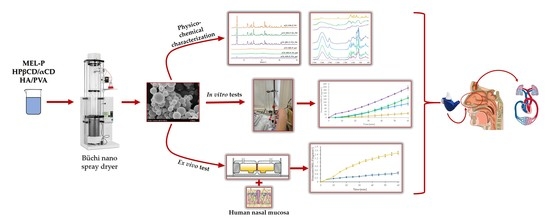
Physico-Chemical, In Vitro and Ex Vivo Characterization of Meloxicam Potassium-Cyclodextrin Nanospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
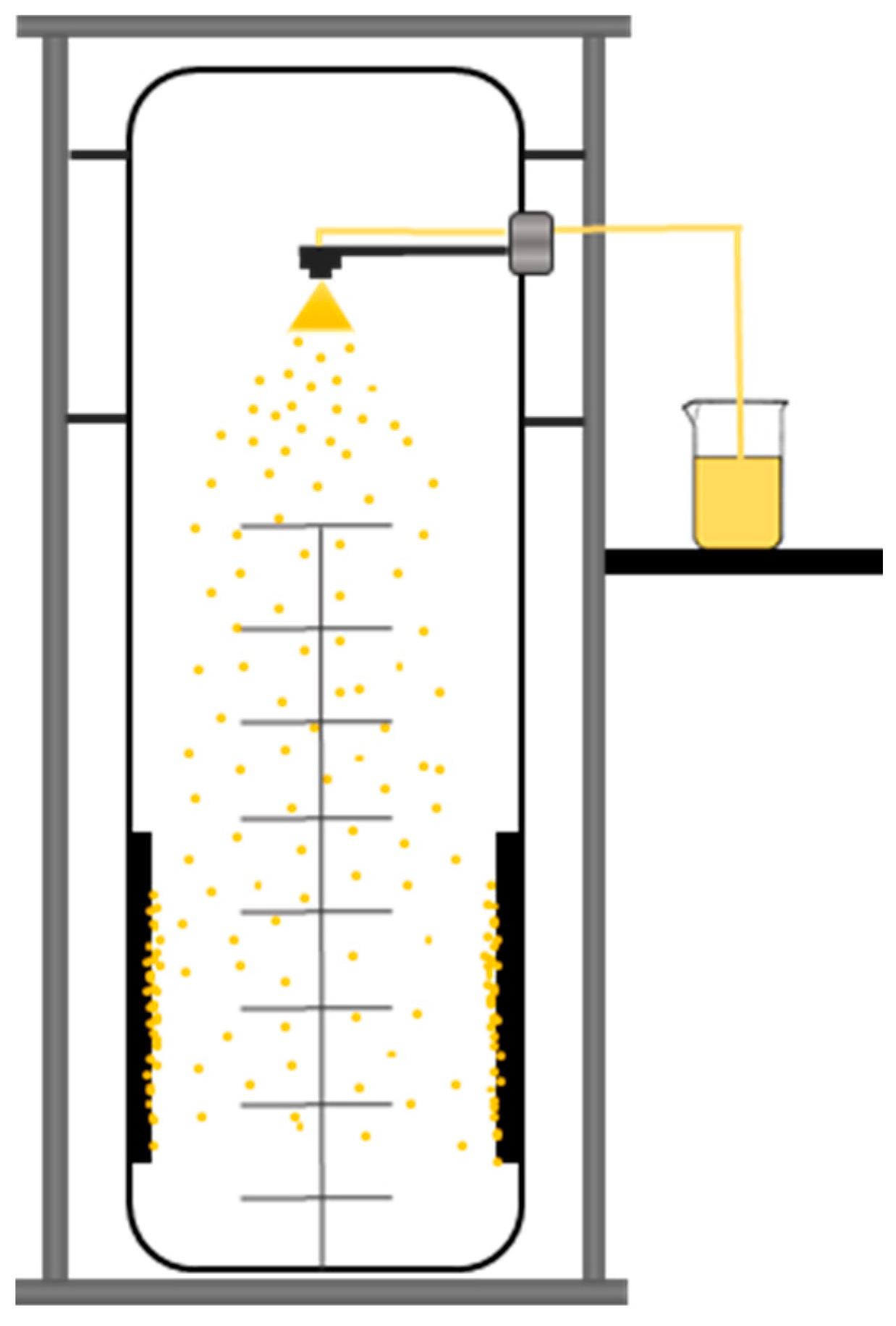
2.2.1. Preparation of the Spray Dried Samples
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. X-ray Powder Diffraction (XRPD)
2.2.5. Fourier-Transformed Infrared Spectroscopy (FT-IR)
2.2.6. Mucoadhesivity
2.2.7. In Vitro and Ex Vivo Permeability Studies
2.2.8. In Vitro Cytotoxicity Measurements
2.2.9. Examination of the Anti-Inflammatory Effect of Compounds in In Vitro Experiments
2.2.10. Total RNA Extraction and cDNA Synthesis
2.2.11. qPCR Amplification of IL-6, COX-2, IL-1b, Actb
3. Results and Discussion
3.1. Particle Size and Morphology
3.2. Thermal Properties
3.3. Structural Characterization
3.4. Secondary Interactions
3.5. Mucoadhesivity
3.6. In Vitro and Ex Vivo Permeability
3.7. In Vitro Cytotoxicity and IL-6, COX-2, IL-1b Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ugwoke, M.I.; Agu, R.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Mathias, N.R.; Hussain, M.A. Non-invasive Systemic Drug Delivery: Developability Considerations for Alternate Routes of Administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery—possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.-I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S.; Illum, L. Absorption Enhancers for Nasal Drug Delivery. Clin. Pharmacokinet. 2003, 42, 1107–1128. [Google Scholar] [CrossRef] [PubMed]
- Kublik, H.; Vidgren, M. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2007, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Alhalaweh, A.; Andersson, S.; Velaga, S.P. Preparation of zolmitriptan–chitosan microparticles by spray drying for nasal delivery. Eur. J. Pharm. Sci. 2009, 38, 206–214. [Google Scholar] [CrossRef]
- Chahal, H.; Matthews, S.; Jones, M. Effect of process conditions on spray dried calcium carbonate powders for thermal spraying. Ceram. Int. 2021, 47, 351–360. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; Pignatello, R., Musumeci, T., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-064-2. [Google Scholar]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B: Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, H.; Janaswamy, S. (Eds.) Natural Polymers for Drug Delivery; CABI: Oxfordshire, UK; Boston, MA, USA, 2017; ISBN 978-1-78064-447-9. [Google Scholar]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B.H. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef]
- Jambhekar, S.; Casella, R.; Maher, T. The physicochemical characteristics and bioavailability of indomethacin from β-cyclodextrin, hydroxyethyl-β-cyclodextrin, and hydroxypropyl-β-cyclodextrin complexes. Int. J. Pharm. 2004, 270, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical Applications of Cyclodextrins. 2. In Vivo Drug Delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J. Drug Target. 2009, 17, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Ainurofiq, A.; Choiri, S. Development and optimization of a meloxicam/β-cyclodextrin complex for orally disintegrating tablet using statistical analysis. Pharm. Dev. Technol. 2018, 23, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. 2012, 100B, 1451–1457. [Google Scholar] [CrossRef]
- Bartos, C.; Ambrus, R.; Sipos, P.; Budai-Szűcs, M.; Csányi, E.; Gáspár, R.; Márki, Á.; Seres, A.B.; Sztojkov-Ivanov, A.; Horváth, T.; et al. Study of sodium hyaluronate-based intranasal formulations containing micro- or nanosized meloxicam particles. Int. J. Pharm. 2015, 491, 198–207. [Google Scholar] [CrossRef]
- Sharpe, K.P.; Berkowitz, R.; A Tyndall, W.; Boyer, D.; McCallum, S.W.; Mack, R.J.; Du, W. Safety, Tolerability, and Effect on Opioid Use of Meloxicam IV Following Orthopedic Surgery. J. Pain Res. 2020, 13, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Vasilieva, E.A.; Lenina, O.A.; Nizameev, I.R.; Kadirov, M.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Cationic liposomes mediated transdermal delivery of meloxicam and ketoprofen: Optimization of the composition, in vitro and in vivo assessment of efficiency. Int. J. Pharm. 2021, 605, 120803. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Kovács, A.; Gáspár, R.; Sztojkov-Ivanov, A.; Márki, A.; Janáky, T.; Tömösi, F.; Kecskeméti, G.; Szabo-Revesz, P. Investigation of Absorption Routes of Meloxicam and Its Salt Form from Intranasal Delivery Systems. Molecules 2018, 23, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Prinz, G.; Powell, J.; Greenlee, L.; Almodovar, J. Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Mater. Sci. Eng. C 2021, 118, 111544. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, A.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Aerodynamic properties and in silico deposition of meloxicam potassium incorporated in a carrier-free DPI pulmonary system. Int. J. Pharm. 2017, 520, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Taylor, L.; Edgar, K.J. The role of polymers in oral bioavailability enhancement; a review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Gieszinger, P.; Kiss, T.; Szabó-Révész, P.; Ambrus, R. The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline. Pharmaceutics 2021, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.E.; Kast, C.E.; Bernkop-Schnürch, A. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm. Res. 2002, 19, 602–608. [Google Scholar] [CrossRef]
- Bartos, C.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Physico-Chemical and In Vitro Characterization of Chitosan-Based Microspheres Intended for Nasal Administration. Pharmaceutics 2021, 13, 608. [Google Scholar] [CrossRef]
- Simpson, R.J.; Hammacher, A.; Smith, D.K.; Matthews, J.; Ward, L.D. Interleukin-6: Structure-function relationships. Protein Sci. 1997, 6, 929–955. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Mezei, T.; Mesterházy, N.; Bakó, T.; Porcs-Makkay, M.; Simig, G.; Volk, B. Manufacture of High-Purity Meloxicam via Its Novel Potassium Salt Monohydrate. Org. Process. Res. Dev. 2009, 13, 567–572. [Google Scholar] [CrossRef]
- Mora, P.C.; Cirri, M.; Allolio, B.; Carli, F.; Mura, P. Enhancement of Dehydroepiandrosterone Solubility and Bioavailability by Ternary Complexation with α-Cyclodextrin and Glycine. J. Pharm. Sci. 2003, 92, 2177–2184. [Google Scholar] [CrossRef]
- Ho, B.T.; Joyce, D.C.; Bhandari, B.R. Encapsulation of ethylene gas into α-cyclodextrin and characterisation of the inclusion complexes. Food Chem. 2011, 127, 572–580. [Google Scholar] [CrossRef]
- Garnero, C.; Aiassa, V.; Longhi, M. Sulfamethoxazole:hydroxypropyl-β-cyclodextrin complex: Preparation and characterization. J. Pharm. Biomed. Anal. 2012, 63, 74–79. [Google Scholar] [CrossRef]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabrò, M.; Di Bella, M.; Rustichelli, C.; Gamberini, M.C.; Ficarra, P. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Ji, Q.; Fu, Y.; Zhao, L.; Li, C.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Mohit, V.; Harshal, G.; Neha, D.; Vilasrao, K.; Rajashree, H. A comparative study of complexation methods for cefdinir-hydroxypropyl-β-cyclodextrin system. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 57–66. [Google Scholar] [CrossRef]
- Kaur, I.; Kapil, M.; Smitha, R.; Aggarwal, D. Development of Topically Effective Formulations of Acetazolamide Using HP-β-CD-Polymer Co-Complexes. Curr. Drug Deliv. 2004, 1, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 2004, 14, 35–43. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Bonferoni, M.; Giunchedi, P.; Dalpiaz, A. Versatile Nasal Application of Cyclodextrins: Excipients and/or Actives? Pharmaceutics 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]











| Samples | Distilled Water (mL) | HPβCD (mg) | αCD (mg) | HA (mg) | PVA (mg) | MEL-P (mg) |
|---|---|---|---|---|---|---|
| HPβCD_MEL-P | 10 | 264.79 | - | - | - | 70 |
| HPβCD_MEL-P_HA | 10 | 264.79 | - | 5 | - | 70 |
| HPβCD_MEL-P_PVA | 10 | 264.79 | - | - | 10 | 70 |
| αCD_MEL-P | 10 | - | 167.11 | - | - | 70 |
| αCD_MEL-P_HA | 10 | - | 167.11 | 5 | - | 70 |
| αCD_MEL-P_PVA | 10 | - | 167.11 | - | 10 | 70 |
| Composition | Avarage PS (nm) |
|---|---|
| HPβCD_MEL-P_spd | 871 ± 439 |
| HPβCD_MEL-P_HA_spd | 868 ± 243 |
| HPβCD_MEL-P_PVA_spd | 723 ± 229 |
| αCD_MEL-P_spd | 612 ± 227 |
| αCD_MEL-P_HA_spd | 756 ± 175 |
| αCD_MEL-P_PVA_spd | 799 ± 256 |
| Formulation | Enhancement Ratio |
|---|---|
| HPβCD_MEL-P_spd | 3.33 |
| HPβCD_MEL-P_HA_spd | 4.68 |
| HPβCD_MEL-P_PVA_spd | 7.05 |
| αCD_MEL-P_spd | 1.75 |
| αCD_MEL-P_HA_spd | 1.61 |
| αCD_MEL-P_PVA_spd | 7.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, P.; Ambrus, R.; Szabó-Révész, P.; Kókai, D.; Burián, K.; Bella, Z.; Fenyvesi, F.; Bartos, C. Physico-Chemical, In Vitro and Ex Vivo Characterization of Meloxicam Potassium-Cyclodextrin Nanospheres. Pharmaceutics 2021, 13, 1883. https://doi.org/10.3390/pharmaceutics13111883
Varga P, Ambrus R, Szabó-Révész P, Kókai D, Burián K, Bella Z, Fenyvesi F, Bartos C. Physico-Chemical, In Vitro and Ex Vivo Characterization of Meloxicam Potassium-Cyclodextrin Nanospheres. Pharmaceutics. 2021; 13(11):1883. https://doi.org/10.3390/pharmaceutics13111883
Chicago/Turabian StyleVarga, Patrícia, Rita Ambrus, Piroska Szabó-Révész, Dávid Kókai, Katalin Burián, Zsolt Bella, Ferenc Fenyvesi, and Csilla Bartos. 2021. "Physico-Chemical, In Vitro and Ex Vivo Characterization of Meloxicam Potassium-Cyclodextrin Nanospheres" Pharmaceutics 13, no. 11: 1883. https://doi.org/10.3390/pharmaceutics13111883
APA StyleVarga, P., Ambrus, R., Szabó-Révész, P., Kókai, D., Burián, K., Bella, Z., Fenyvesi, F., & Bartos, C. (2021). Physico-Chemical, In Vitro and Ex Vivo Characterization of Meloxicam Potassium-Cyclodextrin Nanospheres. Pharmaceutics, 13(11), 1883. https://doi.org/10.3390/pharmaceutics13111883