The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation and Stability Study
2.3. Water Activity
2.4. Liquid Chromatography
2.5. Liquid Chromatography-Mass Spectrometry
2.6. Asymmetrical Flow Field-Flow Fractionation
2.7. Small Angle Neutron Scattering
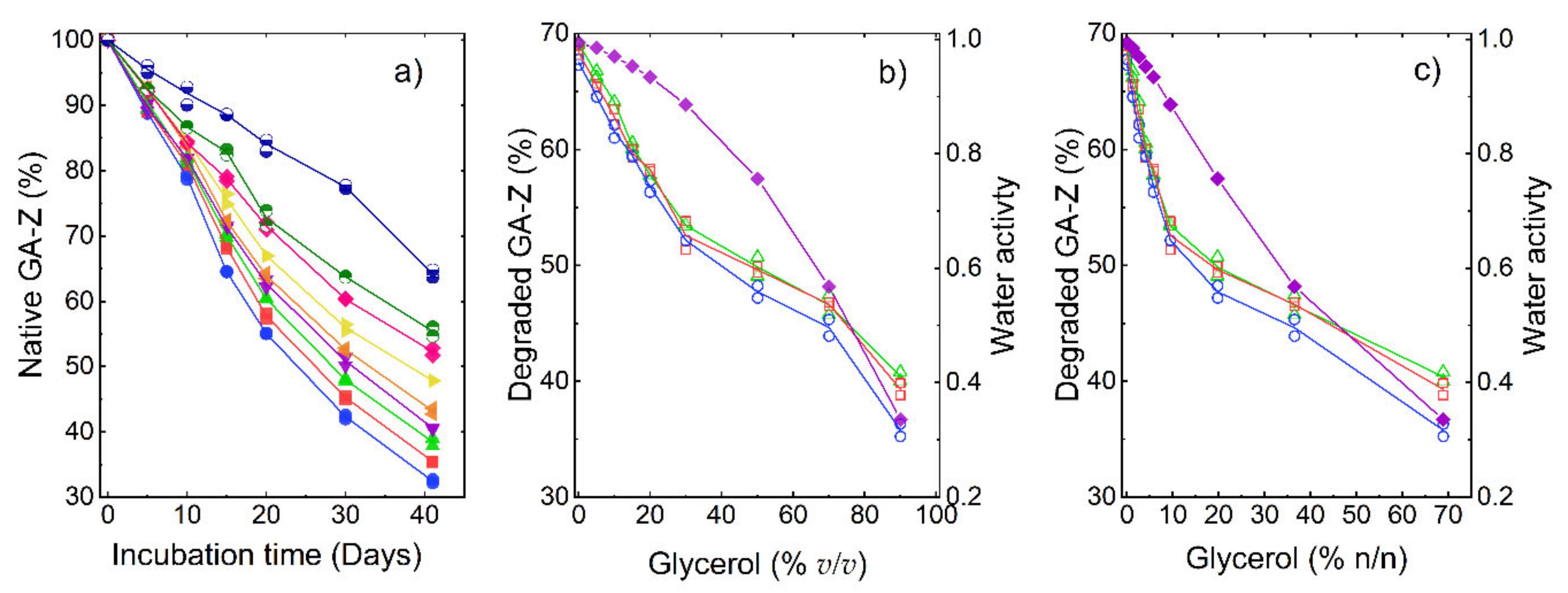
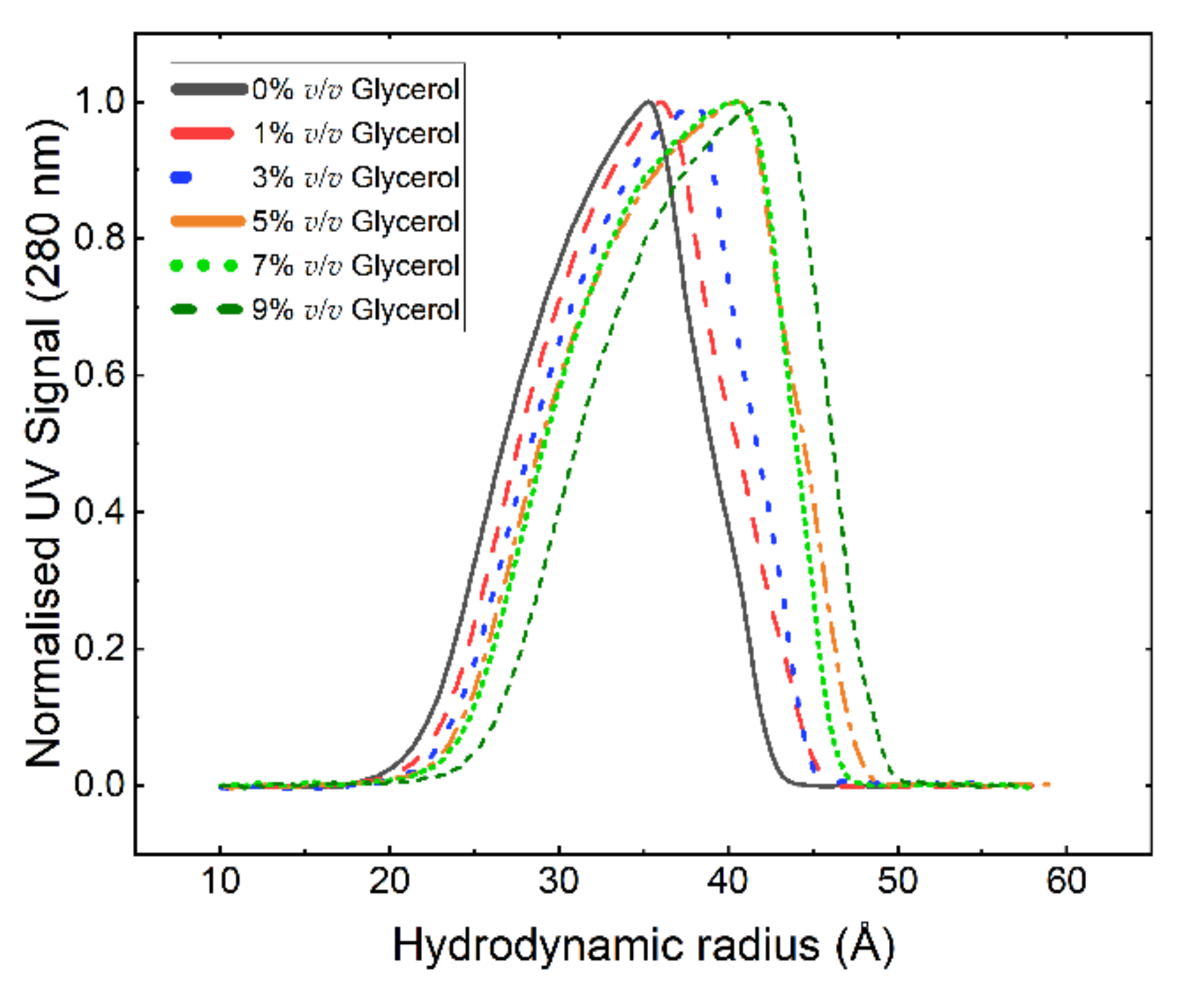
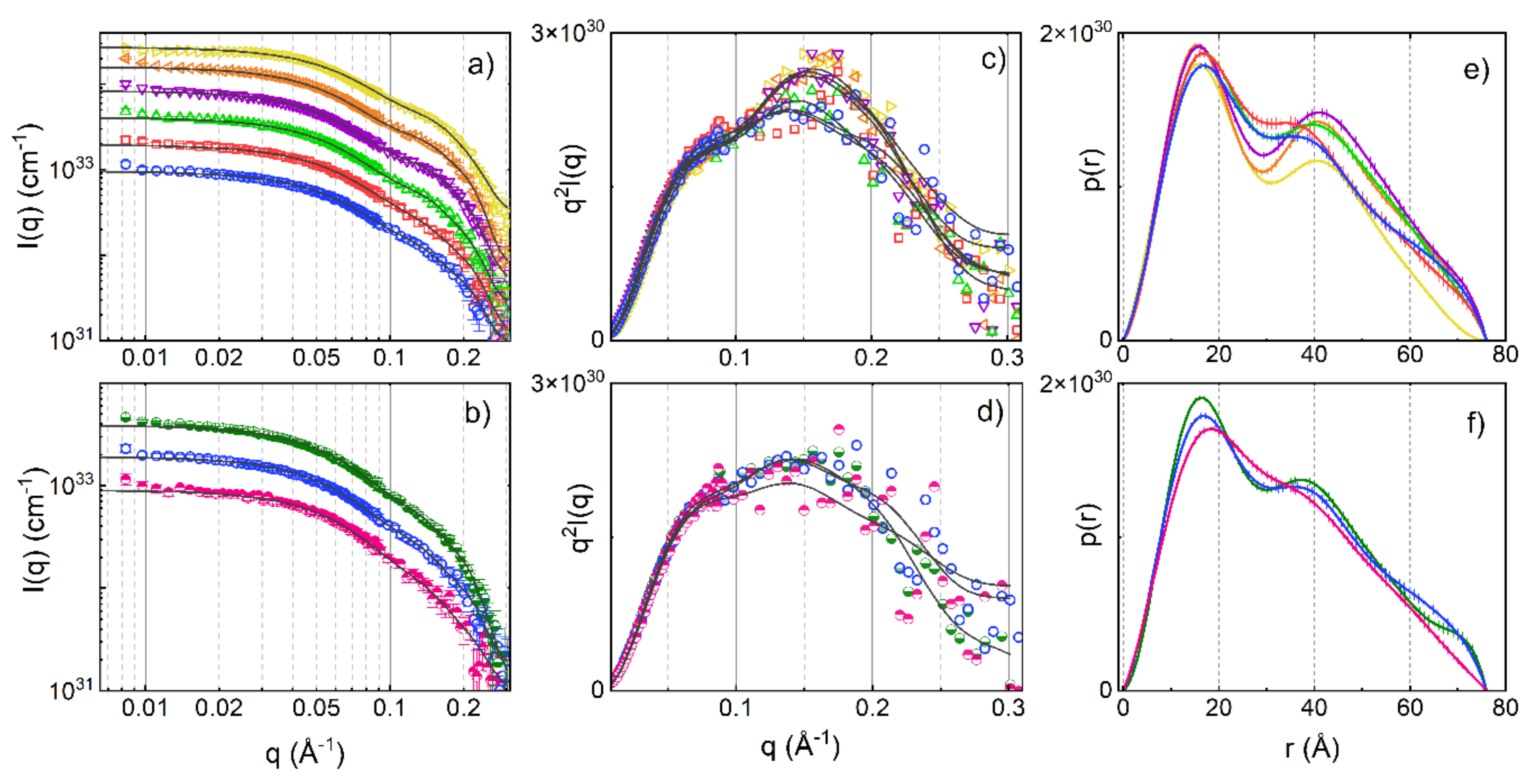
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reubsaet, J.L.; Beijnen, J.H.; Bult, A.; van Maanen, R.J.; Marchal, J.A.; Underberg, W.J. Analytical techniques used to study the degradation of proteins and peptides: Chemical instability. J. Pharm. Biomed. Anal. 1998, 17, 955–978. [Google Scholar] [CrossRef]
- Aswad, D.W.; Paranandi, M.V.; Schurter, B.T. Isoaspartate in peptides and proteins: Formation, significance, and analysis. J. Pharm. Biomed. Anal. 2000, 21, 1129–1136. [Google Scholar] [CrossRef]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of protein pharmaceuticals: An update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.-Y. Deamidation: A source of microheterogeneity in pharmaceutical proteins. Trends Biotechnol. 1992, 10, 364–369. [Google Scholar] [CrossRef]
- Cleland, J.L.; Powell, M.F.; Shire, S.J. The development of stable protein formulations: A close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carr. Syst. 1993, 10, 307–377. [Google Scholar]
- Li, R.; D’Souza, A.J.; Laird, B.B.; Schowen, R.L.; Borchardt, R.T.; Topp, E.M. Effects of solution polarity and viscosity on peptide deamidation. J. Pept. Res. 2000, 56, 326–334. [Google Scholar] [CrossRef]
- Nilsson, M.R.; Driscoll, M.; Raleigh, D.P. Low levels of asparagine deamidation can have a dramatic effect on aggregation of amyloidogenic peptides: Implications for the study of amyloid formation. Protein Sci. 2002, 11, 342–349. [Google Scholar] [CrossRef]
- Takata, T.; Oxford, J.T.; Demeler, B.; Lampi, K.J. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Sci. 2008, 17, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S. Propensity for spontaneous succinimide asparaginyl residues in cellular proteins. Int. J. Pept. Protein Res. 1987, 30, 808–821. [Google Scholar] [CrossRef]
- Brennan, T.; Clarke, S. Spontaneous degradation of polypeptides at aspartyl and asparaginyl residues: Effects of the solvent dielectric. Protein Sci. 1993, 2, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Schowen, R.L. Secondary structure and protein deamidation. J. Pharm. Sci. 1999, 88, 8–13. [Google Scholar] [CrossRef]
- Hirai, M.; Ajito, S.; Sugiyama, M.; Iwase, H.; Takata, S.I.; Shimizu, N.; Igarashi, N.; Martel, A.; Porcar, L. Direct Evidence for the Effect of Glycerol on Protein Hydration and Thermal Structural Transition. Biophys. J. 2018, 115, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, C.L.; Friedman, A.R.; Kubiak, T.M.; Donlan, M.E.; Borchardt, R.T. Effect of secondary structure on the rate of deamidation of several growth hormone releasing factor analogs. Int. J. Pept. Protein Res. 1993, 42, 497–503. [Google Scholar] [CrossRef]
- Xie, M.; Shahrokh, Z.; Kadkhodayan, M.; Henzel, W.J.P.; Michael, F.; Borchardt, R.T.; Schowen, R.L. Asparagine deamidation in recombinant human lymphotoxin: Hindrance by three-dimensional structures. J. Pharm. Sci. 2003, 92, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Huang, M.; Lewis, M.J.; Hu, P. Structure Based Prediction of Asparagine Deamidation Propensity in Monoclonal Antibodies. MAbs 2018, 10, 901–912. [Google Scholar] [CrossRef]
- Vagenende, V.; Yap, M.G.; Trout, B.L. Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef]
- Ronsin, O.; Caroli, C.; Baumberger, T. Preferential hydration fully controls the renaturation dynamics of collagen in water-glycerol solvents. Eur. Phys. J. E Soft Matter 2017, 40, 55. [Google Scholar] [CrossRef]
- Gekko, K.; Timasheff, S.N. Mechanism of protein stabilization by glycerol: Preferential hydration in glycerol-water mixtures. Biochemistry 1981, 20, 4667–4676. [Google Scholar] [CrossRef]
- Scharnagl, C.; Reif, M.; Friedrich, J. Stability of proteins: Temperature, pressure and the role of the solvent. Biochim. Biophys. Acta 2005, 1749, 187–213. [Google Scholar] [CrossRef]
- Bradbury, S.L.; Jakoby, W.B. Glycerol as an enzyme-stabilizing agent: Effects on aldehyde dehydrogenase. Proc. Natl. Acad. Sci. USA 1972, 69, 2373–2376. [Google Scholar] [CrossRef] [Green Version]
- Blackshear, P.J.; Rohde, T.D.; Palmer, J.L.; Wigness, B.D.R.; William, M. Buchwald, Henry. Glycerol prevents insulin precipitation and interruption offFlow in an implantable insulin infusion pump. Diabetes Care 1983, 6, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chanasattru, W.; Decker, E.A.; Julian McClements, D. Impact of cosolvents (polyols) on globular protein functionality: Ultrasonic velocity, density, surface tension and solubility study. Food Hydrocoll. 2008, 22, 1475–1484. [Google Scholar] [CrossRef]
- Priev, A.; Almagor, A.; Yedgar, S.; Gavish, B. Glycerol decreases the volume and compressibility of protein Interior. Biochemistry 1996, 35, 2061–2066. [Google Scholar] [CrossRef]
- Knubovets, T.; Osterhout, J.J.; Connolly, P.J.; Klibanov, A. Structure, thermostability, and conformational flexibility of hen egg-white lysozyme dissolved in glycerol. Biochemistry 1999, 96, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Timasheff, S.N. Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry 2002, 41, 13473–13482. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N.; Neil, L.J.C.P.E.P.T. The interaction of tubulin and other proteins with structure-stabilizing solvents. J. Colloid Interface Sci. 1976, 55, 658–663. [Google Scholar] [CrossRef]
- Na, G.C.T.; Serge, N. Interaction of calf brain tubulin with glycerol. J. Mol. Biol. 1981, 151, 165–178. [Google Scholar] [CrossRef]
- Shukla, D.; Shinde, C.; Trout, B.L. Molecular computations of preferential interaction coefficients of proteins. Journal of Phys. Chem. 2009, 113, 12546–12554. [Google Scholar] [CrossRef]
- Stahl, S.; Graslund, T.; Eriksson Karlstrom, A.; Frejd, F.Y.; Nygren, P.A.; Lofblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Frejd, F.Y.; Kim, K.T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef] [Green Version]
- Bello, J.B.H.R. Chemical modification and cross-linking of proteins by impurities in glycerol. Arch. Biochem. Biophys. 1976, 172, 608–610. [Google Scholar] [CrossRef]
- Choi, J.; Wahlgren, M.; Ek, V.; Elofsson, U.; Fransson, J.; Nilsson, L.; Terry, A.; Soderberg, C.A.G. Characterization of binding between model protein GA-Z and human serum albumin using asymmetrical flow field-flow fractionation and small angle X-ray scattering. PLoS ONE 2020, 15, e0242605. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, K.-G.; Nilsson, L. Flow FFF–Basics and Key Applications. In Field-Flow Fractionation in Biopolymer Analysis; Williams, S.K.R., Caldwell, K.D., Eds.; Springer Vienna: Vienna, Austria, 2012; pp. 1–21. [Google Scholar]
- Litzén, A.; Wahlund, K.-G. Zone broadening and dilution in rectangular and trapezoidal asymmetrical flow field-flow fractionation channels. Anal. Chem. 1991, 63, 1001–1007. [Google Scholar] [CrossRef]
- Magnusson, E.; Hakansson, A.; Janiak, J.; Bergenstahl, B.; Nilsson, L. Hydrodynamic radius determination with asymmetrical flow field-flow fractionation using decaying cross-flows. Part II. Experimental evaluation. J. Chromatogr. A 2012, 1253, 127–133. [Google Scholar] [CrossRef]
- Heniz Maier-Leibnitz Zentrum; Radulescu, A.; Szekely, N.K.; Appavou, M.-S. KWS-2: Small angle scattering diffractometer. J. Large-Scale Res. Facil. 2015, 1, A29. [Google Scholar] [CrossRef] [Green Version]
- Rubinson, K.A. Practical corrections for p(H,D) measurements in mixed H2O/D2O biological buffers. Anal. Methods 2017, 9, 2744–2750. [Google Scholar] [CrossRef]
- Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr. 1977, 10, 415–421. [Google Scholar] [CrossRef]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- BioSAXS Group. GNOM. Available online: https://www.embl-hamburg.de/biosaxs/gnom.html (accessed on 18 March 2021).
- Petoukhov, M.V.; Svergun, D.I. Analysis of X-ray and neutron scattering from biomacromolecular solutions. Curr Opin Struct Biol 2007, 17, 562–571. [Google Scholar] [CrossRef]
- SasView for Small Angle Scattering Analysis. Available online: https://www.sasview.org/ (accessed on 18 March 2021).
- Kaya, H. Scattering from cylinders with globular end-caps. J. Appl. Crystallogr. 2004, 37, 223–230. [Google Scholar] [CrossRef]
- Kaya, H.; de Souza, N.-R. Scattering from capped cylinders. Addendum. J. Appl. Crystallogr. 2004, 37, 508–509. [Google Scholar] [CrossRef] [Green Version]
- Kotlarchyk, M.; Stephens, R.B.H.; John, S. Study of Schultz distribution to model polydispersity of microemulsion droplets. Am. Chem. Soc. 1988, 92, 1538–1553. [Google Scholar]
- Yang, H.; Zubarev, R.A. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis 2010, 31, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Kori, Y.; Patel, R.; Neill, A.; Liu, H. A conventional procedure to reduce Asn deamidation artifacts during trypsin peptide mapping. J. Chromatogr. B 2016, 1009–1010, 107–113. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 83rd ed.; Lide, D.R., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Svergun, D.I.; Koch, M.H.J. Small-angle scattering studies of biological macromolecules in solution. Inst. Phys. Publ. 2003, 66, 1735–1782. [Google Scholar] [CrossRef]
- Puž, V.; Pavšič, M.; Lenarčič, B.; Djinović-Carugo, K. Conformational plasticity and evolutionary analysis of the myotilin tandem Ig domains. Sci. Rep. 2017, 7, 3993. [Google Scholar] [CrossRef]
- RCSB PDB. 1PRB. Available online: https://www.wwpdb.org/pdb?id=pdb_00001prb (accessed on 18 March 2021).
- RCSB PDB. 2B88. Available online: https://www.wwpdb.org/pdb?id=pdb_00002b88 (accessed on 18 March 2021).
- Lendel, C.; Dogan, J.; Hard, T. Structural basis for molecular recognition in an affibody:affibody complex. J. Mol. Biol. 2006, 359, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.U.; Châeau, M.d.; Wikström, M.; Forsén, S.; Drakenberg, T.; Björck, L. Solution structure of the albumin-binding GA module: A versatile bacterial protein domain. J. Mol. Biol. 1997, 266, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Rose, A.S.; Koča, J.; Burley, S.; Velankar, S. Mol*: Towards a common library and tools for web molecular graphics. In Proceedings of the Workshop on Molecular Graphics and Visual Analysis of Molecular Data, Brno, Czech Republic, 3 November 2018; pp. 29–33. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramm, I.; Sanchez-Fernandez, A.; Choi, J.; Lang, C.; Fransson, J.; Schagerlöf, H.; Wahlgren, M.; Nilsson, L. The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation. Pharmaceutics 2021, 13, 1853. https://doi.org/10.3390/pharmaceutics13111853
Ramm I, Sanchez-Fernandez A, Choi J, Lang C, Fransson J, Schagerlöf H, Wahlgren M, Nilsson L. The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation. Pharmaceutics. 2021; 13(11):1853. https://doi.org/10.3390/pharmaceutics13111853
Chicago/Turabian StyleRamm, Ingrid, Adrian Sanchez-Fernandez, Jaeyeong Choi, Christian Lang, Jonas Fransson, Herje Schagerlöf, Marie Wahlgren, and Lars Nilsson. 2021. "The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation" Pharmaceutics 13, no. 11: 1853. https://doi.org/10.3390/pharmaceutics13111853
APA StyleRamm, I., Sanchez-Fernandez, A., Choi, J., Lang, C., Fransson, J., Schagerlöf, H., Wahlgren, M., & Nilsson, L. (2021). The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation. Pharmaceutics, 13(11), 1853. https://doi.org/10.3390/pharmaceutics13111853








