Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Nanoparticles
2.2. Measurement of Antigen-Capturing Capacity
2.3. Cell Culture
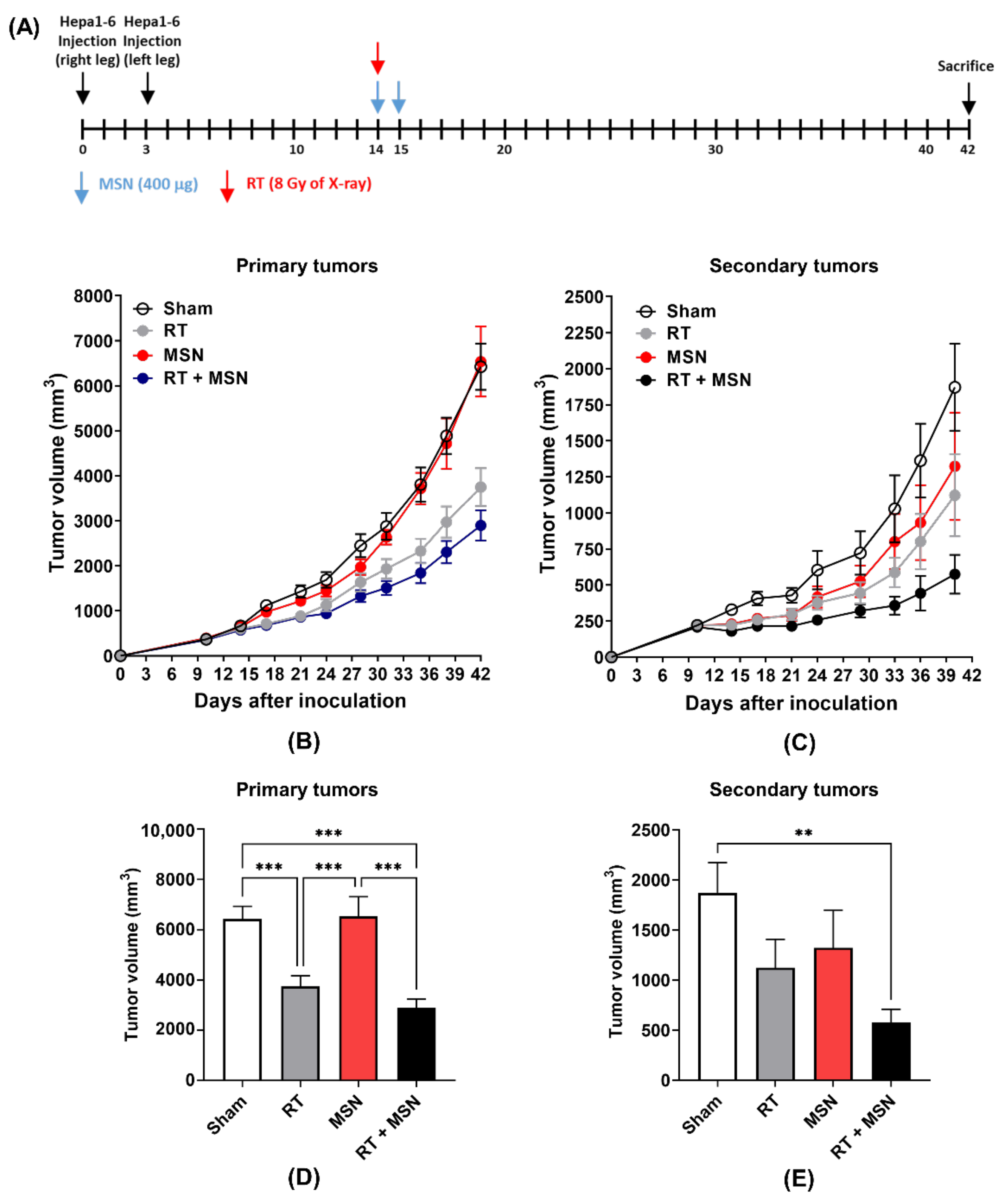
2.4. Measurement of Tumor Growth in Mice Co-Treated with MSNs and Radiation
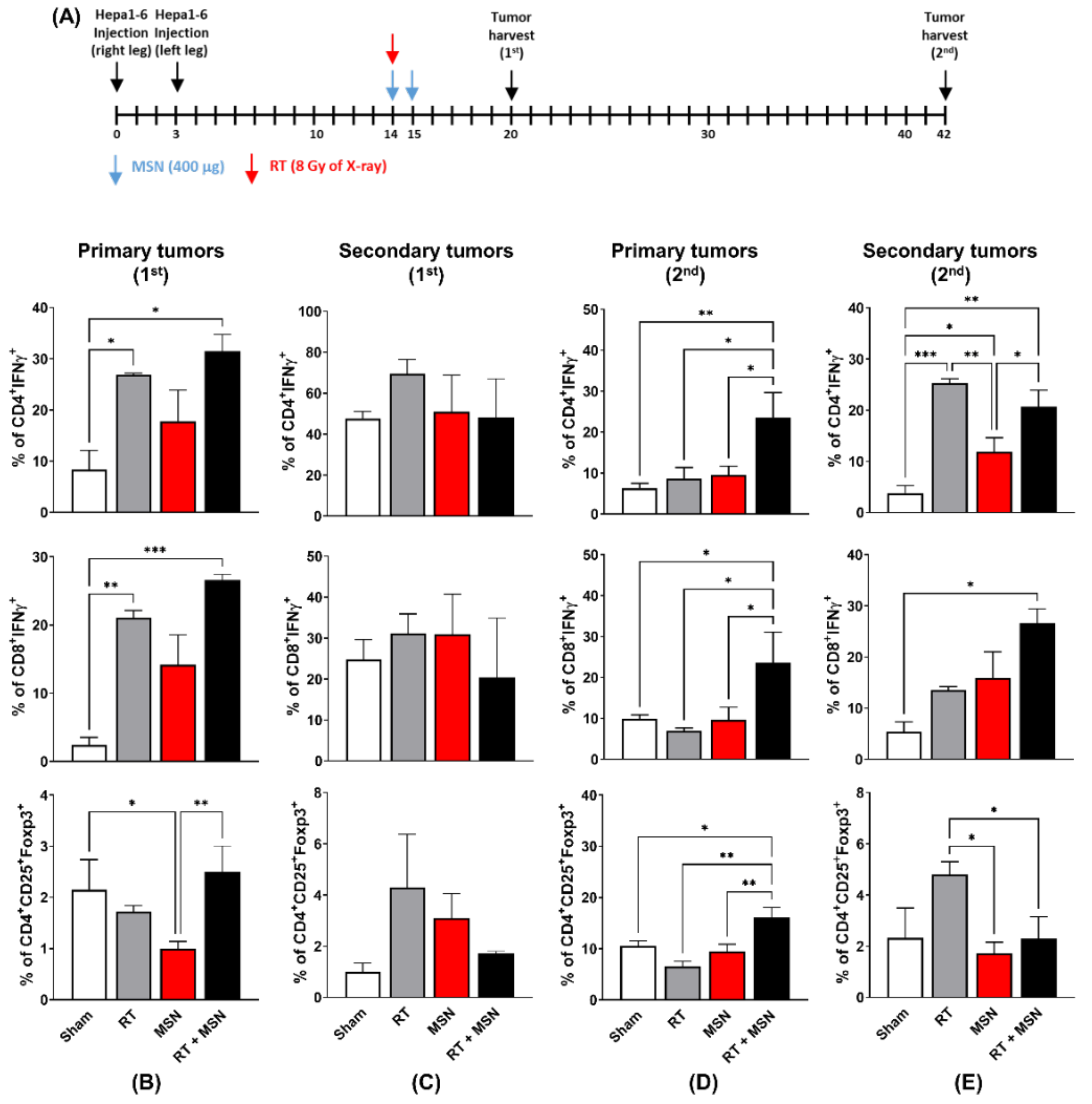
2.5. Flow Cytometry Analysis
2.6. Pre-Incubation of MSNs with Irradiated Cell Conditioned Medium
2.7. Cellular Uptake of MSNs
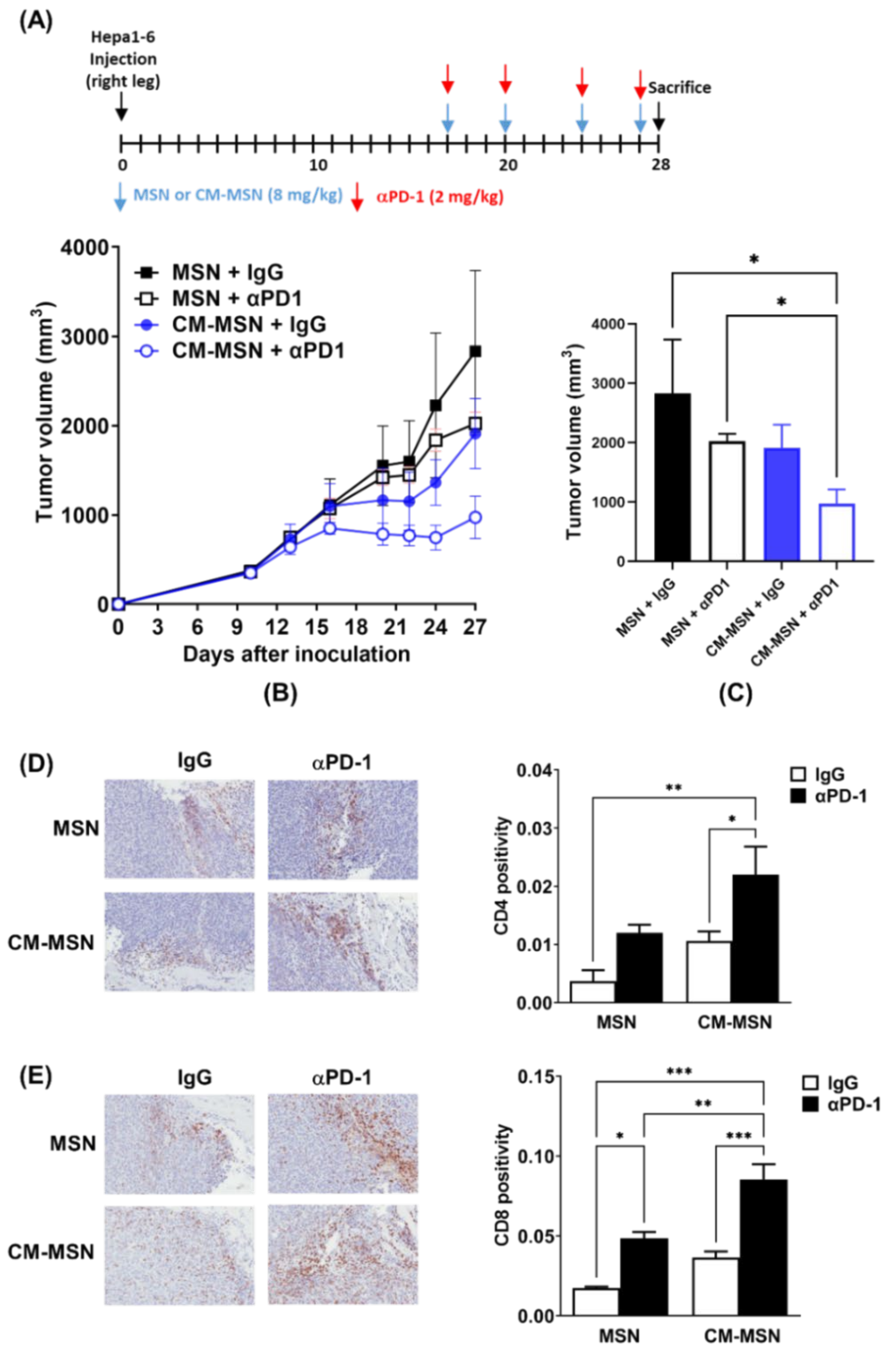
2.8. Measurement of Tumor Growth in Mice Co-Treated with CM-MSNs and Anti-PD1
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Characterization of MSNs
3.2. Effects of Intratumorally Injected MSNs on Radiation-Induced Abscopal Tumor Growth
3.3. Effects of Intratumorally Injected MSNs on Tumor-Infiltrating Lymphocytes
3.4. Effects of MSNs on Dendritic Cell Activation
3.5. Effects of CM-MSNs on Hepa1-6 Tumor Growth in a Syngeneic Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Khan, A.A.; Liu, Z.-K.; Xu, X. Recent advances in immunotherapy for hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Lin, J.; Long, J.; Yang, X.; Yang, X.; Lu, X.; Sang, X.; Zhao, H. T lymphocytes in hepatocellular carcinoma immune microenvironment: Insights into human immunology and immunotherapy. Am. J. Cancer Res. 2020, 10, 4585–4606. [Google Scholar] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, G.S.; Cho, W.K.; Park, H.C. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2416–2429. [Google Scholar] [CrossRef]
- Yu, J.I.; Lee, S.J.; Lee, J.; Lim, H.Y.; Paik, S.W.; Yoo, G.S.; Choi, C.; Park, H.C. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med. 2019, 8, 6986–6994. [Google Scholar] [CrossRef]
- Yoo, G.S.; Ahn, W.-G.; Kim, S.-Y.; Kang, W.; Choi, C.; Park, H.C. Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study. Clin. Mol. Hepatol. 2021, 27, 144–156. [Google Scholar] [CrossRef]
- Lurje, I.; Werner, W.; Mohr, R.; Roderburg, C.; Tacke, F.; Hammerich, L. In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 650486. [Google Scholar] [CrossRef]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, S.; Chatterjee, S.; Das, J.; Sil, P.C. Nanoparticles as Smart Carriers for Enhanced Cancer Immunotherapy. Front. Chem. 2020, 8, 8. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.-C.; Park, S.P.; Moon, W.K.; et al. Uniform Mesoporous Dye-Doped Silica Nanoparticles Decorated with Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence Imaging, and Drug Delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Shin, S.-W.; Yang, K.; Lee, M.; Moon, J.; Son, A.; Kim, Y.; Choi, S.; Kim, D.-H.; Choi, C.; Lee, N.; et al. Manganese Ferrite Nanoparticles Enhance the Sensitivity of Hepa1-6 Hepatocellular Carcinoma to Radiation by Remodeling Tumor Microenvironments. Int. J. Mol. Sci. 2021, 22, 2637. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Yoshiyuki, K.; Watanabe, Y.; Sogo, Y.; Ohno, T.; Tsuji, N.M.; Ito, A. Comprehensive Mechanism Analysis of Mesoporous-Silica-Nanoparticle-Induced Cancer Immunotherapy. Adv. Health Mater. 2016, 5, 1169–1176. [Google Scholar] [CrossRef]
- Hong, X.; Zhong, X.; Du, G.; Hou, Y.; Zhang, Y.; Zhang, Z.; Gong, T.; Zhang, L.; Sun, X. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 2020, 6, eaaz4462. [Google Scholar] [CrossRef]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy. ACS Central Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Fehres, C.M.; Unger, W.W.J.; Egarcia-Vallejo, J.J.; Kooyk, Y.E. Understanding the Biology of Antigen Cross-Presentation for the Design of Vaccines Against Cancer. Front. Immunol. 2014, 5, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, G. Dendritic cells in cancer immunotherapy. Eur. J. Immunol. 2010, 40, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichon, T.; Kulach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comiskey, M.C.; Dallos, M.C.; Drake, C.G. Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr. Oncol. Rep. 2018, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.S.P.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2016, 39, 137–152. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.J. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front. Immunol. 2020, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.D.; Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, M.; Huang, Z.; Yu, J.; Meng, X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: A focus on the mechanisms, advances, and future challenges. J. Hematol. Oncol. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Accounts Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Iturrioz-Rodríguez, N.; A Correa-Duarte, M.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benezra, M.; Medina, O.P.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Choi, C.; Cho, H.; Ahn, W.-G.; Kim, S.-Y.; Shin, S.-W.; Kim, Y.; Jang, T.; Lee, N.; Park, H.C. Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models. Pharmaceutics 2021, 13, 1811. https://doi.org/10.3390/pharmaceutics13111811
Yang K, Choi C, Cho H, Ahn W-G, Kim S-Y, Shin S-W, Kim Y, Jang T, Lee N, Park HC. Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models. Pharmaceutics. 2021; 13(11):1811. https://doi.org/10.3390/pharmaceutics13111811
Chicago/Turabian StyleYang, Kyungmi, Changhoon Choi, Hayeong Cho, Won-Gyun Ahn, Shin-Yeong Kim, Sung-Won Shin, Yeeun Kim, Taekyu Jang, Nohyun Lee, and Hee Chul Park. 2021. "Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models" Pharmaceutics 13, no. 11: 1811. https://doi.org/10.3390/pharmaceutics13111811
APA StyleYang, K., Choi, C., Cho, H., Ahn, W.-G., Kim, S.-Y., Shin, S.-W., Kim, Y., Jang, T., Lee, N., & Park, H. C. (2021). Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models. Pharmaceutics, 13(11), 1811. https://doi.org/10.3390/pharmaceutics13111811





