Magnetoliposomes Based on Magnetic/Plasmonic Nanoparticles Loaded with Tricyclic Lactones for Combined Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Manganese Ferrite/Gold Nanoparticles
2.1.1. Manganese Ferrite Nanoparticles Preparation
2.1.2. Gold Nanoparticles Preparation
2.1.3. Synthesis of Gold-Seed Manganese Ferrite Nanoparticles
2.1.4. Synthesis of Gold-Coated Manganese Ferrite Core-Shell Nanoparticles
2.2. Preparation of Magnetoliposome-Type Structures
2.3. Preparation of Giant Unilamellar Vesicles (GUVs)
2.4. Spectroscopic Measurements
2.4.1. Absorption and Fluorescence Spectra
2.4.2. Drug Encapsulation Efficiency
2.5. Characterization of Structural and Magnetic Properties
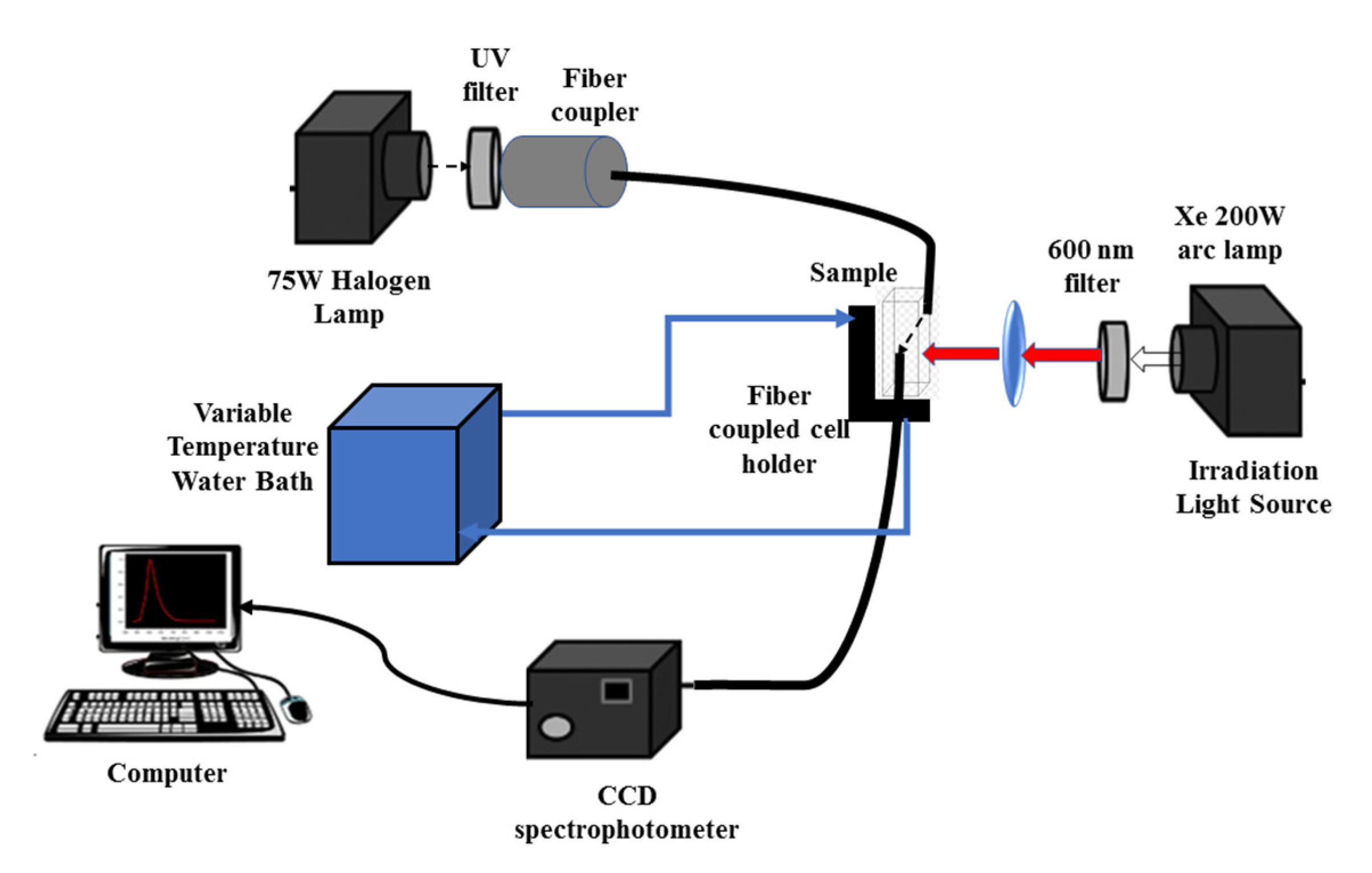
2.6. Measurement of the Photothermal Effect
2.7. Studies in Cell Lines
3. Results and Discussion
3.1. Photophysical Properties of the Antitumor Compounds
3.2. Nanoparticles Synthesis and Characterization
3.2.1. Synthesis of Manganese Ferrite/Gold Nanoparticles
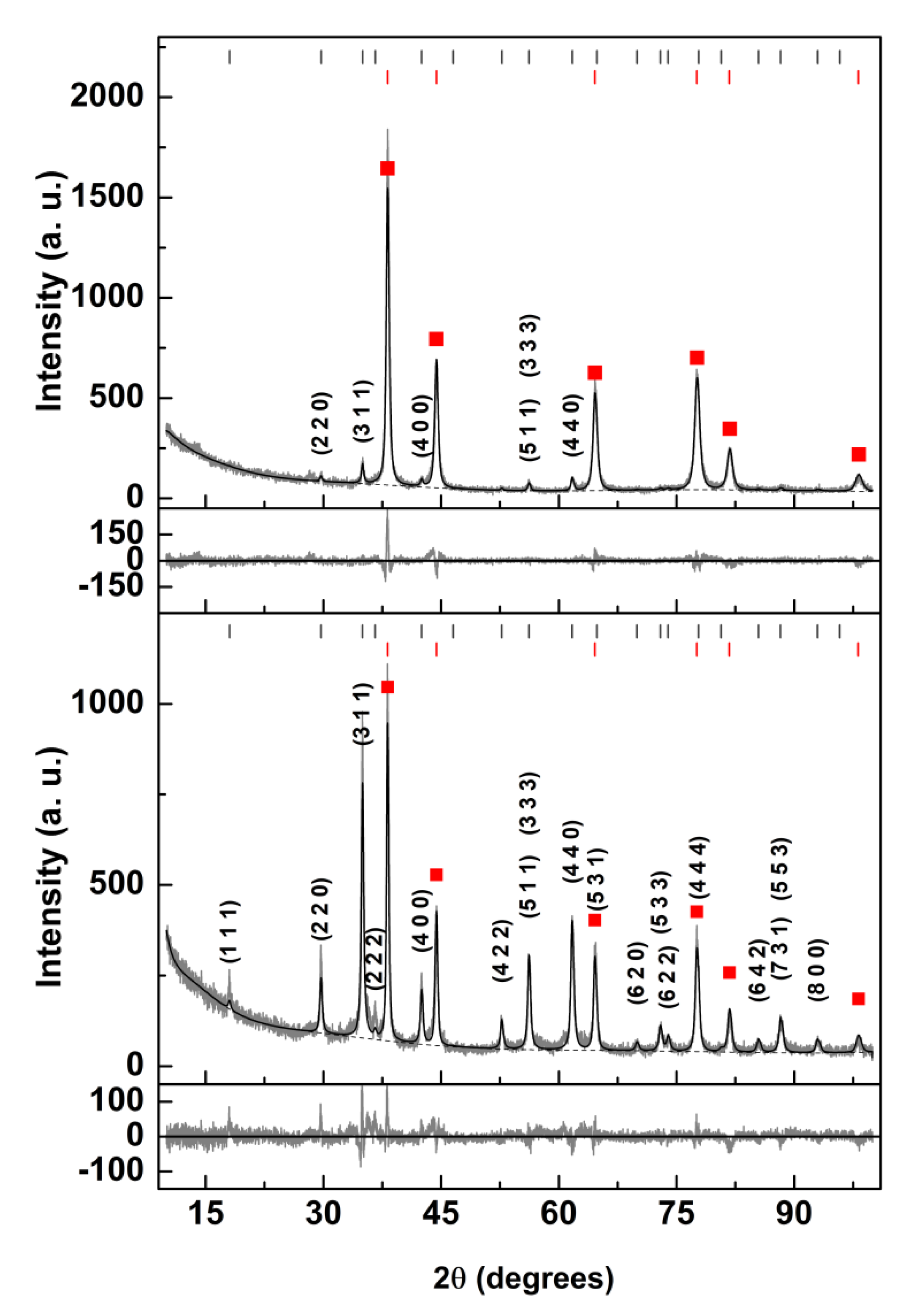
3.2.2. XRD Analysis
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Magnetic Properties
3.3. Drug-Loaded Magnetoliposomes
3.4. Cytotoxicity Assays in Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Bremer-Hoffmann, S.; Amenta, V.; Rossi, F. Nanomedicines in the European translational process. Eur. J. Nanomed. 2015, 7, 191–202. [Google Scholar] [CrossRef]
- Chang, E.H.; Harford, J.B.; Eatin, M.A.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticles drug delivery systems: An excellent carrier for tumor peptide vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond.) 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Gobbo, O.L.; Sjaatsad, K.; Radomski, M.K.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Datta, N.R.; Krishnan, S.; Speiser, D.E.; Neufeld, E.; Kuster, N.; Bodis, S.; Hofmann, H. Magnetic nanoparticle-induced hyperthermia with appropriate payloads: Paul Ehrlich’s “magic (nano)bullet” for cancer theranostics? Cancer Treat. Rev. 2016, 50, 217–227. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Garcia, M.P.F.; Guedes, A.; Tavares, P.B.; Grenèche, J.M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) nanoparticles: Tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Cabrera, L.I.; Somoza, A.; Marco, J.F.; Serna, C.J.; Morales, M.P. Synthesis and surface modification of uniform MFe2O4 (M = Fe, Mn, and Co) nanoparticles with tunable sizes and functionalities. J. Nanopart. Res. 2012, 14, 873. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmad, M.Z.; Kazmi, I.; Akhter, S.; Afzal, M.; Gupta, G.; Sinha, V.R. Emergence of Nanomedicine as cancer-targeted magic bullets: Recent development and need to address the toxicity apprehension. Curr. Drug Discov. Technol. 2012, 9, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kafrouni, L.; Savadogo, O. Recent progress on magnetic nanoparticles for magnetic hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.; An, S.; Yi, D. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Yeh, Y.; Creran, B.; Rotello, V. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Carneiro, M.H.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health B 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Stockman, M. Nanoplasmonics: The physics behind the applications. Phys. Today 2011, 64, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.; Iatì, M. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Larsen, G.K.; Farr, W.; Murph, S.E.H. Multifunctional Fe2O3-Au nanoparticles with different shapes: Enhanced catalysis, photothermal effects, and magnetic recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Stafford, S.; Garcia, R.S.; Gun’ku, Y.K. Multimodal magnetic-plasmonic nanoparticles for biomedical applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Reguera, J.; Curcio, A.; Muñoz--Noval, A.; Kuttner, C.; Van de Walle, A.; Liz--Marzán, L.M.; Wilhelm, C. Janus Magnetic--Plasmonic Nanoparticles for Magnetically Guided and Thermally Activated Cancer Therapy. Small 2020, 16, 1904960. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Vale-Silva, L.; Pinto, E.; Nascimento, M.S.-J. Novel [6-(hetero)arylamino]thieno [3,2-b]pyridines: Synthesis and antitumoral activities. Eur. J. Med. Chem. 2010, 45, 5732–5738. [Google Scholar] [CrossRef]
- Machado, V.A.; Peixoto, D.R.; Costa, H.J.C.; Froufe, R.C.; Calhelha, R.M.V.; Abreu, I.C.F.R.; Ferreira, R.; Soares, M.-J.R.P. Queiroz, Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorg. Med. Chem. 2015, 23, 6497–6509. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.M.; Buisson, P.; Pereira, J.M.; Pinheiro, I.M.; Fernández-Marcelo, T.; Vasconcelos, M.H.; Berteina-Raboin, S.; Queiroz, M.-J.R.P. Synthesis of novel 8-(het)aryl-6H-pyrano[4′,3′:4,5]thieno[3,2-b] pyridines by 6-endo-dig cyclization of Sonogashira products and halolactonizations with Cu salts/NXS. Preliminary antitumor evaluation. Tetrahedron 2019, 75, 1387–1397. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Brown, K.R.; Natan, M.J. Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir 1998, 14, 726–728. [Google Scholar] [CrossRef]
- Tamba, Y.; Terashima, H.; Yamazaki, M. A membrane filtering method for the purification of giant unilamellar vesicles. Chem. Phys. Lipids 2011, 164, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim. Biophys. Acta 2002, 1564, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.F.; Graça, V.C.; Calhelha, R.C.; Ferreira, I.C.F.R.; Santos, P.F. Aminosquaraines as potential photodynamic agents: Synthesis and evaluation of in vitro cytotoxicity. Bioorg. Med. Chem. Lett. 2017, 27, 4467–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, M.-J.R.P.; Peixoto, D.; Rodrigues, A.R.O.; Mendes, P.M.F.; Costa, C.N.C.; Coutinho, P.J.G.; Castanheira, E.M.S. New 1,3-diarylureas linked by C–C Suzuki coupling to the methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate moiety: Synthesis and fluorescence studies in solution and in lipid membranes. J. Photochem. Photobiol. A Chem. 2013, 255, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.N.C.; Hortelão, A.C.L.; Ramos, J.M.F.; Oliveira, A.D.S.; Calhelha, R.C.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. A new antitumoral heteroarylaminothieno[3,2-b]pyridine derivative: Its incorporation into liposomes and interaction with proteins monitored by fluorescence. Photochem. Photobiol. Sci. 2014, 13, 1730–1740. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Almeida, B.G.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thieno[3,2-b]pyridin-7-arylamines: Photophysical and biological studies. RSC Adv. 2017, 7, 15352–15361. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for antitumor drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.; Kiely, C.J.; Bethell, D.; Schiffrin, D.J. Self-Organization of Nanosized Gold Particles. Chem. Mater. 1998, 10, 922–926. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of multifunctional liposomes containing magnetic/plasmonic MnFe2O4/Au core/shell nanoparticles. Pharmaceutics 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, C.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of novel magnetoliposomes containing nickel ferrite nanoparticles covered with gold for applications in thermotherapy. Materials 2020, 13, 815. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xu, X.; Liu, X. Influence of dielectric core, embedding medium and size on the optical properties of gold nanoshells. Solid State Commun. 2008, 146, 7–11. [Google Scholar] [CrossRef]
- Qian, X.; Bai, J. Theoretical Studies of the Optical Properties of Hollow Spherical Metallic Nanoshells. J. Comput. Theor. Nanosci. 2013, 10, 2354–2360. [Google Scholar] [CrossRef]
- Li, R.; Gu, X.; Liang, X.; Hou, S.; Hu, D. Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acid and Hydrogen Peroxide. Materials 2019, 12, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, J.; Friedel, P.; Kleeberg, R. Commission on Powder Diffraction Newsletter; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998; pp. 5–8. [Google Scholar]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallog. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, D.; Casula, M.F.; Falqui, A.; Loche, D.; Mountjoy, G.; Sangregorio, C.; Corrias, A. A structural and magnetic investigation of the inversion degree in ferrite nanocrystals MFe2O4 (M = Mn, Co, Ni). J. Phys. Chem. C 2009, 113, 8606–8615. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-based plasmonic magnetogels: A supramolecular composite nanosystem for multimodal cancer therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; John, S.A.; Sagara, T. Simultaneous Determination of Paracetamol and Ascorbic Acid Using Tetraoctylammonium Bromide Capped Gold Nanoparticles Immobilized on 1,6-Hexanedithiol Modified Au Electrode. Electrochim. Acta 2009, 54, 6837–6843. [Google Scholar] [CrossRef]
- Crespo, P.; Litrán, R.; Rojas, T.C.; Multigner, M.; Fuente, J.M.; Sanchez-Lopez, J.C.; García, M.A.; Hernando, A.; Penades, S.; Fernández, A. Permanent magnetism, magnetic anisotropy, and hysteresis of thiol-capped gold nanoparticles. Phys. Rev. Lett. 2004, 93, 087204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, J. Magnetic Properties of Materials; McGraw Hill: New York, NY, USA, 1971; p. 89. ISBN 978-0070584457. [Google Scholar]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C.; Devlin, E.; Kostikas, A. Size-dependent magnetic properties of MnFe2O4 fine particles synthesized by coprecipitation. Phys. Rev. B Condens. Matter 1996, 54, 9288–9296. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anticancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
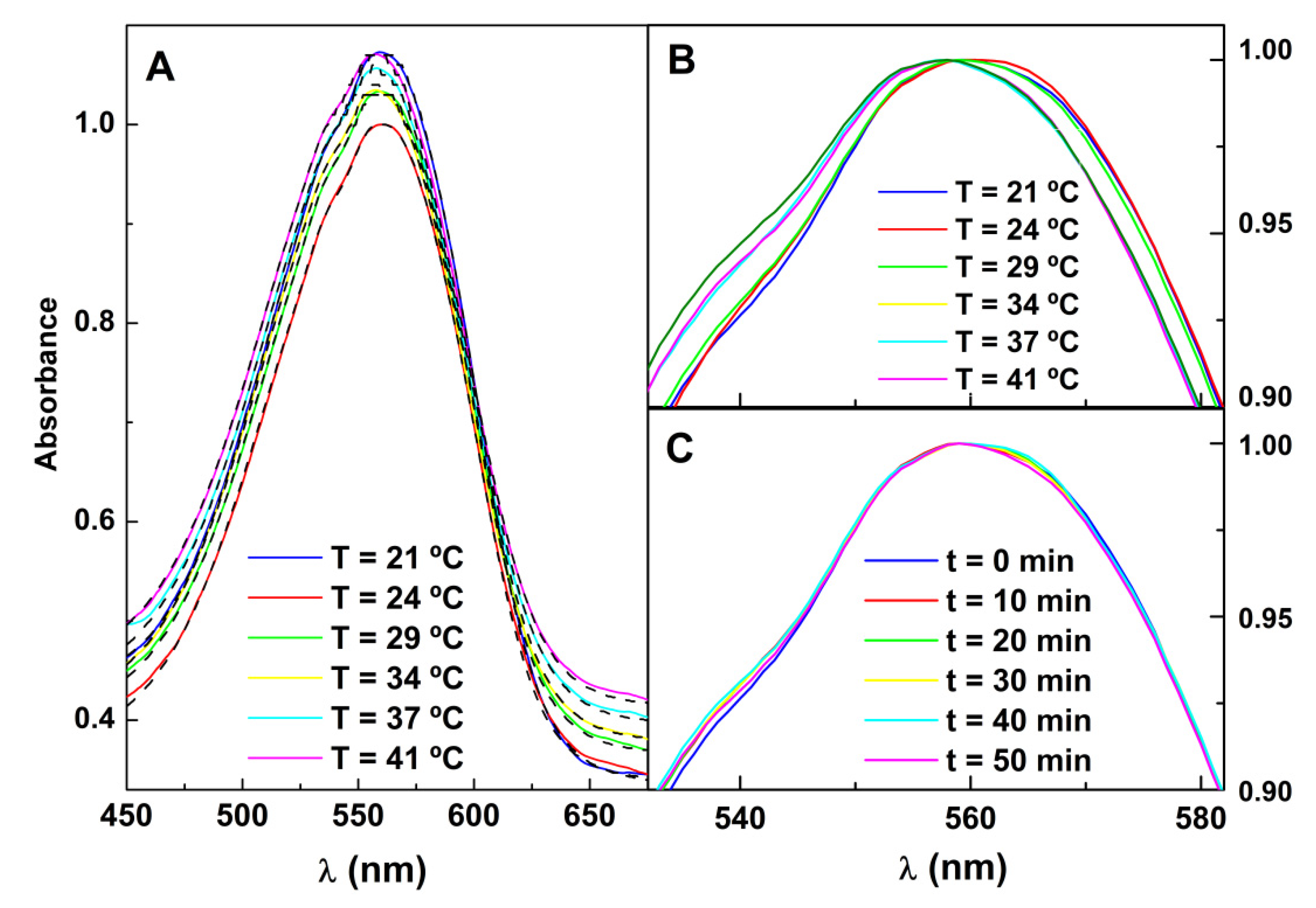
- Kawski, A.; Kukliński, B.; Bojarski, P. Photophysical Properties and Thermochromic Shifts of Electronic Spectra of Nile Red in Selected Solvents. Excited States Dipole Moments. Chem. Phys. 2009, 359, 58–64. [Google Scholar] [CrossRef]












| GI50 (μM) [28] | Compound 1 | Compound 2 |
|---|---|---|
| HCT-15 | 7.4 ± 1.1 | 12.6 ± 2.9 |
| NCI-H460 | 7.3 ± 0.1 | 11.4 ± 0.9 |
| Solvent | λabs/nm (ε/104 M−1 cm−1) | λem/nm | ||
|---|---|---|---|---|
| Compound 1 | Compound 2 | Compound 1 | Compound 2 | |
| Chloroform | 369 (1.33) | 403 (1.05) | 425 | 523 |
| Ethyl acetate | 364 (1.41) | 405 (1.16) | 427 | 532 |
| Ethanol | 365 (1.33) | 415 (1.10) | 430 | 581 |
| Acetonitrile | 363 (1.50) | 403 (1.21) | 428 | 568 |
| Sample | Ox,y,z (*) | i (**) | Phase Size (nm) Lattice Constant (nm) | Au (wt%) | RP | χ2 | |
|---|---|---|---|---|---|---|---|
| MnFe2O4 | Au | ||||||
| Au@MnFe2O4 | 0.3865 | 0.2 (+) | 26.7 0.8498 | 41.9 0.4078 | 12.8 | 10.5 | 1.96 |
| MnFe2O4/Au | 0.3885 | 0.2 (+) | 28.8 0.8497 | 27.9 0.4078(+) | 74.7 | 9.4 | 1.62 |
| T (K) | Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Mr/Ms | |
|---|---|---|---|---|---|
| MnFe2O4 [39] | 300 | 6.30 | 0.58 | 36.00 | 0.02 |
| MnFe2O4 NPs decorated with Au NPs | 5 | 247.47 | 17.68 | 71.02 | 0.25 |
| 300 | 46.23 | 2.17 | 33.66 | 0.06 | |
| MnFe2O4/Au core/shell NPs | 5 | 173.82 | 1.93 | 14.43 | 0.13 |
| 300 | 45.95 | 0.55 | 6.89 | 0.08 |
| Type of NPs | Size ± SD (nm) | PdI ± SD | ||
|---|---|---|---|---|
| After Preparation | After 7 Days | After Preparation | After 7 Days | |
| Magnetoliposomes with decorated NPs | 124 ± 28 | 136 ± 34 | 0.28 ± 0.08 | 0.32 ± 0.062 |
| Magnetoliposomes with core-shell NPs | 132 ± 21 | 147 ± 28 | 0.23 ± 0.04 | 0.26 ± 0.05 |
| Irradiation Time (min). | Magnetoliposomes with Decorated NPs | Magnetoliposomes with Core/Shell NPs |
|---|---|---|
| 0 | 21 °C | 21 °C |
| 10 | 21.6 °C | 21.8 °C |
| 20 | 22.5 °C | 23.1 °C |
| 30 | 22.8 °C | 23.8 °C |
| 40 | 22.9 °C | 23.9 °C |
| 50 | 22.9 °C | 24.0 °C |
| System | EE% ± SD% | |
|---|---|---|
| Compound 1 | Compound 2 | |
| Magnetoliposomes with core-shell NPs | 99.3 ± 0.8 | 99.9 ± 0.5 |
| Magnetoliposomes with decorated NPs | 98.5 ± 1.3 | 99.8 ± 0.6 |
| System | GI50 ± SD (μM) | |
|---|---|---|
| Compound 1 | Compound 2 | |
| Drug-loaded magnetoliposomes with decorated NPs | --- | --- |
| Drug-loaded magnetoliposomes with core-shell NPs | 0.11 ± 0.02 | 0.12 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; et al. Magnetoliposomes Based on Magnetic/Plasmonic Nanoparticles Loaded with Tricyclic Lactones for Combined Cancer Therapy. Pharmaceutics 2021, 13, 1905. https://doi.org/10.3390/pharmaceutics13111905
Rio ISR, Rodrigues ARO, Rodrigues JM, Queiroz M-JRP, Calhelha RC, Ferreira ICFR, Almeida BG, Pires A, Pereira AM, Araújo JP, et al. Magnetoliposomes Based on Magnetic/Plasmonic Nanoparticles Loaded with Tricyclic Lactones for Combined Cancer Therapy. Pharmaceutics. 2021; 13(11):1905. https://doi.org/10.3390/pharmaceutics13111905
Chicago/Turabian StyleRio, Irina S. R., Ana Rita O. Rodrigues, Juliana M. Rodrigues, Maria-João R. P. Queiroz, R. C. Calhelha, Isabel C. F. R. Ferreira, Bernardo G. Almeida, Ana Pires, André M. Pereira, João P. Araújo, and et al. 2021. "Magnetoliposomes Based on Magnetic/Plasmonic Nanoparticles Loaded with Tricyclic Lactones for Combined Cancer Therapy" Pharmaceutics 13, no. 11: 1905. https://doi.org/10.3390/pharmaceutics13111905
APA StyleRio, I. S. R., Rodrigues, A. R. O., Rodrigues, J. M., Queiroz, M.-J. R. P., Calhelha, R. C., Ferreira, I. C. F. R., Almeida, B. G., Pires, A., Pereira, A. M., Araújo, J. P., Castanheira, E. M. S., & Coutinho, P. J. G. (2021). Magnetoliposomes Based on Magnetic/Plasmonic Nanoparticles Loaded with Tricyclic Lactones for Combined Cancer Therapy. Pharmaceutics, 13(11), 1905. https://doi.org/10.3390/pharmaceutics13111905









