Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DDS
2.2.1. Preparation of MS
2.2.2. Preparation of Micelles
2.3. Characterization of DDS
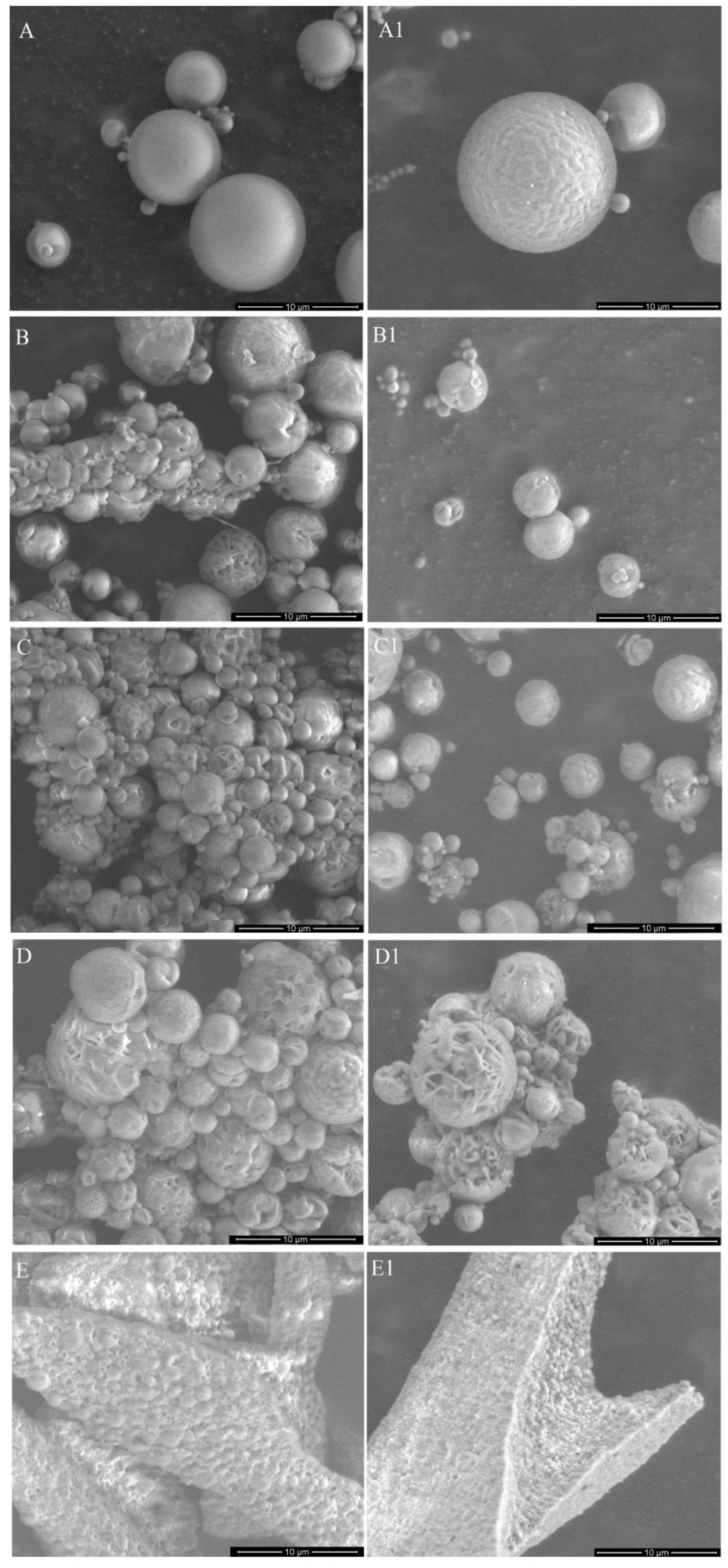
2.3.1. Microscopic Evaluation
2.3.2. FTIR Analysis
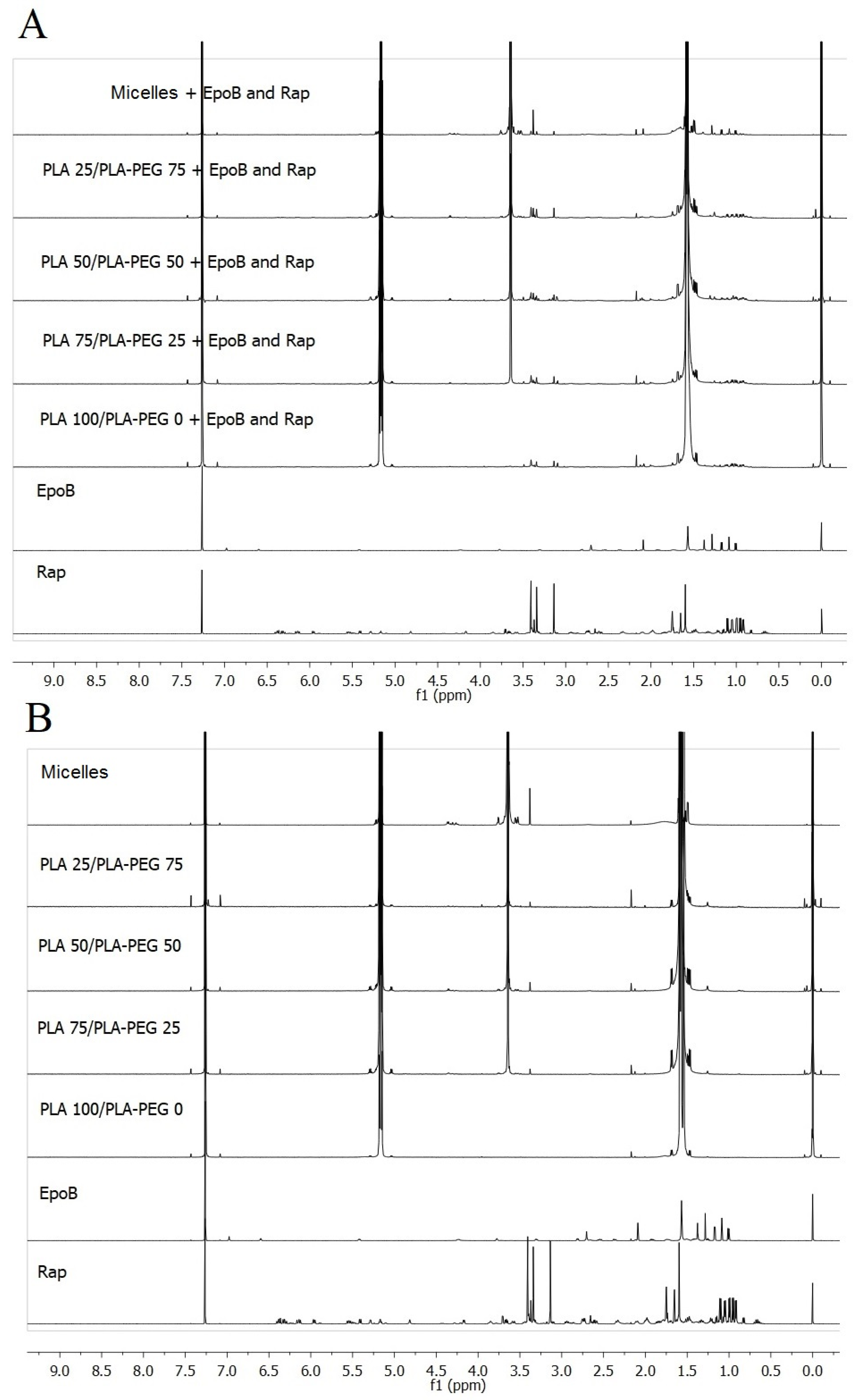
2.3.3. NMR Analysis
2.3.4. HPLC Measurements
2.4. Encapsulation Efficiency
2.5. In Vitro Release Studies
2.5.1. Drug Release from MS
2.5.2. Drug Release from Micelles
2.6. In Vitro Cytotoxicity Studies
2.7. Statistical Analysis
3. Results
3.1. Characteristics of DDS
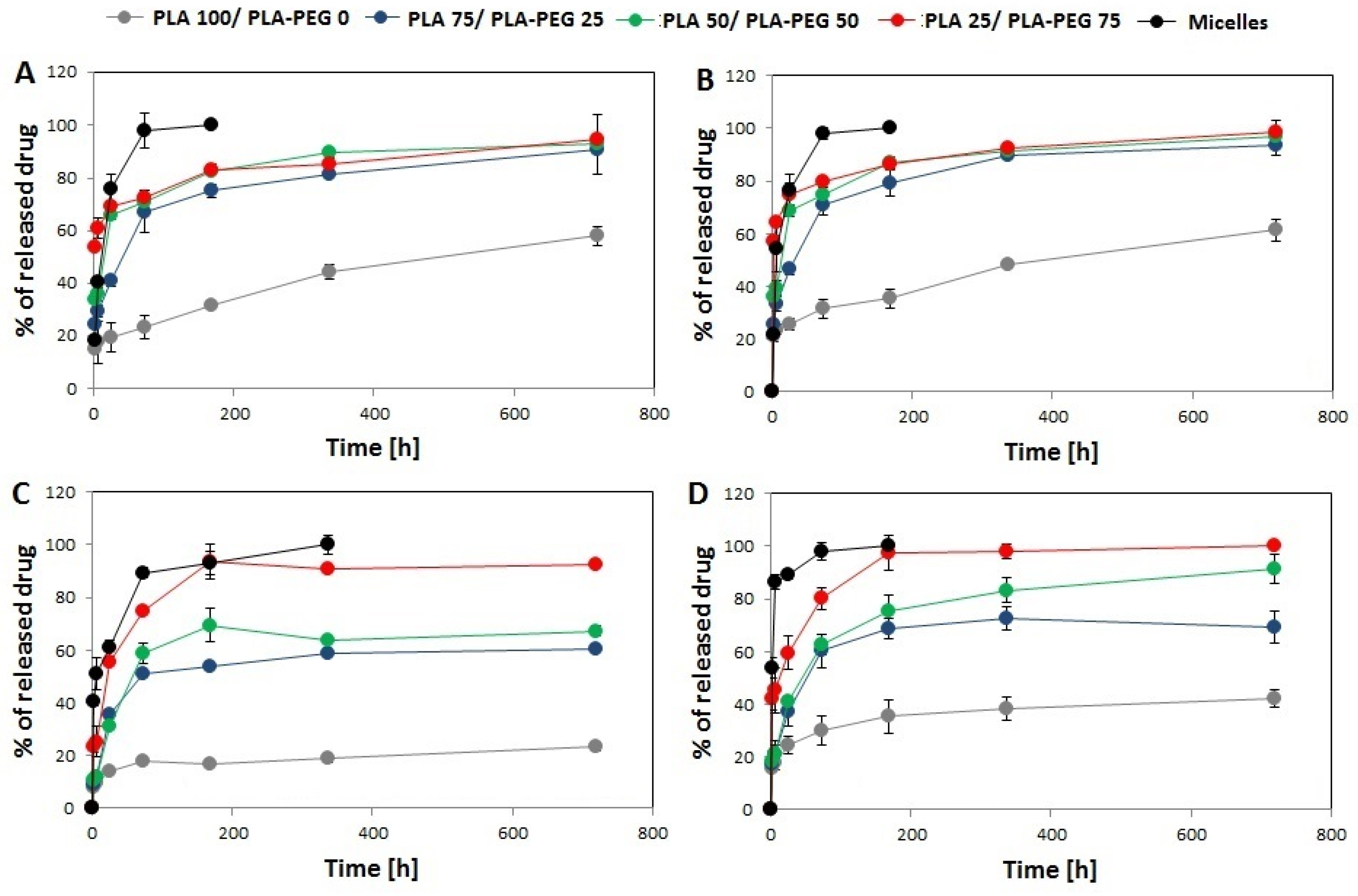
3.2. In Vitro Drug Release
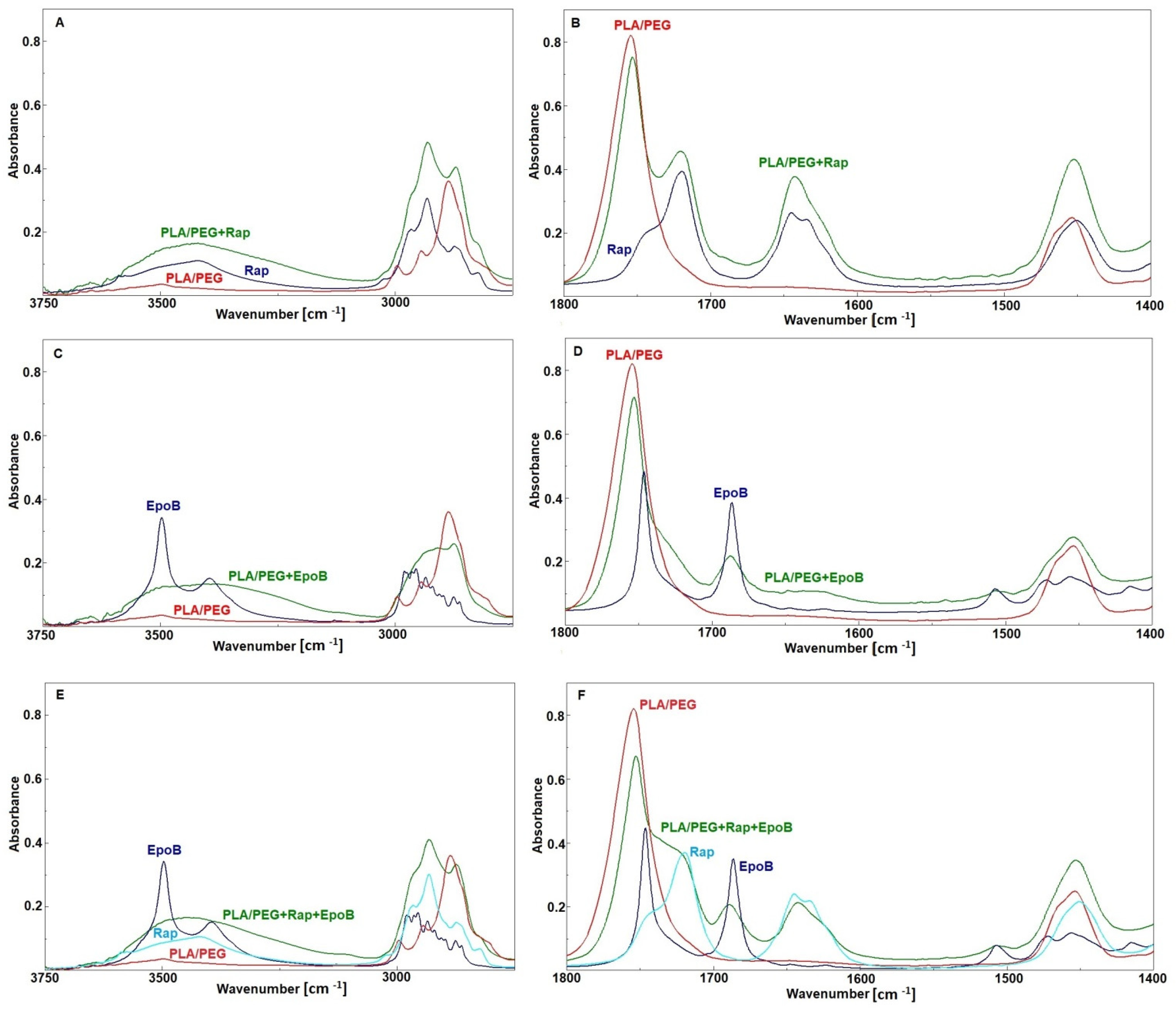
3.3. FTIR Analysis
3.4. Cytotoxic Activity of EpoB and Rap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.-C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) Hydrogels for Biomedical Applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Cegłowski, M.; Jerca, V.V.; Jerca, F.A.; Hoogenboom, R. Reduction-Responsive Molecularly Imprinted Poly(2-isopropenyl-2-oxazoline) for Controlled Release of Anticancer Agents. Pharmaceutics 2020, 12, 506. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular imprinted polymers as drug delivery vehicles. Drug Deliv. 2016, 23, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG-PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(ethylene glycol)-Polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajdel, A.; Wilczok, A.; Jelonek, K.; Kaps, A.; Musiał-Kulik, M.; Kasperczyk, J. Cytotoxic effect of targeted biodegradable epothilone B and rapamycin co-loaded nanocarriers on breast cancer cells. J. Biomed. Mater. Res. Part A 2021, 109, 1693–1700. [Google Scholar] [CrossRef]
- Tomao, F.; Papa, A.; Zaccarelli, E.; Rossi, L.; Caruso, D.; Minozzi, M.; Vici, P.; Frati, L.; Tomao, S. Triple-negative breast cancer: New perspectives for targeted therapies. OncoTargets Ther. 2015, 8, 177–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirkhani Namagerdi, A.; d’Angelo, D.; Ciani, F.; Iannuzzi, C.A.; Napolitano, F.; Avallone, L.; De Laurentiis, M.; Giordano, A. Triple-Negative Breast Cancer Comparison With Canine Mammary Tumors From Light Microscopy to Molecular Pathology. Front. Oncol. 2020, 10, 563779. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of care and promising new agents for triple negative metastatic breast cancer. Cancers 2014, 6, 2187–2223. [Google Scholar] [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Ekizceli, G.; Uluer, E.T.; Inan, S. Investigation of the effects of rapamycin on the mTOR pathway and apoptosis in metastatic and non-metastatic human breast cancer cell lines. Bratisl. Lek. Listy 2020, 121, 308–315. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, X.; Li, C.; Zhang, X.; Li, C.L. Rapamycin promotes the anticancer action of dihydroartemisinin in breast cancer MDA-MB-231 cells by regulating expression of Atg7 and DAPK. Oncol. Lett. 2018, 15, 5781–5786. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Hu, J.; Obayemi, J.D.; Malatesta, K.; Košmrlj, A.; Soboyejo, W.O. Enhanced cellular uptake of LHRH-conjugated PEG-coated magnetite nanoparticles for specific targeting of triple negative breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. BCR 2002, 4, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Obayemi, J.D.; Danyuo, Y.; Dozie-Nwachukwu, S.; Odusanya, O.S.; Anuku, N.; Malatesta, K.; Yu, W.; Uhrich, K.E.; Soboyejo, W.O. PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for anticancer drug release: Effects of particle size on drug release kinetics and cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 51–65. [Google Scholar] [CrossRef]
- Dozie-Nwachukwu, S.O.; Danyuo, Y.; Obayemi, J.D.; Odusanya, O.S.; Malatesta, K.; Soboyejo, W.O. Extraction and encapsulation of prodigiosin in chitosan microspheres for targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 268–278. [Google Scholar] [CrossRef]
- Liang, X.; Chen, X.; Zhao, G.; Tang, T.; Dong, W.; Wang, C.; Zhang, J.; Liao, Z. Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells. Pharmaceutics 2018, 10, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012, 33, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Sukarto, A.; Amsden, B.G. Low melting point amphiphilic microspheres for delivery of bone morphogenetic protein-6 and transforming growth factor-β3 in a hydrogel matrix. J. Control. Release Off. J. Control. Release Soc. 2012, 158, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Nguyen, T.H.N.; Orchel, A.; Chodurek, E.; Paduszyński, P.; Jaworska-Kik, M.; Chrobak, E.; Bębenek, E.; et al. Bioresorbable filomicelles for targeted delivery of betulin derivative—In vitro study. Int. J. Pharm. 2019, 557, 43–52. [Google Scholar] [CrossRef]
- Jelonek, K.; Karpeta, P.; Jaworska, J.; Pastusiak, M.; Wlodarczyk, J.; Kasperczyk, J.; Dobrzynski, P. Comparison of extraction methods of sirolimus from polymeric coatings of bioresorbable vascular scaffolds. Mater. Lett. 2018, 214, 220–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Jelonek, K.; Li, S.M.; Kaczmarczyk, B.; Marcinkowski, A.; Orchel, A.; Musial-Kulik, M.; Kasperczyk, J. Multidrug PLA-PEG filomicelles for concurrent delivery of anticancer drugs-The influence of drug-drug and drug-polymer interactions on drug loading and release properties. Int. J. Pharm. 2016, 510, 365–374. [Google Scholar] [CrossRef]
- Zhang, F.; Aaltonen, J.; Tian, F.; Saville, D.J.; Rades, T. Influence of particle size and preparation methods on the physical and chemical stability of amorphous simvastatin. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2009, 71, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Li, H.; Shimizu, H.; Nishida, C.; Ogura, H.; Ortiz de Montellano, P.R.; Poulos, T.L. Crystal structures of epothilone D-bound, epothilone B-bound, and substrate-free forms of cytochrome P450epoK. J. Biol. Chem. 2003, 278, 44886–44893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.H.; Kwon, G.S. Pre-clinical evaluation of a themosensitive gel containing epothilone B and mTOR/Hsp90 targeted agents in an ovarian tumor model. J. Control. Release Off. J. Control. Release Soc. 2017, 268, 176–183. [Google Scholar] [CrossRef]
- Shin, D.H.; Kwon, G.S. Epothilone B-based 3-in-1 polymeric micelle for anticancer drug therapy. Int. J. Pharm. 2017, 518, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.K.; Dizon, D.S. Medical therapy of endometrial cancer: Current status and promising novel treatments. Drugs 2012, 72, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Jelonek, K.; Kaczmarczyk, B.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Dobrzyński, P.; Kasperczyk, J. The influence of drug-polymer interactions on release of antirestenotic agent from bioresorbable scaffolds. Mater. Lett. 2018, 223, 82–85. [Google Scholar] [CrossRef]

| Name of DDS | Composition of DDS | |
|---|---|---|
| Amount of PLA (%) | Amount of PLA–PEG (%) | |
| Microspheres | ||
| PLA 100/PLA–PEG 0 | 100 | 0 |
| PLA 75/PLA–PEG 25 | 75 | 25 |
| PLA 50/PLA–PEG 50 | 50 | 50 |
| PLA 25/PLA–PEG 75 | 25 | 75 |
| PLA 0/PLA–PEG 100 | 0 | 100 |
| Micelles | ||
| Micelles | 0 | 100 |
| Name of DDS | EE of EpoB (%) | EE of Rap (%) |
|---|---|---|
| PLA 100/PLA–PEG 0 + EpoB | 8.9 ± 1.2 | - |
| PLA 100/PLA–PEG 0 + Rap | - | 83.0 ± 4.2 |
| PLA 100/PLA–PEG 0 + EpoB and Rap | 13.1 ± 2.3 | 48.1 ± 1.5 |
| PLA 75/PLA–PEG 25 + EpoB | 7.2 ± 2.3 | - |
| PLA 75/PLA–PEG 25 + Rap | - | 96.0 ± 3.7 |
| PLA 75/PLA–PEG 25 + EpoB and Rap | 9.4 ± 1.9 | 54.2 ± 0.9 |
| PLA 50/PLA–PEG 50 + EpoB | 2.9 ± 0.2 | |
| PLA 50/PLA–PEG 50 + Rap | - | 95.0 ± 4.7 |
| PLA 50/PLA–PEG 50 + EpoB and Rap | 4.2 ± 1.2 | 59.7 ± 1.3 |
| PLA 25/PLA–PEG 75 + EpoB | 6.8 ± 3.6 | - |
| PLA 25/PLA–PEG 75 + Rap | - | 91.0 ± 8.9 |
| PLA 25/PLA–PEG 75 + EpoB and Rap | 7.2 ± 2.7 | 53.9 ± 1.8 |
| Micelles + EpoB | 43.8 ± 3.8 | |
| Micelles + Rap | - | 96.5 ± 3.3 |
| Micelles + EpoB and Rap | 41.0 ± 3.8 | 40.3 ± 0.7 |
| Name of DDS | Korsmeyer-Peppas (R2) | Peppas-Sahlin (R2) | ||
|---|---|---|---|---|
| EpoB | Rap | EpoB | Rap | |
| PLA 100/PLA–PEG 0 + EpoB | 0.945 | - | 0.952 | - |
| PLA 100/PLA–PEG 0 + Rap | - | 0.951 | - | 0.960 |
| PLA 100/PLA–PEG 0 + EpoB and Rap | 0.995 | 0.975 | 0.995 | 0.975 |
| PLA 75/PLA–PEG 25 + EpoB | 0.977 | - | 0.992 | - |
| PLA 75/PLA–PEG 25 + Rap | - | 0.976 | - | 0.985 |
| PLA 75/PLA–PEG 25 + EpoB and Rap | 0.924 | 0.909 | 0.988 | 0.971 |
| PLA 50/PLA–PEG 50 + EpoB | 0.972 | - | 0.985 | - |
| PLA 50/PLA–PEG 50 + Rap | - | 0.969 | - | 0.982 |
| PLA 50/PLA–PEG 50 + EpoB and Rap | 0.972 | 0.876 | 0.992 | 0.961 |
| PLA 25/PLA–PEG 75 + EpoB | 0.999 | - | 0.999 | - |
| PLA 25/PLA–PEG 75 + Rap | - | 0.996 | - | 0.997 |
| PLA 25/PLA–PEG 75 + EpoB and Rap | 0.968 | 0.925 | 0.977 | 0.982 |
| Micelles + EpoB | 0.956 | - | 0.996 | - |
| Micelles + Rap | - | 0.919 | - | 0.988 |
| Micelles + EpoB and Rap | 0.966 | 0.982 | 0.976 | 0.982 |
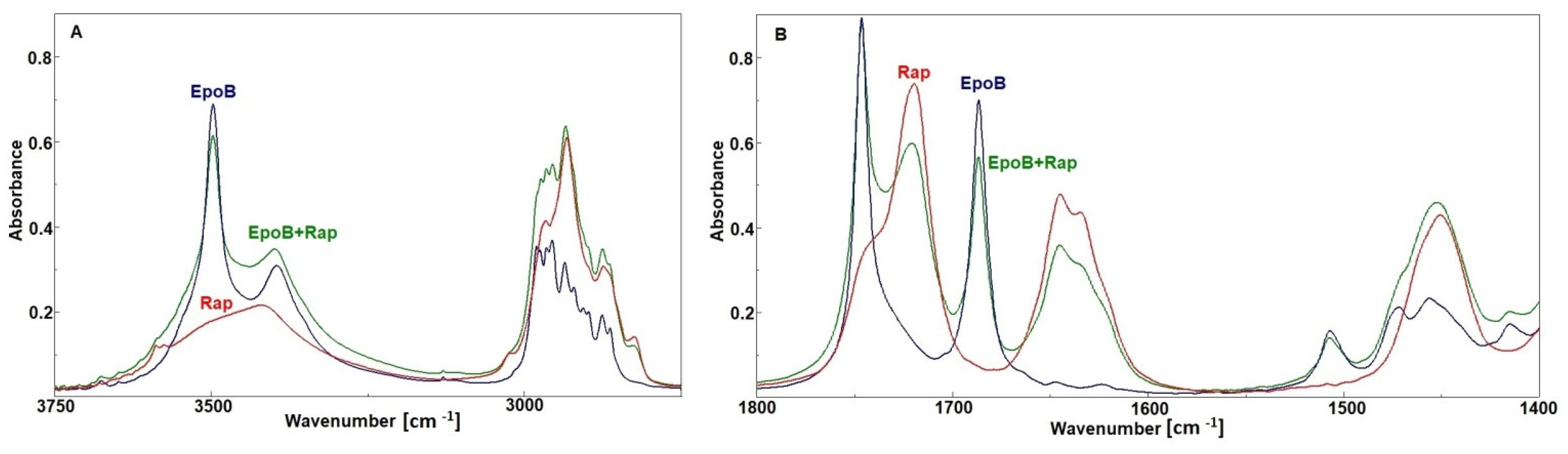
| PLA–PEG | Rap | EpoB | Assignment |
|---|---|---|---|
| 3500 | 3588 3577 sh 3419 | 3497 3395 | Overtonevν OH |
| 3000–2700 | 3000–2700 | 3000–2700 | ν CH, CH2 |
| 1754 | ν C=O ester PLA | ||
| 1720 1645 1634 | 1746 1742 sh 1686 | ν C=O ester free ν C=O ester bonded ν C=O ketone ν C=O amide (I amide bands) |
| Drugs Concentration | Free Drugs | Micelles PLA100/PLA–PEG0 | Microspheres PLA100/PLA–PEG0 | Microspheres PLA50/PLA–PEG50 |
|---|---|---|---|---|
| Control (blank) | 100.00 ± 0.44 | 100.00 ± 0.29 | 100.00 ± 0.45 | 100.00 ± 0.24 |
| EpoB 10 nM | 24.67 ± 1.11 * | 18.77 ± 1.23 * | 100.25 ± 0.54 | 19.58 ± 1.32 * |
| Rap 4 nM | 99.22 ± 1.42 | 98.89 ± 1.72 | 98.42 ± 0.51 | 97.63 ± 1.22 |
| EpoB 10 nM + Rap 4 nM | 19.52 ± 1.23 * | 14.18 ± 1.75 * | 97.29 ± 0.97 | 16.25 ± 1.39 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelonek, K.; Zajdel, A.; Wilczok, A.; Kaczmarczyk, B.; Musiał-Kulik, M.; Hercog, A.; Foryś, A.; Pastusiak, M.; Kasperczyk, J. Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells. Pharmaceutics 2021, 13, 1881. https://doi.org/10.3390/pharmaceutics13111881
Jelonek K, Zajdel A, Wilczok A, Kaczmarczyk B, Musiał-Kulik M, Hercog A, Foryś A, Pastusiak M, Kasperczyk J. Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells. Pharmaceutics. 2021; 13(11):1881. https://doi.org/10.3390/pharmaceutics13111881
Chicago/Turabian StyleJelonek, Katarzyna, Alicja Zajdel, Adam Wilczok, Bożena Kaczmarczyk, Monika Musiał-Kulik, Anna Hercog, Aleksander Foryś, Małgorzata Pastusiak, and Janusz Kasperczyk. 2021. "Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells" Pharmaceutics 13, no. 11: 1881. https://doi.org/10.3390/pharmaceutics13111881






