Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors?
Abstract
:1. Introduction
1.1. Phytocannabinoids
1.2. The Endocannabinoid System
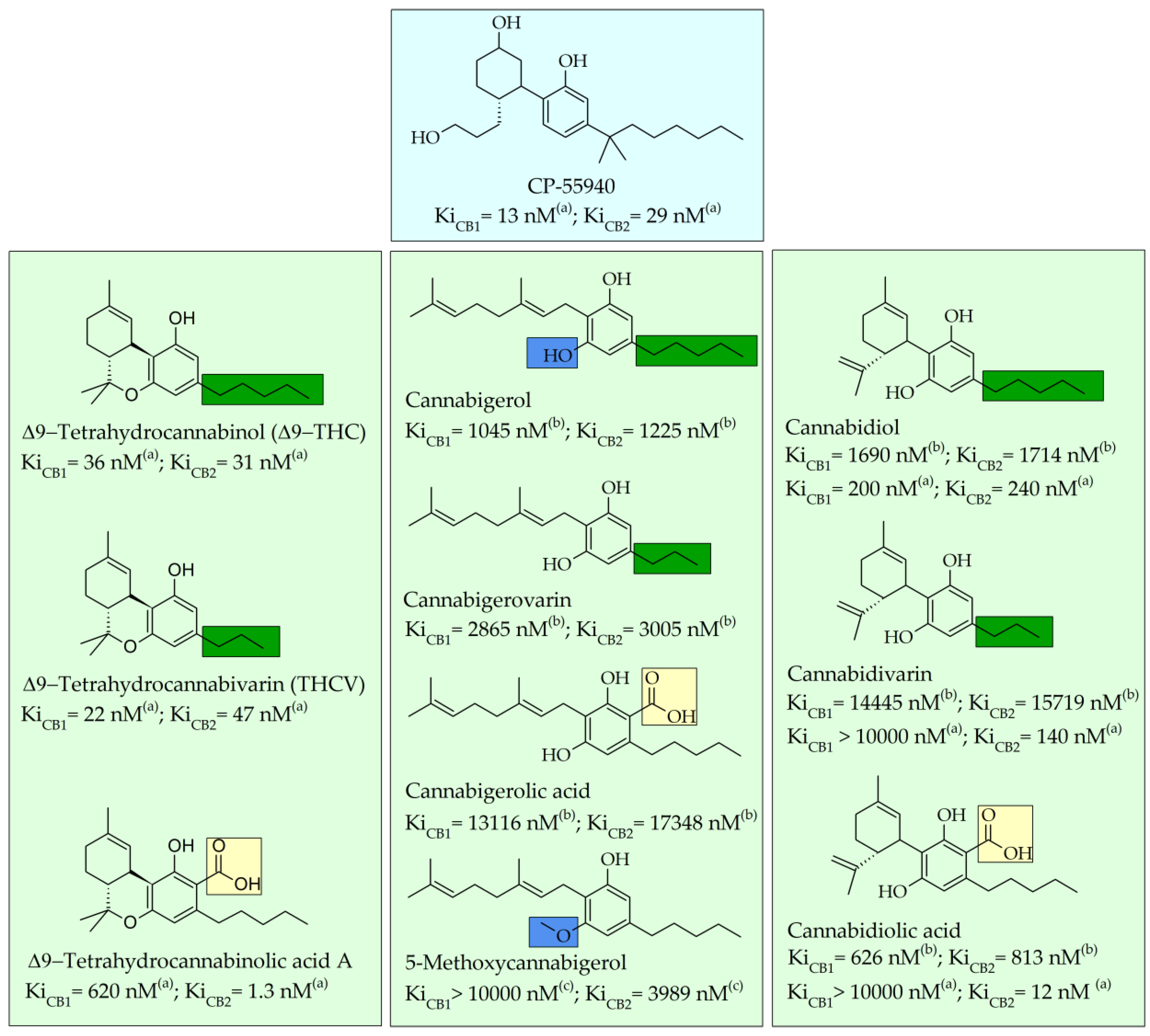
2. Major Phytocannabinoids: Cannabigerol-, Cannabidiol-, and Tetrahydrocannabinol-Type Compounds
2.1. Structure–Affinity Relationship of Cannabinoid Receptors
2.2. Pharmacological Effects
2.2.1. Cannabigerol (CBG)-Type Compounds
2.2.2. Cannabidiol (CBD)-Type Compounds
| Class | Compound | Experimental Model | Findings | Reference |
|---|---|---|---|---|
| CBG | CBG | Mouse model of intestinal inflammation induced with the intracolonic administration of DNBS | Anti-inflammatory effect associated with the downregulation of inflammatory cytokines interleukin-1β, interleukin-10, and interferon-γ and reduction in iNOS expression. | [77] |
| CBG CBGA CBGV | In vitro HEK-293 cells stably overexpressing rat recombinant TRPV3 or TRPV4 | CBGV and CBGA desensitize TRPV3 to the action of carvacrol at concentrations of EC50 = 0.8 and 7.4 µM. CBGV, CBGA, and CBG desensitize TRPV4 to the action of 4α-phorbol-12,13-didecanoate(4α-PDD) with EC50 values of 1.3–5.4 µM. These compounds desensitize TRPV3 and TRPV4 channels at lower doses than those at which they stimulate these channels. | [76] | |
| CBG CBGV | HEK-293 cells stably overexpressing human TRPV1 | CBG and CBGV stimulated and desensitized human TRPV1. | [72] | |
| CBG CBGA | COX-1 enzyme, purified from ram seminal vesicles and COX-2 enzyme, purified from sheep placental cotyledons | Inhibition of more than 30% of COX -1 and 292 COX -2 in a concentration-dependent manner. | [78] | |
| CBG | Computational model of α2A, α2B, and α2C isoforms of murine and human 304 adrenoceptors | Affinity for the receptor appeared higher than that of the α2-adrenergic receptor agonist clonidine. | [152] | |
| CBG | CBG | Mouse skin melanoma cells | Significant antitumor activity (inhibitory concentration (ICs0) = 31.31 gg/mL) in in vitro assay. | [117] |
| CBG | Human oral epithelioid carcinoma 308 cell lines (KB) and NIH 3T3 fibroblasts | CBG exhibited the highest growth inhibitory activity against the cancer cell lines. | [118] | |
| CBG | HEK-293 encoding the rat TRPM8 and overexpressing high levels of TRPM8 | Potent TRPM8 antagonist (IC50 = 0.16 ± 0.02). | [72] | |
| CBG | CBG | Two human colon adenocarcinoma cell lines (Caco-2 and HCT 116, ATCC); Mouse azoxymethane (AOM) model of colon carcinogenesis | CBG inhibits the growth of CRC cells mainly via a pro-apoptotic mechanism and hinders the development and the growth of colon carcinogenesis in vivo. | [111] |
| Mouse brain membranes | CBG activates α2-adrenoreceptors and blocks 5-HT1A receptors, antagonizing the 5-HT1A receptor agonist R-(+)-8-hydroxy-2-(di-n-propylamino) tetralin. | [86] | ||
| CBG | TMEV (Thaler’s murine encephalomyelitis virus)-induced demyelinating disease (TMEV-IDD) in SJL/J mice | Anti-inflammatory and neuroprotective effects through the inhibition of IL-1β and IL-6 cytokines, and downregulation of PGE2 synthesis. CBG and CBG-quinone inhibited the microglia inflammatory response, protected neurons from toxic insults. | [124] | |
| CBG | CBG | Mouse model of Huntington’s disease (HD), created using 3-Nitropropionate i.p. repeated administration | Neuroprotective effects by downregulating the proinflammatory markers COX-2, 367 iNOS, IL-6, and TNF-α, by preventing neuronal degradation, downregulating disease-associated genes SgKL and CD44, and normalizing specific protein-1 levels. | [125] |
| CBG | In vitro model of neuro inflammation on NSC-34 motor neurons | Pretreatment with CBG (7.5 μM) improved viability in treated cells through the inhibition of cell apoptosis, reduction in IL-1β, TNF-α, IFN-γ, and PPAR-γ proinflammatory protein levels, reduction in oxidative stress, and upregulation of Nrf-2 levels. | [74] | |
| CBG | MC65 human neuron-like cell lines treated to induce intra-neuronal Alzheimer’s disease cell alterations | CBG blocked cell death, reduced oxidative damage, and prevented neurons from accumulating toxic β-amyloid protein. | [126] | |
| CBG | Male Lister hooded rats | Doses between 120 and 140 mg/ kg of CBG induced a dose-dependent increase in food intake, increased the number of meals taken, decreased the latency until the first meal, and improved locomotor activity. | [128] | |
| CBG | Standard S. aureus strain (ATCC 25923) and a clinical isolate (XU212) MRSA strain | Antibacterial properties. | [110] | |
| Methicillin-resistant S. aureus 404 (MRSA) strain; murine systemic infection model caused by MRSA | In vitro disruption of the cytoplasmatic membrane of MRSA. In vivo efficacy against MRSA. | [129] | ||
| CBG | Keratinocyte proliferation assay | CBG had an inhibitory action on keratinocyte proliferation in a CB1/CB2 receptor-independent manner. | [130] | |
| Human keratinocytes (HaCaT cells) | CBG acted as a transcriptional repressor controlling cell proliferation and differentiation through a mechanism that involved increasing DNA methylation on the keratin-10 gene. | [131] | ||
| CBG CBGA | Human recombinant and pig kidney aldose reductase | Both compounds showed statistically significant ALR2 inhibitory activity by being able to interact with the major active site of the enzyme. | [79] | |
| CBG | CBG | HEK-293 cells stably overexpressing human TRPV1 | Stimulates and desensitizes TRPV1 channels with an of EC50 = 21.0 ± 1.25. | [72] |
| Colon cancer cells and normal colon cell lines | Cytotoxic activity on colon cancer cells, but reduced activity on normal colon cell lines. | [120] | ||
| CBGV | HEK-293 cells encoding the rat TRPV2 and expressing high levels of TRPV2 | Antagonizes TRPV2 channels with an EC50 = 1.7 μM. | [72] | |
| CBD | CBD | Murine (mouse) model of depression | CBD reduced immobility time in mice undergoing forced swimming test, the effect being similar to that produced by antidepressants such as imipramine. | [85] |
| CBD | Mouse model of autism spectrum disorders | 10–20 mg/kg acute administration of CBD determined an improvement in social behavior. | [89] | |
| CBD | Alzheimer’s disease mouse model | 20 mg/kg sub-chronic administration of CBD reversed cognitive deficits in object recognition memory and social recognition memory. | [153] | |
| CBD | PTSD determined by yohimbine HCl (Tocris) administration in Wistar rats | 10 mg/kg acute administration came with therapeutic benefits for post-traumatic stress disorder symptoms. | [154] | |
| CBD | Human breast cancer cell lines MDA-MB231 and MDA-MB436 | Significantly decreased Id-1 expression in metastatic breast cancer cells, leading to the downregulation of tumor aggressiveness. | [155] | |
| CBD | CBDV | HEK-293 cells stably overexpressing human TRPV1 HEK-293 cells encoding the rat TRPV2 and expressing high levels of TRPV2 HEK-293 cells over- expressingTRPA1 HEK-293 encoding the rat TRPM8 and overexpressing high levels of TRPM8 | Stimulates TRPV1 channels. Stimulates TRPV2 channels. Stimulates TRPA1 channels. Antagonizes TRPM8 channels. | [72] |
| hGPR55-HEK293 cells | Antagonizes GPR55 channels. | [148] | ||
| CBD | 43-day-old rats received d,l-AMPH (4 mg/kg, i.p.) or vehicle in the conditioned place preference (CPP) paradigm (8 days), when each experimental group was re-assigned to receive CBD at two different doses (5 or 10 mg/kg, i.p) or control, for 5 days | CBD treatment prevented amphetamine relapse behavior in rats that had previously exhibited amphetamine-conditioned place preference, modulated immunoreactivity of dopaminergic targets in the prefrontal cortex and ventral striatum, areas with major involvement in drug dependence. CBD maintains dopamine transport levels. | [27] | |
| CBD | Mouse genetic model of Dravet syndrome (DS) | CBD reduced the frequency, severity, and duration of spontaneous seizures through the antagonization of GPR55 receptors. | [89] | |
| Mecp2 mutant mice, a model of Rett syndrome (RTT) | CBDV rescues recognition memory deficits in Mecp2 mutant mice and delays the appearance of neurological defects. | [149] | ||
| Mouse model for Rett syndrome, caused by mutations in the MECP2 gene | CBDV proved to attenuate brain alterations, restore the compromised general status, increase sociability, and partially restore motor coordination in treated mice. Molecularly, CBDV has antagonistic properties on GPR55. | [144] | ||
| CBD | Double AD transgenic mouse model (APP/PS1) | CBD inhibited tau hyperphosphorylation and reduced Aβ production. | [26] | |
| CBD | CBD | Wistar rat model of neuropathic pain (Bennet and Xie’s NP model (1988)) | CBD modulates chronic neuropathic pain and depression-specific behavior by activating 5-HT1A and CB1 receptors in the prefrontal cortex. | [83] |
| CBDV | Autism-like behavior models through prenatal valproic acid exposure in rats | CBDV ameliorated behavioral abnormalities, restored hippocampal endocannabinoid signaling, and decreased neuroinflammation. | [149] | |
| CBDV | In vitro model of ischemic stroke obtained by exposing cells to ischemic conditions through oxygen–glucose deprivation | CBDV has neuroprotective and anti-inflammatory properties. | [151] | |
| CBDV | IBD mouse model of DNBS- and DSS-induced colitis | CBDV (orally or intraperitoneally) reduced the specific signs of colon inflammation–neutrophil infiltration, and increased colon weight and intestinal permeability. | [91] | |
| Human colonic tissues from children with active ulcerative colitis | In vitro treatment with CBGV produced a significant reduction in the proinflammatory cytokine levels (IL-1β). | |||
| CBDA | Mouse model of Dravet syndrome (Scn1aRX/+ mice) | CBDA exhibited significant anticonvulsant properties through a mechanism that could involve the 5-HT1A, GPR55, or TRPV1 receptors. | [136] | |
| Rodent models of carrageenan-induced inflammatory pain | I.p. administration of CBDA at 60 min before carrageenan produced anti-inflammatory and anti-hyperalgesia effects. | [135] | ||
| MDA-MB-231 breast cancer cell model | CBDA inhibited cell migration through a mechanism that is supposed to involve the activation of RhoA and through the inhibition of cAMP-dependent protein kinase A. | [94] | ||
| Rat models of acute lithium chloride-induced nausea | CBDA suppresses nausea and vomiting in rats through the activation of the serotonin 1A receptor (5-HT1A). | [92,93] | ||
| THC | Δ9-THC | Murine model of concanavalin A (ConA)-induced hepatitis | Intraperitoneal administration of THC inhibited hepatitis by significant decrease in liver enzymes and reduced liver tissue injury. THC treatment significantly suppressed inflammatory cytokines in ConA-induced hepatitis. | [156] |
| Δ9-THC | Splenocytes of C57BL/6 mice | In vitro THC treatment significantly reduced proliferative response to mitogens, including anti-CD3 monoclonal antibodies (mAbs), concanavalin A (Con A), and lipopolysaccharide (LPS). | [157] | |
| Δ9-THC | Sprague Dawley male rats | Δ9-THC therapy inhibited acetylcholinesterase, reduced amyloid-β levels and hippocampal neurogenesis, and induced brain-derived neurotrophic factor release through mixed CB1 and CB2 modulation. | [9,117] | |
| Δ9-THC | Genes encoding human, mouse, and rat TRPV2 | Δ9-THC is a potent TRPV2 agonist. | [101] | |
| Δ8-THC | Water-deprived albino rats | Groups treated with 5.0 and 10.0 mg/kg of Δ8-THC reduced intake of food at 1 day post-injection. | [158] | |
| THCV | Rat recombinant TRPV3- and TRPV4-expressing HEK-293 cells | Stimulates TRPV3 with high efficacy (50–70% of the effect of ionomycin) and potency (EC50 = 3.7 μM) and TRPV4 with moderate-high efficacy (30–60% of the effect of ionomycin) and potency (EC50 = 0.9–6.4 μM) [76]. | [76] | |
| Δ9-THCA | HEK-293T, Neuro-2a (N2a), STHdh Q7/Q7, and STHdh Q111/Q111 cells, which express either a wild-type or a mutated form of the huntingtin protein | Δ9-THCA activated PPARγ and increased mitochondrial mass in neuroblastoma N2a cells and prevented cytotoxicity induced by serum deprivation in STHdh Q111/Q111 cells and by mutHtt-q94 in N2a cells. Δ9-THCA showed potent neuroprotective activity, worth consideration for the treatment of Huntington’s disease and possibly other neurodegenerative and neuroinflammatory diseases. | [104] | |
| Δ9-THCA-A | Mouse model of HFD significantly induced obesity | Administration of Δ9-THCA-A reduced fat mass and body weight gain, markedly ameliorating glucose intolerance and insulin resistance, and largely preventing liver steatosis, adipogenesis, and macrophage infiltration in fat tissues. | [159] |
| Class | Compounds | Clinical Study | Results | Reference |
|---|---|---|---|---|
| CBD THC | CBD THC THC + CBD | A 4-way, double-blind, placebo-controlled crossover design study in cannabis users. 48 volunteers, CBD (16 mg), THC (8 mg), THC + CBD (8 mg + 16 mg), and placebo, by inhalation. | CBD improved emotional facial affect recognition at 60% emotional intensity. THC was detrimental to the recognition of ambiguous faces of 40% intensity. THC alone and combined THC+CBD equally increased feelings of being “stoned”. | [145] |
| CBD | CBD | Double-blind, placebo-controlled trial. 120 children and young adults with the Dravet syndrome and drug-resistant seizures, CBD oral solution, 20 mg/kg of body weight/day or placebo, in addition to standard antiepileptic treatment. | The median frequency of convulsive seizures per month decreased from 12.4 to 5.9 with cannabidiol, as compared with a decrease from 14.9 to 14.1 with placebo. The percentage of patients who had at least a 50% reduction in convulsive seizure frequency was 43% with cannabidiol and 27% with placebo. | [160] |
| CBD | Double-blind, randomized clinical trial in 199 children with Dravet syndrome on cannabidiol (10 or 20 mg/kg/d) or matched placebo for 14 weeks. | Convulsive seizure frequency compared with baseline was reduced by 48.7% in the 10 mg/kg/d cannabidiol group and 45.7% in the 20 mg/kg/d cannabidiol group vs. 26.9% in the placebo group. | [161] | |
| CBD | Double-blind, placebo-controlled trial conducted at 30 clinical centers; we randomly assigned patients with Lennox–Gastaut syndrome. 225 patients were enrolled; 76 patients were assigned to the 20 mg cannabidiol group, 73 to the 10 mg cannabidiol group, and 76 to the placebo group. | The median percent reduction from baseline in drop seizure frequency during the treatment period was 41.9% in the 20 mg cannabidiol group, 37.2% in the 10 mg cannabidiol group, and 17.2% in the placebo group. | [162] | |
| CBD | Double-blind, placebo-controlled, randomized crossover trial in 39 healthy young subjects. A single dose of cannabidiol e-liquid (0.25 mL, 5% cannabidiol, 12.5 mg cannabidiol) and once placebo for vaping after learning 15 unrelated nouns. | Cannabidiol enhanced verbal episodic memory performance (placebo: 7.03 [2.34]; cannabidiol 7.71 [2.48]). | [163] | |
| CBDV | Case–control, placebo-controlled, randomized, double-blind, repeated-measures, crossover study on 34 subjects with autism spectrum disorder. | CBDV shifts subcortical levels of the brain’s primary excitatory metabolite glutamate both in the neurotypical and autistic brain; however, there may be significant response variability in ASD. | [142] |
2.2.3. Tetrahydrocannabinol (THC)-Type Compounds
3. Structure Modulation to Obtain New Pharmacological Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Abel, E.L.; Emboden, W. Marihuana: The First Twelve Thousand Years. Q Rev. Biol. 1981, 56, 514. [Google Scholar]
- Tamba, B.I.; Stanciu, G.D.; Urîtu, C.M.; Rezus, E.; Stefanescu, R.; Mihai, C.T.; Luca, A.; Rusu-zota, G.; Leon-constantin, M.M.; Cojocaru, E.; et al. Challenges and Opportunities in Preclinical Research of Synthetic Cannabinoids for Pain Therapy. Medicina 2020, 56, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgeman, I.; Bar-Sela, G. Cannabis for cancer–illusion or the tip of an iceberg: A review of the evidence for the use of Cannabis and synthetic cannabinoids in oncology. Expert Opin. Investig. Drugs 2019, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Farag, S.; Kayser, O. The Cannabis Plant: Botanical Aspects. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 3–12. ISBN 9780128008270. [Google Scholar]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.A.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 103, pp. 1–36. [Google Scholar]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Hively, R.L.; Mosher, W.A.; Hoffmann, F.W. Isolation of trans-Δ 6 -tetrahydrocannabinol from marijuana. J. Am. Chem. Soc. 1966, 88, 1832–1833. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The structure and synthesis of cannabigerol, a new hashish constituent. Proc. Chem. Soc. 1964, 82, 82. [Google Scholar]
- Gaoni, Y.; Mechoulam, R. Cannabichromene, a new active principle in hashish. Chem. Commun. 1966, 1, 20–21. [Google Scholar] [CrossRef]
- Ghosh, R.; Todd, A.R.; Wilkinson, S. Cannabis indica. Part V. The synthesis of cannabinol. J. Chem. Soc. 1940, 1, 1393–1396. [Google Scholar] [CrossRef]
- Adams, R.; Pease, D.C.; Clark, J.H. Isolation of cannabinol, cannabidiol and quebrachitol from red oil of Minnesota Wild Hemp. J. Am. Chem. Soc. 1940, 62, 2194–2196. [Google Scholar] [CrossRef]
- Ginneken, C.; Vree, T.; Breimer, D.; Thijssen, H.; Rossum, J. Cannabinodiol, a new hashish consituent, identified by gaschromatography-mass spectrometry. In Proceedings of the International Symposium on Gas Chromatografy Mass Spectrometry, Isle Elba, Italy, 17–19 May 1972; pp. 111–129. [Google Scholar]
- Robert, J.J.C.; Ludwig Bercht, C.A.; van Ooyen, R.; Spronck, H.J.W. Cannabinodiol: Conclusive identification and synthesis of a new cannabinoid from Cannabis sativa. Phytochemistry 1977, 16, 595–597. [Google Scholar] [CrossRef]
- Korte, F.; Sieper, H. Zur chemischen klassifizierung von pflanzen. XXIV. Untersuchung von Haschisch-Inhaltsstoffen durch Dünnschichtchromatographie. J. Chromatogr. A 1964, 13, 90–98. [Google Scholar] [CrossRef]
- Crombie, L.; Ponsford, R.; Shani, A.; Yagnitinsky, B.; Mechoulam, R. Hashish components. Photochemical production of cannabicyclol from cannabichromene. Tetrahedron Lett. 1968, 9, 5771–5772. [Google Scholar] [CrossRef]
- Bercht, C.A.L.; Lousberg, R.J.J.; Küppers, F.J.E.M.; Salemink, C.A.; Vree, T.B.; Van Rossum, J.M. Cannabis. VII. Identification of cannabinol methyl ether from hashish. J. Chromatogr. A 1973, 81, 163–166. [Google Scholar] [CrossRef]
- Chan, W.R.; Magnus, K.E.; Watson, H.A. The structure of cannabitriol. Experientia 1976, 32, 283–284. [Google Scholar] [CrossRef]
- Friedrich-Fiechtl, J.; Spiteller, G. Neue cannabinoide-1. Tetrahedron 1975, 31, 479–487. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Deiana, S. Potential Medical Uses of Cannabigerol: A Brief Overview. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 958–967. ISBN 9780128008270. [Google Scholar]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Pina, S.D.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef]
- Metz, V.G.; da Rosa, J.L.O.; Rossato, D.R.; Milanesi, L.H.; Burger, M.E.; Pase, C.S. Cannabidiol prevents amphetamine relapse and modulates D1- and D2-receptor levels in mesocorticolimbic brain areas of rats. Eur. Neuropsychopharmacol. 2021, 50, 23–33. [Google Scholar] [CrossRef]
- Friedman, L.K.; Wongvravit, J.P. Anticonvulsant and neuroprotective effects of cannabidiol during the juvenile period. J. Neuropathol. Exp. Neurol. 2018, 77, 904–919. [Google Scholar] [CrossRef]
- Hawes, E.M.; Lee, C.R.; Brackney, D.E.; Ensley, T.G.; Kidd, J.; Page, C. Cannabidiol Products: Review of the Regulatory and Clinical Considerations. J. Nurse Pract. 2020, 16, 747–755. [Google Scholar] [CrossRef]
- Martínez-Peña, A.A.; Perono, G.A.; Gritis, S.A.; Sharma, R.; Selvakumar, S.; Walker, O.S.; Gurm, H.; Holloway, A.C.; Raha, S. The impact of early life exposure to cannabis: The role of the endocannabinoid system. Int. J. Mol. Sci. 2021, 22, 8576. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Academic Press Inc.: Oxford, UK, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Hall, W.; MacDonald, C.; Currow, D. Cannabinoids and cancer: Causation, remediation, and palliation. Lancet. Oncol. 2005, 6, 35–42. [Google Scholar] [CrossRef]
- Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Bifulco, M. The endocannabinoid signaling system in cancer. Trends Pharmacol. Sci. 2013, 34, 273–282. [Google Scholar] [CrossRef]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, S.H.; Zurier, R.B. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009, 11, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The role of the endocannabinoid system in pain. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar] [PubMed]
- Taylor, A.H.; Ang, C.; Bell, S.C.; Konje, J.C. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum. Reprod. Update 2007, 13, 501–513. [Google Scholar] [CrossRef]
- Gillies, R.; Lee, K.; Vanin, S.; Laviolette, S.R.; Holloway, A.C.; Arany, E.; Hardy, D.B. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod. Toxicol. 2020, 94, 84–91. [Google Scholar] [CrossRef]
- Sun, X.; Dey, S.K. Endocannabinoid Signalingin Female Reproduction. ACS Chem. Neurosci. 2012, 3, 349. [Google Scholar] [CrossRef] [Green Version]
- Warshak, C.R.; Regan, J.; Moore, B.; Magner, K.; Kritzer, S.; Van Hook, J. Association between marijuana use and adverse obstetrical and neonatal outcomes. J. Perinatol. 2015, 35, 991–995. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid system components: Overview and tissue distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12. [Google Scholar]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Doyle, M.E.; Liu, Q.R.; Cimbro, R.; Santa-Cruz Calvo, S.; Ghosh, S.; Cieala, A.; Moaddel, R.; Carlson, O.D.; et al. Human CB1 receptor isoforms, present in hepatocytes and β-cells, are involved in regulating metabolism. Sci. Rep. 2016, 6, 33302. [Google Scholar] [CrossRef] [Green Version]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodríguez De Fonseca, F.; et al. Cannabinoid CB 1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Trembovler, V.; Di Marzo, V.; Petrosino, S.; Leo, G.; Alexandrovich, A.; Regev, A.; Casap, N.; Shteyer, A.; Ledent, C.; et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008, 22, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.R.; Pan, C.H.; Hishimoto, A.; Li, C.Y.; Xi, Z.X.; Llorente-Berzal, A.; Viveros, M.P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Bi, G.H.; Li, X.; Li, J.; Qu, H.; Zhang, S.J.; Li, C.Y.; Onaivi, E.S.; Gardner, E.L.; Xi, Z.X.; et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 2015, 40, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021, 6, 607–615. [Google Scholar]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Ababei, D.C.; Bild, V.; Bild, W.; Paduraru, L.; Gutu, M.M.; Tamba, B.-I. Renal contributions in the pathophysiology and neuropathological substrates shared by chronic kidney disease and Alzheimer’s disease. Brain Sci. 2020, 10, 563. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agró, A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003, 10, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 51–56. [Google Scholar] [CrossRef]
- Nicolussi, S.; Gertsch, J. Endocannabinoid transport revisited. Vitam. Horm. 2015, 98, 441–485. [Google Scholar]
- Manz, K.M.; Ghose, D.; Turner, B.D.; Taylor, A.; Becker, J.; Grueter, C.A.; Grueter, B.A. Calcium-Permeable AMPA Receptors Promote Endocannabinoid Signaling at Parvalbumin Interneuron Synapses in the Nucleus Accumbens Core. Cell Rep. 2020, 32, 107971. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Phil. Trans. R. Soc. B 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 101, 20405. [Google Scholar]
- Linciano, P.; Citti, C.; Luongo, L.; Belardo, C.; Maione, S.; Vandelli, M.A.; Forni, F.; Gigli, G.; Laganà, A.; Montone, C.M.; et al. Isolation of a high-affinity cannabinoid for the human CB1 receptor from a medicinal Cannabis sativa variety: Δ9-tetrahydrocannabutol, the butyl homologue of Δ9-tetrahydrocannabinol. J. Nat. Prod. 2020, 83, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Husni, A.S.; McCurdy, C.R.; Radwan, M.M.; Ahmed, S.A.; Slade, D.; Ross, S.A.; ElSohly, M.A.; Cutler, S.J. Evaluation of phytocannabinoids from high-potency Cannabis sativa using in vitro bioassays to determine structure–activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Med. Chem. Res. 2014, 23, 4295–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthaler, S.; Pöhn, B.; Kolmanz, C.; Nguyen Huu, C.; Krewenka, C.; Huber, A.; Kranner, B.; Rausch, W.D.; Moldzio, R. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 2014, 46, 49–56. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Marzo, V.D. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, R.L.; Salgado-Rohner, C.J.; Hallak, J.E.C.; De Souza Crippa, J.A.; Coimbra, N.C. Involvement of prelimbic medial prefrontal cortex in panic-like elaborated defensive behaviour and innate fear-induced antinociception elicited by GABAA receptor blockade in the dorsomedial and ventromedial hypothalamic nuclei: Role of the endocannabinoid. Int. J. Neuropsychopharmacol. 2013, 16, 1781–1798. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef] [Green Version]
- Lopatriello, A.; Caprioglio, D.; Minassi, A.; Schiano Moriello, A.; Formisano, C.; De Petrocellis, L.; Appendino, G.; Taglialatela-Scafati, O. Iodine-mediated cyclization of cannabigerol (CBG) expands the cannabinoid biological and chemical space. Bioorg. Med. Chem. 2018, 26, 4532–4536. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Orlando, P.; Schiano Moriello, A.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Giofrè, S.V.; Galati, E.M.; Monforte, M.T.; Cicero, N.; D’Angelo, V.; Grassi, G.; Circosta, C. Inhibition of aldose reductase activity by Cannabis sativa chemotypes extracts with high content of cannabidiol or cannabigerol. Fitoterapia 2018, 127, 101–108. [Google Scholar] [CrossRef]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Santoni, G.; Maggi, F.; Morelli, M.B.; Santoni, M.; Marinelli, O. Transient Receptor Potential Cation Channels in Cancer Therapy. Med. Sci. 2019, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Urits, I.; Gress, K.; Charipova, K.; Habib, K.; Lee, D.; Lee, C.; Jung, J.W.; Kassem, H.; Cornett, E.; Paladini, A.; et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 463–477. [Google Scholar] [CrossRef]
- Malvestio, R.B.; Medeiros, P.; Negrini-Ferrari, S.E.; Oliveira-Silva, M.; Medeiros, A.C.; Padovan, C.M.; Luongo, L.; Maione, S.; Coimbra, N.C.; de Freitas, R.L. Cannabidiol in the prelimbic cortex modulates the comorbid condition between the chronic neuropathic pain and depression-like behaviour in rats: The role of medial prefrontal cortex 5-HT1A and CB1 receptors. Brain Res. Bull. 2021, 174, 323–338. [Google Scholar] [CrossRef]
- Joca, S.; Silote, G.P.; Sartim, A.; Sales, A.; Guimarães, F.; Wegener, G. Putative effects of cannabidiol in depression and synaptic plasticity. In The Neuroscience of Depression; Elsevier: Amsterdam, The Netherlands, 2021; pp. 459–467. [Google Scholar]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT 1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link between diabetes and Alzheimer’s disease due to the shared amyloid aggregation and deposition involving both neurodegenerative changes and neurovascular damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [Green Version]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.-H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Bolognini, D.; Rock, E.M.; Cluny, N.L.; Cascio, M.G.; Limebeer, C.L.; Duncan, M.; Stott, C.G.; Javid, F.A.; Parker, L.A.; Pertwee, R.G. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 2013, 168, 1456–1470. [Google Scholar] [CrossRef] [Green Version]
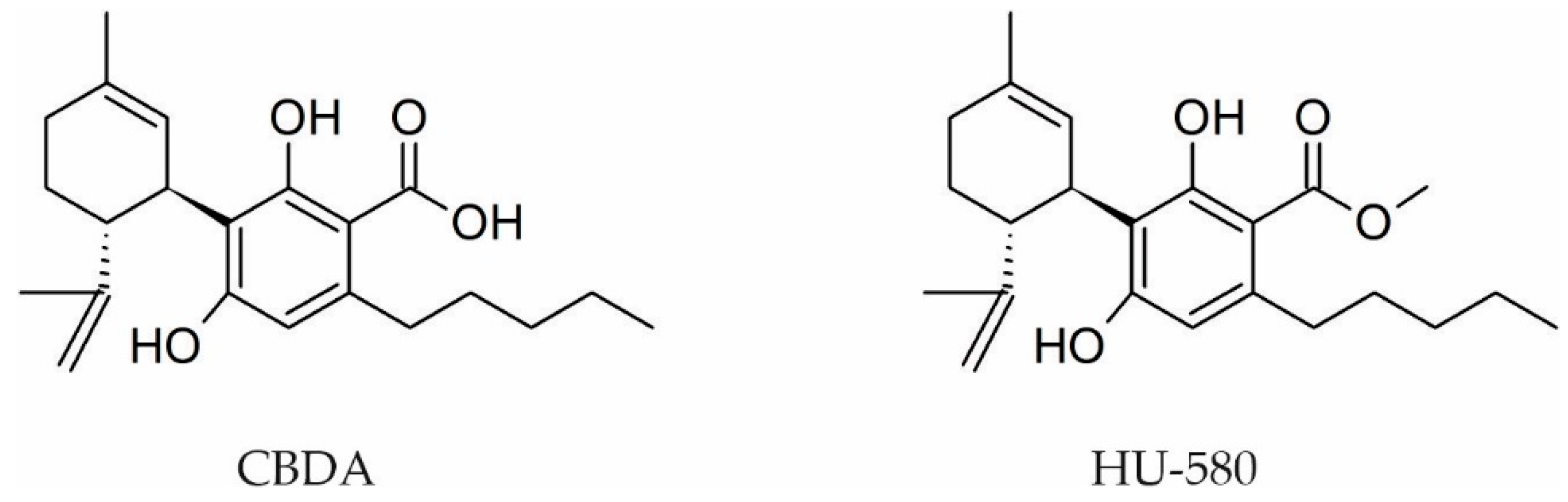
- Rock, E.M.; Sullivan, M.T.; Collins, S.A.; Goodman, H.; Limebeer, C.L.; Mechoulam, R.; Parker, L.A. Evaluation of repeated or acute treatment with cannabidiol (CBD), cannabidiolic acid (CBDA) or CBDA methyl ester (HU-580) on nausea and/or vomiting in rats and shrews. Psychopharmacology 2020, 237, 2621–2631. [Google Scholar] [CrossRef]
- Takeda, S.; Okajima, S.; Miyoshi, H.; Yoshida, K.; Okamoto, Y.; Okada, T.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; Aramaki, H. Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration. Toxicol. Lett. 2012, 214, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Δ9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Butovsky, E.; Juknat, A.; Goncharov, I.; Elbaz, J.; Eilam, R.; Zangen, A.; Vogel, Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Δ9-tetrahydrocannabinol. J. Neurochem. 2005, 93, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Lafenêtre, P. Roles of the Endocannabinoid System in Learning and Memory. Curr. Top. Behav. Neurosci. 2009, 1, 201–230. [Google Scholar] [PubMed]
- Sido, J.M.; Yang, X.; Nagarkatti, P.S.; Nagarkatti, M. Δ9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J. Leukoc. Biol. 2015, 97, 677–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eubanks, L.M.; Rogers, C.J.; Beuscher, A.E.; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 2006, 3, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Li, Y.; Liu, H.; Bai, G.; Mayl, J.; Lin, X.; Sutherland, K.; Nabar, N.; Cai, J. The potential therapeutic effects of THC on Alzheimer’s disease. J. Alzheimers Dis 2014, 42, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Wang, Y.; Flores, C.M.; Qin, N. Activation Properties of Heterologously Expressed Mammalian TRPV2. J. Biol. Chem. 2007, 282, 15894–15902. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Marzo, V. Di Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef]
- Showalter, V.M.; Compton, D.R.; Martin, B.R.; Abood, M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): Identification of cannabinoid receptor subtype selective ligands. J. Pharmacol Exp. Ther 1996, 8, 989–999. [Google Scholar]
- Nadal, X.; Río, C.; del Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sánchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S.; et al. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef] [Green Version]
- Grunfeld, Y.; Ei, H. Psychopharmacological activity of the active constituents of hashish and some related cannabinoids. Psychopharmacologia 1969, 14, 200–210. [Google Scholar] [CrossRef]
- Zirpel, B.; Degenhardt, F.; Martin, C.; Kayser, O.; Stehle, F. Engineering yeasts as platform organisms for cannabinoid biosynthesis. J. Biotechnol. 2017, 259, 204–212. [Google Scholar] [CrossRef]
- Shoyama, Y.; Yagi, M.; Nishioka, I.; Yamauchi, T. Biosynthesis of cannabinoid acids. Phytochemistry 1975, 14, 2189–2192. [Google Scholar] [CrossRef]
- Shoyama, Y.; Hirano, H.; Makino, H.; Umekita, N. The isolation and structure of four new propyl cannabis acids, tetrahydrocannabivarinic acid, cannabidivarinic acid, cannabichromevarinic acid and cannabigerovarinic acid from Thai Cannabis, ‘Meao Variant’. Chem. Pharm. Bull. 1977, 25, 2306–2311. [Google Scholar] [CrossRef] [Green Version]
- Elsohly, H.N.; Turner, C.E.; Clark, A.M.; Elsohly, M.A. Synthesis and Antimicrobial Activities of Certain Cannabichromene and Cannabigerol Related Compounds. J. Pharm. Sci. 1982, 71, 1319–1323. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, P.G.; Preti, D.; Materazzi, S.; Geppetti, P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J. Med. Chem. 2010, 53, 5085–5107. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Morita, T.; Mitsuyama, K.; Yamasaki, H.; Mori, A.; Yoshimura, T.; Araki, T.; Morita, M.; Tsuruta, K.; Yamasaki, S.; Kuwaki, K.; et al. Gene expression of transient receptor potential channels in peripheral blood mononuclear cells of inflammatory bowel disease patients. J. Clin. Med. 2020, 9, 2643. [Google Scholar] [CrossRef]
- International Cannabinoid Research Society. 22nd Annual Symposium of the International Cannabinoid Research Society; International Cannabinoid Research Society: Freiburg im Breisgau, Germany, 2012; ISBN 9780965805353. [Google Scholar]
- Baek, S.-H.; Seok Han, D.; Nam Yook, C.; Chae Kim, Y.; Suk Kwak, J. Synthesis and antitumor activity of cannabigerol. Arch. Pharm. Res. 1996, 19, 228–230. [Google Scholar] [CrossRef]
- Hwa Baek, S.; Ok Kim, Y.; Suk Kwag, J.; Eun Choi, K.; Young Jung, W.; Seok Han, D. Boron Trifluoride Etherate on Silica-A Modified Lewis Acid Reagent (VII). In Antitumor Activity of Cannabigerol Against Human Oral Epitheloid Carcinoma Cells; Bentham Science Publishers: Sharjah, United Arab Emirates, 1998; Volume 21. [Google Scholar]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, cancer and immunity—implication of TRPV1 channel. Front. Oncol. 2019, 9, 1087–1103. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of synergistic interaction between cannabis-derived compounds for cytotoxic activity in colorectal cancer cell lines and colon polyps that induces apoptosis-related cell death and distinct gene expression. Cannabis Cannabinoid Res. 2018, 3, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kössl, M.; Holsboer, F.; Zieglgänsberger, W.; Landgraf, R.; Lutz, B.; Wotjak, C.T. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J. Neurosci. 2007, 27, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Di Marzo, V.; Berretta, N.; Matias, I.; Maccarrone, M.; Bernardi, G.; Mercuri, N.B. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J. Neurosci. 2003, 23, 3136–3144. [Google Scholar] [CrossRef] [Green Version]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Sagredo, O. Neuroprotective properties of cannabigerol in Huntington’s disease: Studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics 2015, 12, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Schubert, D.; Kepchia, D.; Liang, Z.; Dargusch, R.; Goldberg, J.; Maher, P. Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 7719–7730. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Rusu, R.N.; Bild, V.; Filipiuc, L.E.; Tamba, B.I.; Ababei, D.C. Systemic actions of SGLT2 inhibition on chronic mTOR activation as a shared pathogenic mechanism between alzheimer’s disease and diabetes. Biomedicines 2021, 9, 576. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef] [Green Version]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; Macnair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzi, J.F.C.; Sales, A.J.; Guimarães, F.S.; Joca, S.R.L.; Crippa, J.A.S.; Del Bel, E. Cannabidiol prevents disruptions in sensorimotor gating induced by psychotomimetic drugs that last for 24-h with probable involvement of epigenetic changes in the ventral striatum. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110352. [Google Scholar] [CrossRef]
- Khan, A.A.; Shekh-Ahmad, T.; Khalil, A.; Walker, M.C.; Ali, A.B. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br. J. Pharmacol. 2018, 175, 2097–2115. [Google Scholar] [CrossRef] [Green Version]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of cannabidiolic acid and ∆9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. [Google Scholar] [CrossRef]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef] [Green Version]
- Vollner, L.; Bieniek, D.; Korte, F. Cannabidivarin, a new hashish constituent. Tetrahedron Lett. 1969, 3, 145–147. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [Green Version]
- Shrader, S.H.; Tong, Y.-G.; Duff, M.B.; Freedman, J.H.; Song, Z.-H. Involvement of dopamine receptor in the actions of non-psychoactive phytocannabinoids. Biochem. Biophys. Res. Commun. 2020, 533, 1366–1370. [Google Scholar] [CrossRef]
- Martin, L.J.; Banister, S.D.; Bowen, M.T. Understanding the complex pharmacology of cannabidiol: Mounting evidence suggests a common binding site with cholesterol. Pharmacol. Res. 2021, 166, 105508. [Google Scholar] [CrossRef]
- Sylantyev, S.; Jensen, T.P.; Ross, R.A.; Rusakov, D.A. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. USA 2013, 110, 5193–5198. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, C.M.; Voinescu, B.; Lythgoe, D.; Horder, J.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; Edden, R.A.E.; et al. Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): A single dose trial during magnetic resonance spectroscopy. Transl. Psychiatry 2019, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Zamberletti, E.; Gabaglio, M.; Piscitelli, F.; Brodie, J.S.; Woolley-Roberts, M.; Barbiero, I.; Tramarin, M.; Binelli, G.; Landsberger, N.; Kilstrup-Nielsen, C.; et al. Cannabidivarin completely rescues cognitive deficits and delays neurological and motor defects in male Mecp2 mutant mice. J. Psychopharmacol. 2019, 33, 894–907. [Google Scholar] [CrossRef]
- Vigli, D.; Cosentino, L.; Raggi, C.; Laviola, G.; Woolley-Roberts, M.; De Filippis, B. Chronic treatment with the phytocannabinoid Cannabidivarin (CBDV) rescues behavioural alterations and brain atrophy in a mouse model of Rett syndrome. Neuropharmacology 2018, 140, 121–129. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Schafer, G.; Gardener, C.; Das, R.K.; Morgan, C.J.A.; Curran, H.V. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 2015, 25, 325–334. [Google Scholar] [CrossRef] [Green Version]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, V.; Bialer, M.; Perucca, E. Cannabidiol in the treatment of epilepsy: Current evidence and perspectives for further research. Neuropharmacology 2021, 185, 108442. [Google Scholar] [CrossRef] [PubMed]
- Anavi-Goffer, S.; Baillie, G.; Irving, A.J.; Gertsch, J.; Greig, I.R.; Pertwee, R.G.; Ross, R.A. Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J. Biol. Chem. 2012, 287, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin treatment ameliorates Autism-like behaviors and restores hippocampal endocannabinoid system and glia alterations induced by prenatal valproic acid exposure in rats. Front. Cell. Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, S.; Barker, V.D.; Adams, A.A. Effects of cannabidiol on the in vitro lymphocyte pro-inflammatory cytokine production of senior horses. J. Equine Vet. Sci. 2021, 103, 103668. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. Protective effects of cannabidivarin and cannabigerol on cells of the blood–brain barrier under ischemic conditions. Cannabis Cannabinoid Res. 2021, 6, 315–326. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Gazarini, L.; Stern, C.A.J.; Piornedo, R.R.; Takahashi, R.N.; Bertoglio, L.J. PTSD-Like Memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. Int. J. Neuropsychopharmacol. 2015, 18, pyu026. [Google Scholar] [CrossRef]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef] [Green Version]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharmacol. 2008, 74, 20–33. [Google Scholar] [CrossRef]
- McKallip, R.J.; Lombard, C.; Martin, B.R.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J. Pharmacol. Exp. Ther. 2002, 302, 451–465. [Google Scholar] [CrossRef]
- Jarbe, T.U.C.; Henriksson, B.G. Acute effects of two tetrahydrocannabinols (Δ9-THC and Δ8-THC) on water intake in water deprived rats: Implications for behavioral studies on marijuana compounds. Psychopharmacologia 1973, 30, 315–322. [Google Scholar] [CrossRef]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [Green Version]
- Hotz, J.; Fehlmann, B.; Papassotiropoulos, A.; de Quervain, D.J.; Schicktanz, N.S. Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. J. Psychiatr. Res. 2021, 143, 327–333. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Alghetaa, H.; Sultan, M.; Singh, N.P.; Nagarkatti, P.; Nagarkatti, M. Administration of Δ9-tetrahydrocannabinol (THC) post-staphylococcal enterotoxin B exposure protects mice from acute Respiratory Distress syndrome and toxicity. Front. Pharmacol. 2020, 11, 893. [Google Scholar] [CrossRef]
- Suliman, N.A.; Taib, C.N.M.; Moklas, M.A.M.; Basir, R. Delta-9-tetrahydrocannabinol (∆9-THC) induce neurogenesis and improve cognitive performances of male Sprague Dawley rats. Neurotox. Res. 2018, 33, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.R.; Ali, D.W. Pharmacology of medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar]
- Boggs, D.L.; Nguyen, J.D.; Morgenson, D.; Taffe, M.A.; Ranganathan, M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar] [PubMed]
- Iwamura, H.; Suzuki, H.; Ueda, Y.; Kaya, T.; Inaba, T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001, 296, 420–425. [Google Scholar] [PubMed]
- Rinaldi-Carmona, M.; Barth, F.; Héaulme, M.; Shire, D.; Calandra, B.; Congy, C.; Martinez, S.; Maruani, J.; Néliat, G.; Caput, D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994, 350, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Bayewitch, M.; Rhee, M.-H.; Avidor-Reiss, T.; Breuer, A.; Mechoulam, R.; Vogel, Z. (—)-Δ9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. Int. J. Biol. Chem. 1996, 271, 9902–9905. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Stevenson, L.A.; Wease, K.N.; Price, M.R.; Baillie, G.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB receptor antagonist. Br. J. Pharmacol. 2005, 146, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Huffman, J.W.; Liddle, J.; Yu, S.; Aung, M.M.; Abood, M.E.; Wiley, J.L.; Martin, B.R. 3-(1′,1′-Dimethylbutyl)-1-deoxy-Δ 8 -THC and related compounds: Synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 1999, 7, 2905–2914. [Google Scholar] [CrossRef]
- Busch-Petersen, J.; Hill, W.A.; Fan, P.; Khanolkar, A.; Xie, X.-Q.; Tius, M.A.; Makriyannis, A. Unsaturated side chain β-11-hydroxyhexahydrocannabinol analogs. J. Med. Chem. 1996, 39, 3790–3796. [Google Scholar] [CrossRef]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Rock, E.M.; Kopstick, R.L.; Limebeer, C.L.; Parker, L.A. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br. J. Pharmacol. 2013, 170, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Sanz, G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ9-Tetrahydrocannabinolic Acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.C.M.; Korthout, H.A.A.J.; van Meeteren-Kreikamp, A.P.; Ehlert, K.A.; Wang, M.; van der Greef, J.; Rodenburg, R.J.T.; Witkamp, R.F. Unheated Cannabis sativa extracts and its major compound THC-acid have potential immuno-modulating properties not mediated by CB1 and CB2 receptor coupled pathways. Int. Immunopharmacol. 2006, 6, 656–665. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Zulfiqar, F.; ElSohly, M.A. Cannabinoid ester constituents from high-potency. Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Mcpartland, J.M.; Macdonald, C.; Young, M.; Grant, P.S.; Furkert, D.P.; Glass, M. Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2017, 2, 87–95. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H. An update on Non-CB1, Non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz Iii, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Mechoulam, R.; Feigenbaum, J.J.; Lander, N.; Segal, M.; Järbe, T.U.C.; Hiltunen, A.J.; Consroe, P. Enantiomeric cannabinoids: Stereospecificity of psychotropic activity. Experientia 1988, 44, 762–764. [Google Scholar] [CrossRef]
- LaBuda, C.J.; Koblish, M.; Little, P.J. Cannabinoid CB2 receptor agonist activity in the hindpaw incision: Model of postoperative pain. Eur. J. Pharmacol. 2005, 527, 172–174. [Google Scholar] [CrossRef]
- Shevyrin, V.A.; Morzherin, Y.Y. Cannabinoids: Structures, effects, and classification. Russ. Chem. Bull. 2015, 64, 1249–1266. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.R.; Jefferson, R.; Winckler, R.; Wiley, J.L.; Huffman, J.W.; Crocker, P.J.; Saha, B.; Razdan, R.K. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J. Pharm. Exper. Therapeutics 1999, 290, 1065–1079. [Google Scholar]
- Bow, E.W.; Rimoldi, J.M. The structure-function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect. Med. Chem. 2016, 8, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Auwärter, V. JMS Letter. J. Mass Spectrom. 2009, 44, 832–837. [Google Scholar] [CrossRef]
- Vardakou, I.; Pistos, C.; Spiliopoulou, C. Spice drugs as a new trend: Mode of action, identification and legislation. Toxicol. Lett. 2010, 197, 157–162. [Google Scholar] [CrossRef]
- Dresen, S.; Ferreirós, N.; Pütz, M.; Westphal, F.; Zimmermann, R.; Auwärter, V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J. Mass Spectrom. 2010, 45, 1186–1194. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2021; ISBN 978-92-9497-588-1. ISSN 2314-9086. [Google Scholar]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Tarling, E.J.; Bennett, A.J.; Kendall, D.A.; Randall, M.D. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem. Biophys. Res. Commun. 2005, 337, 824–831. [Google Scholar] [CrossRef]
- Carrillo-Salinas, F.J.; Navarrete, C.; Mecha, M.; Feliú, A.; Collado, J.A.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Guaza, C. A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e94733. [Google Scholar]
- Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; Del Río, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernández-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Gómez-Cañas, M.; Burgaz, S.; Palomares, B.; Gómez-Gálvez, Y.; Palomo-Garo, C.; Campo, S.; Ferrer-Hernández, J.; Pavicic, C.; Navarrete, C.; et al. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: Possible involvement of different binding sites at the PPARγ receptor. J. Neuroinflammation 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Cueto, C.; Santos-García, I.; García-Toscano, L.; Espejo-Porras, F.; Bellido, M.; Fernández-Ruiz, J.; Muñoz, E.; de Lago, E. Neuroprotective effects of the cannabigerol quinone derivative VCE-003.2 in SOD1G93A transgenic mice, an experimental model of amyotrophic lateral sclerosis. Biochem. Pharmacol. 2018, 157, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Aguareles, J.; Paraíso-Luna, J.; Palomares, B.; Bajo-Grañeras, R.; Navarrete, C.; Ruiz-Calvo, A.; García-Rincón, D.; García-Taboada, E.; Guzmán, M.; Muñoz, E.; et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, S.; García, C.; Gómez-Cañas, M.; Muñoz, E.; Fernández-Ruiz, J. Development of an oral treatment with the PPAR-γ-acting cannabinoid VCE-003.2 against the inflammation-driven neuronal deterioration in experimental Parkinson’s disease. Molecules 2019, 24, 2702. [Google Scholar] [CrossRef] [Green Version]
- Hen-Shoval, D.; Amar, S.; Shbiro, L.; Smoum, R.; Haj, C.G.; Mechoulam, R.; Zalsman, G.; Weller, A.; Shoval, G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018, 351, 1–3. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Linher-Melville, K.; Niazmand, M.J.; Sharma, M.; Shahid, A.; Zhu, K.L.; Parzei, N.; Sidhu, J.; Haj, C.; Mechoulam, R.; et al. An evaluation of the anti-hyperalgesic effects of cannabidiolic acid-methyl ester in a preclinical model of peripheral neuropathic pain. Br. J. Pharmacol. 2020, 177, 2712–2725. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Rock, E.M.; Guenther, K.; Limebeer, C.L.; Stevenson, L.A.; Haj, C.; Smoum, R.; Parker, L.A.; Mechoulam, R. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT1A receptor-mediated suppression of nausea and anxiety in rats. Br. J. Pharmacol. 2018, 175, 100–112. [Google Scholar] [CrossRef] [Green Version]

| Class of Compounds | The Number of Compounds in Each Class | The First Representative Compound of the Class | Chemical Structure of the Representative Compound |
|---|---|---|---|
| Δ9-trans-tetrahydrocannabinol | 25 | Δ9-THC—isolated in 1964 by Goani and Mecholum using chromatography techniques [10] |  |
| Δ8-trans-tetrahydrocannabinol | 5 | Δ8-THC—isolated in Maryland in 1966 [11] |  |
| cannabidiol | 10 | CBD-C5—isolated in 1940 from native Minnesota hemp [12] |  |
| cannabigerol | 16 | CBG—isolated in 1964 using florisil chromatography [13] | 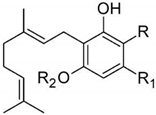 |
| cannabichromene | 9 | CBC—isolated in 1966 by Gaoni Y. [14] | 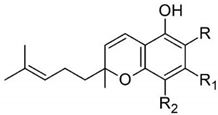 |
| cannabinol | 11 | CBN synthesized by Adams et al. in the US and by Todd’s group in the UK in 1940 [15,16] | 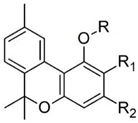 |
| cannabinodiol | 2 | CBND-C3—isolated in 1973 [17] CBND-C5—isolated in 1977 [18] |  |
| cannabicyclol | 3 | CBL—compound was isolated by Korte and Sieper in 1964, and the structure was elucidated by Crombie et al. in 1968 [19,20] |  |
| cannabielsoin | 5 | CBE-C5—isolated in 1973 from Lebanese hashish [21] |  |
| cannabitriol | 9 | CBT-C5—isolated in 1966 from Japanese hemp, but the complete chemical structure was established 10 years later [21,22] | 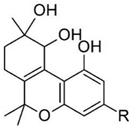 |
| other unclassified types of cannabinoids | 30 | The first ones isolated in 1975 (examples: dehydrocannabifuran DCBF-C5, cannabifuran CBF-C5) [23] |  |
| Class | Compounds | Targets | Effects | Potential Use as/in | References |
|---|---|---|---|---|---|
| CBG | CBG | CB1 | Poor agonist | [70] | |
| CB2 | Partial agonist | [70] | |||
| TRPM8 | Antagonist | Prostatic cancer | [72] | ||
| TRPV1 | Stimulation | Pain and inflammation, breast, skin, colon cancer | [72] | ||
| α2-Adrenoceptor | Agonist | Anti-inflammatory | [25,73] | ||
| IL-1β | Reduction | Neuroinflammation | [74] | ||
| TNF-α | |||||
| IFN-γ | |||||
| PPAR-γ | |||||
| Nrf-2 levels | Upregulation | ||||
| CBG, cyclic CBG | TRPA1 | Activation | Analgesic, anti-inflammatory | [75] | |
| CBG, CBGV, CBGA | TRPV3 TRPV4 | Activation and desensitization | Anti-inflammatory agent in IBD | [76] | |
| iNOS expression | Reduction | Anti-inflammatory | [77] | ||
| SOD | Increased activity | ||||
| Cytokines | Downregulation | ||||
| CBG, CBGA | COX-1, COX-2 | Inhibition | Anti-inflammatory | [78] | |
| PLA2 | Inhibition | ||||
| MAGL | Inhibition | ||||
| CBG CBGA | ALR | Inhibition | Diabetic complications | [79] | |
| PPARα/γ | Full or partial agonist | [80] | |||
| CBGV | TRPV2 | Antagonist | Cancer | [72,81] | |
| CBD | CBD | CB1 | Activation | Chronic neuropathic pain | [82,83] |
| TRPV1 | Agonist | Depression | [84,85] | ||
| 5HT1A | Agonist | ||||
| PPARγ | Agonist | ||||
| CBDV | CB1/CB2 | Indirect inhibition | [86] | ||
| TRPA1 | Stimulation | [72] | |||
| TRPV1 | Desensitization | [87] | |||
| TRPV2 | Stimulation | [88] | |||
| GPR55 | Antagonist | Dravet syndrome, anticonvulsant | [87,89] | ||
| GPR6 | Inverse agonist | [90] | |||
| DAGLα | Inhibition | [86] | |||
| AEA | Inhibition of cellular uptake | [86] | |||
| IL-1β | Reduction | IBD | [91] | ||
| CBDA | 5HT1A | Activation | Nausea | [92,93] | |
| cAMP protein kinase A | Inhibition | Breast cancer | [94] | ||
| THC | Δ9-THC | CB1/CB2 | Activation | Anti-inflammatory | [95] |
| Mixed modulation | Alzheimer | [96,97] | |||
| MDSCs | Induction | Anti-inflammatory | [98] | ||
| AchE | Inhibition | Alzheimer | [99] | ||
| Amyloid-β | Reduction | Alzheimer | [100] | ||
| TRPV2 | Agonist | [101,102] | |||
| TRPV3 | Agonist | [76,101] | |||
| TRPV3 | Agonist | [76,101] | |||
| Δ8-THC | CB1 | Antagonist | Anti-inflammatory | [103] | |
| CB2 | Partial agonist | Mood disorders | [103] | ||
| THCA-A | PPARγ | Stimulation | Obesity | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. https://doi.org/10.3390/pharmaceutics13111823
Filipiuc LE, Ababei DC, Alexa-Stratulat T, Pricope CV, Bild V, Stefanescu R, Stanciu GD, Tamba B-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics. 2021; 13(11):1823. https://doi.org/10.3390/pharmaceutics13111823
Chicago/Turabian StyleFilipiuc, Leontina Elena, Daniela Carmen Ababei, Teodora Alexa-Stratulat, Cosmin Vasilica Pricope, Veronica Bild, Raluca Stefanescu, Gabriela Dumitrita Stanciu, and Bogdan-Ionel Tamba. 2021. "Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors?" Pharmaceutics 13, no. 11: 1823. https://doi.org/10.3390/pharmaceutics13111823
APA StyleFilipiuc, L. E., Ababei, D. C., Alexa-Stratulat, T., Pricope, C. V., Bild, V., Stefanescu, R., Stanciu, G. D., & Tamba, B.-I. (2021). Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics, 13(11), 1823. https://doi.org/10.3390/pharmaceutics13111823








