, G.; Xie, R. Biogenetic acellular dermal matrix maintaining rich interconnected microchannels for accelerated tissue amendment. ACS Appl. Mater. Interfaces 2021, 13, 16048–16061. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, D.-J.; Periasamy, S.; Chuang, C.-T.; Tseng, F.-W.; Kuo, J.-C.; Tarng, Y.-W. Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: Role in accelerated wound healing. Materialia 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Da Silva, K.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Three-dimensional printing of extracellular matrix (ECM)-mimicking scaffolds: A critical review of the current ECM materials. J. Biomed. Mater. Res. Part A 2020, 108, 2324–2350. [Google Scholar] [CrossRef]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.Y.; Neuenschwander, P.; Chou, S.F. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]


| Materials | Developing Methods | Biological Characteristics | Ref. |
|---|---|---|---|
| Gentamicin-SIS | Hydrated SIS in a 40 mg/mL gentamicin solution for 2 min | Anti-E. coli; Anti-S. epidermidis; Anti-methicillin-resistant S. aureus, Anti-P. aeruginosa; Anti-S. marcescens; Anti-S. aureus | [98] |
| Antibiotic-CS-UBM | Dissolved the antibiotic powder (3 mg minocycline/dish or 0.5 mg rifampicin/dish) to the CS: UBM slurry in a 60 mm petri dish | Anti-E. coli; Anti-S. aureus; Adjustable drug release rates and antibacterial effects | [99] |
| Silver NP-ADM | Immersed ADM into a silver NP suspension at concentrations of 0 to 1% for 1 min | Anti-P. aeruginosa; Anti-S. aureus; No significant cytotoxicity | [100] |
| Silver NP-SIS | Immersed SIS into a 50 mg/mL silver NP suspension for 24 h | Anti-P. aeruginosa; Lower expression levels of IL-6 and C-reactive protein, less inflammation, more re-epithelization, and better neovascularization in the wounds of the silver NP-SIS group than that of the pure SIS group. | [101] |
| ZnO NP-AAM | Immersed AAM into a 75 µg/mL ZnO NP suspension for 3 h | Anti-Gram-positive bacteria (S. aureus, S. mutans, E. faecalis, and L. fusiformis); Anti-Gram-negative bacteria (S. sonnei, P. aeruginosa, P. vulgaris, and C. freundii) | [102] |
| THDP-ADM | Coated ADM with a 10 mL THDP solution at concentrations of 0.647, 1.62 and 3.24 mM | Anti-Gram-positive bacteria (S. aureus); Anti-Gram-negative bacteria (E. coli, P. aeruginosa); Endotoxin-blocking property | [103] |
| Dex-SIS AgS-SIS | Electrospun solutions containing Dex-SIS or AgS-SIS | Suppressed macrophage infiltration | [13] |
| CeO2 NP-ADM | Immersed ADM into a CeO2 NP suspension at concentrations of 1 to 20 mg/mL for 24 h | Antioxidant property | [104] |
| CN-CS-ADM | Added CN to the CS-ADM at a concentration of 1.5 mg/mL | Good ROS scavenging property | [105] |
| EGF-HA-DP | Immersed HA-DP into a 1 μg/mL EGF solution for 12 h | Raised wound healing rate; Promoted regeneration of skin appendages; The regeneration of thicker epidermis and dermis layers | [106] |
| Curcumin-SIS | Added SIS to the curcumin solutions at concentrations of 0.1, 0.5 and 1% for 30 min | Anti-E. coli; Anti-S. aureus; Free radical scavenging capability | [107] |
| Honey-ADM | Immersed ADM into the honey solutions at concentrations of 5%, 10%, 15% for 30 min | Anti-E. coli; Anti-S. aureus; Controlled immune response | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da, L.-C.; Huang, Y.-Z.; Xie, H.-Q.; Zheng, B.-H.; Huang, Y.-C.; Du, S.-R. Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing. Pharmaceutics 2021, 13, 1796. https://doi.org/10.3390/pharmaceutics13111796
Da L-C, Huang Y-Z, Xie H-Q, Zheng B-H, Huang Y-C, Du S-R. Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing. Pharmaceutics. 2021; 13(11):1796. https://doi.org/10.3390/pharmaceutics13111796
Chicago/Turabian StyleDa, Lin-Cui, Yi-Zhou Huang, Hui-Qi Xie, Bei-Hong Zheng, Yong-Can Huang, and Sheng-Rong Du. 2021. "Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing" Pharmaceutics 13, no. 11: 1796. https://doi.org/10.3390/pharmaceutics13111796
APA StyleDa, L.-C., Huang, Y.-Z., Xie, H.-Q., Zheng, B.-H., Huang, Y.-C., & Du, S.-R. (2021). Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing. Pharmaceutics, 13(11), 1796. https://doi.org/10.3390/pharmaceutics13111796






