Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candida Strains, Media, and Growth Conditions
2.2. Antifungal Compounds
2.2.1. Ceragenins
2.2.2. Gold Nanoparticles (Au NPs) Functionalized by CSA-13, CSA-44, and CSA-131
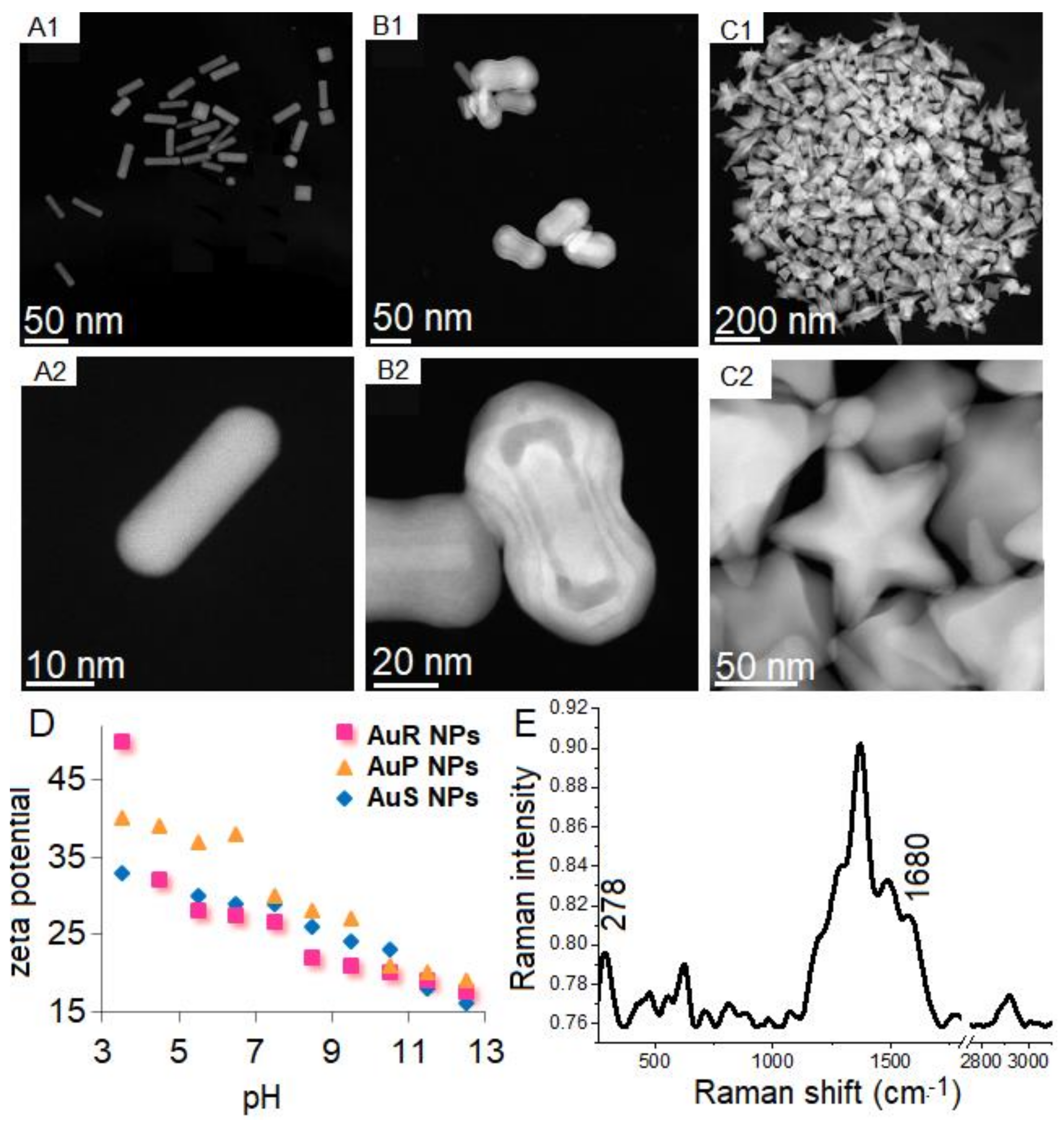
2.2.3. Physicochemical Properties of AuR NPs@CSA-13, AuP NPs@CSA-13, AuS NPs@CSA-13; AuR NPs@CSA-44, AuP NPs@CSA-44 and AuS NPs@CSA-44, and AuR NPs@CSA-131, AuP NPs@CSA-131, AuS NPs@CSA-131 Nanoparticles
2.3. Methods
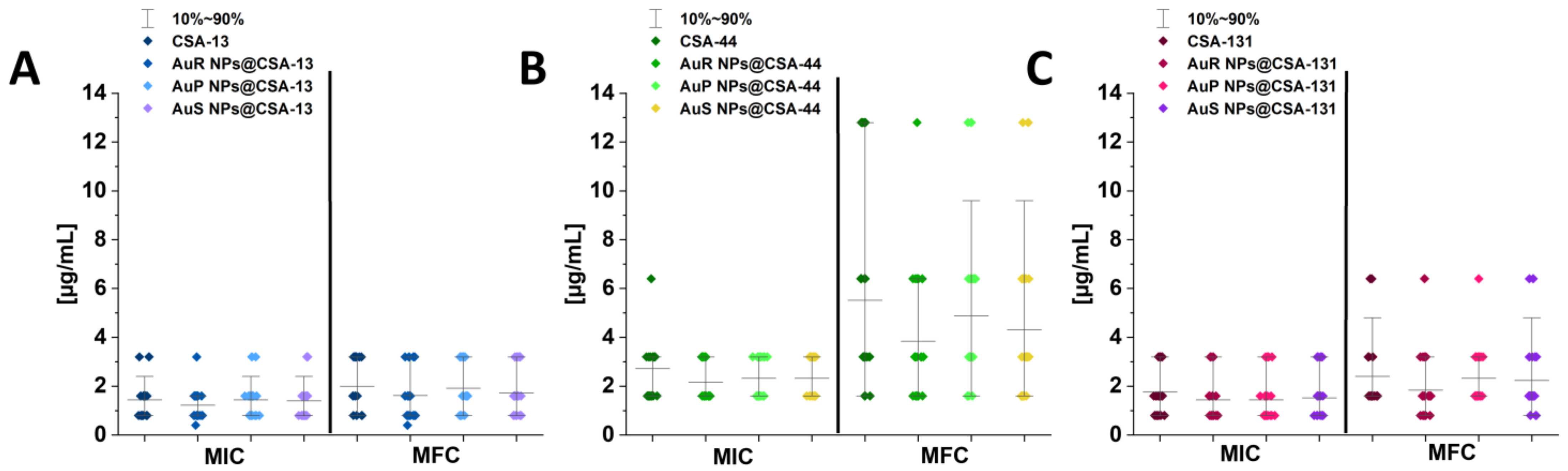
2.3.1. MIC/MFC Measurements
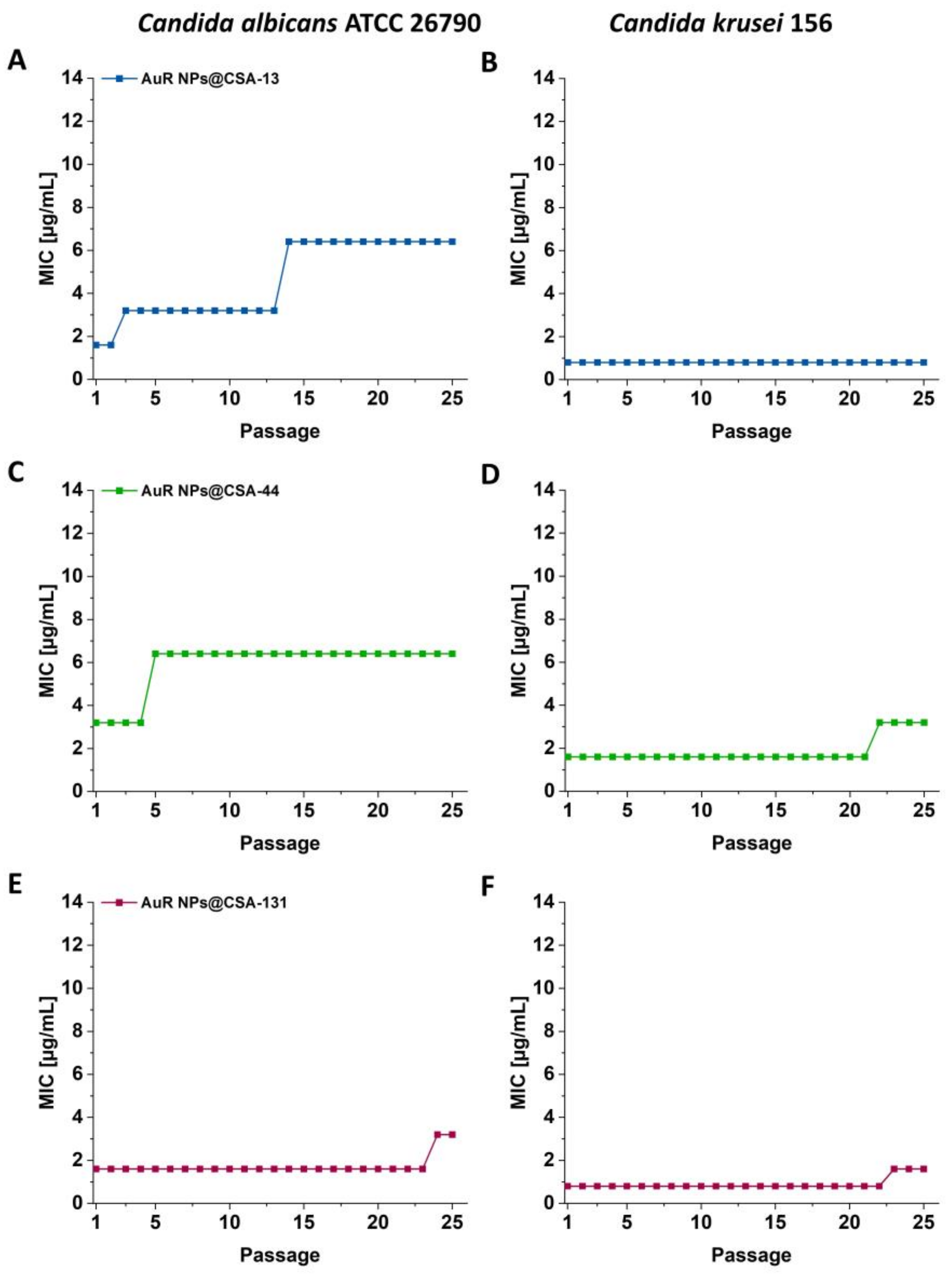
2.3.2. Induction of C. albicans ATCC 26790 and C. krusei 156 Resistance to AuR NPs@CSA-13, AuR NPs@CSA-44, and AuR NPs@CSA-131
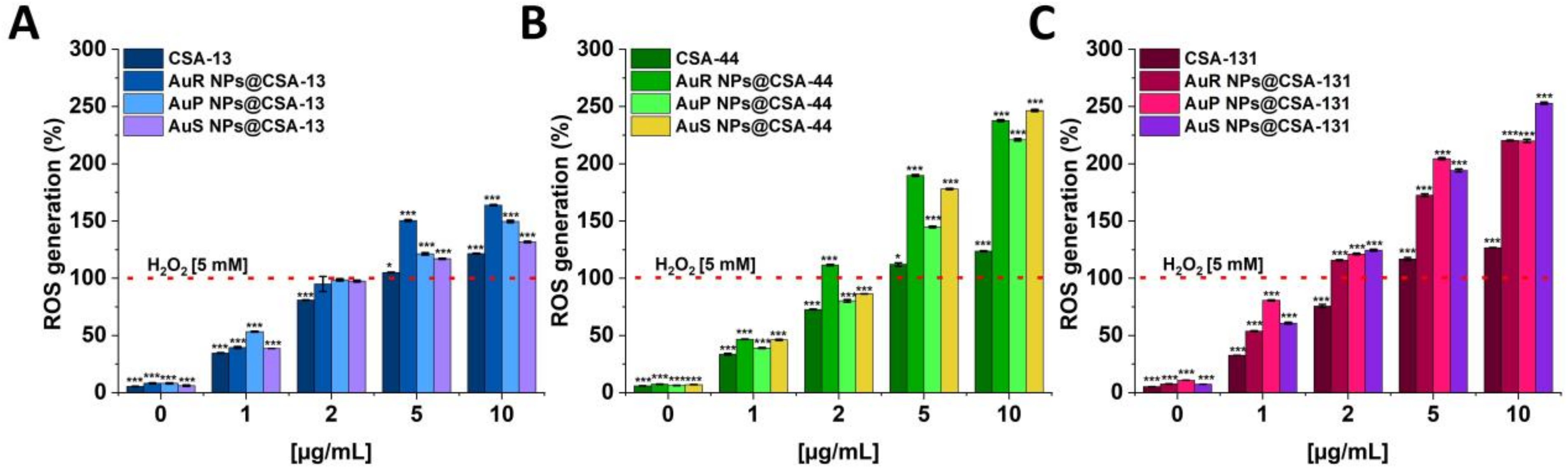
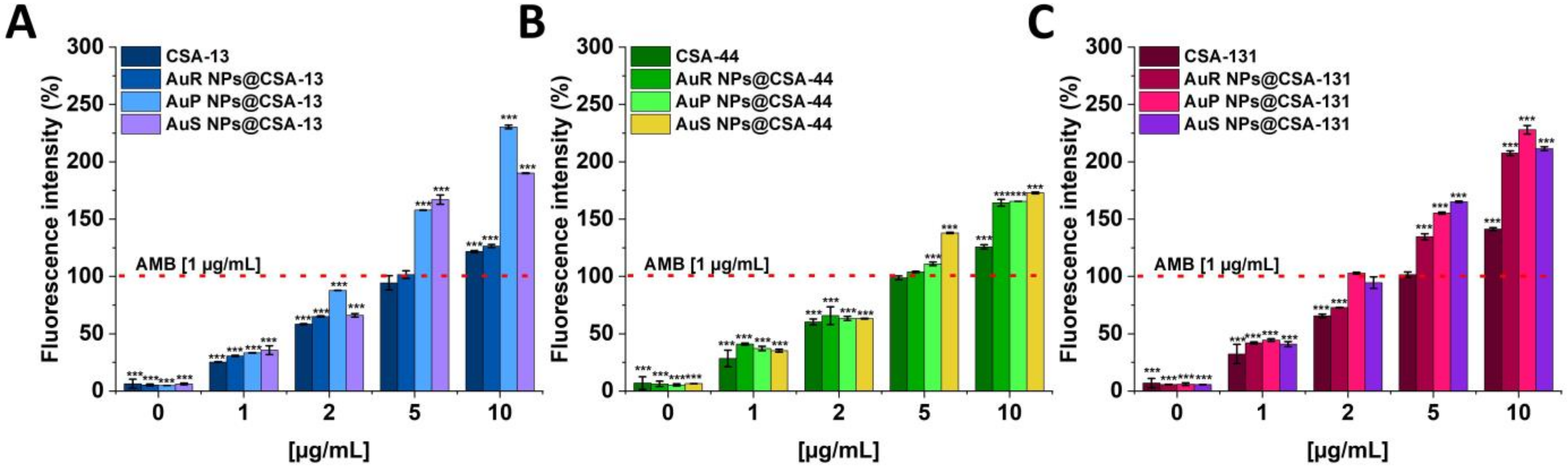
2.3.3. ROS Generation Assessment
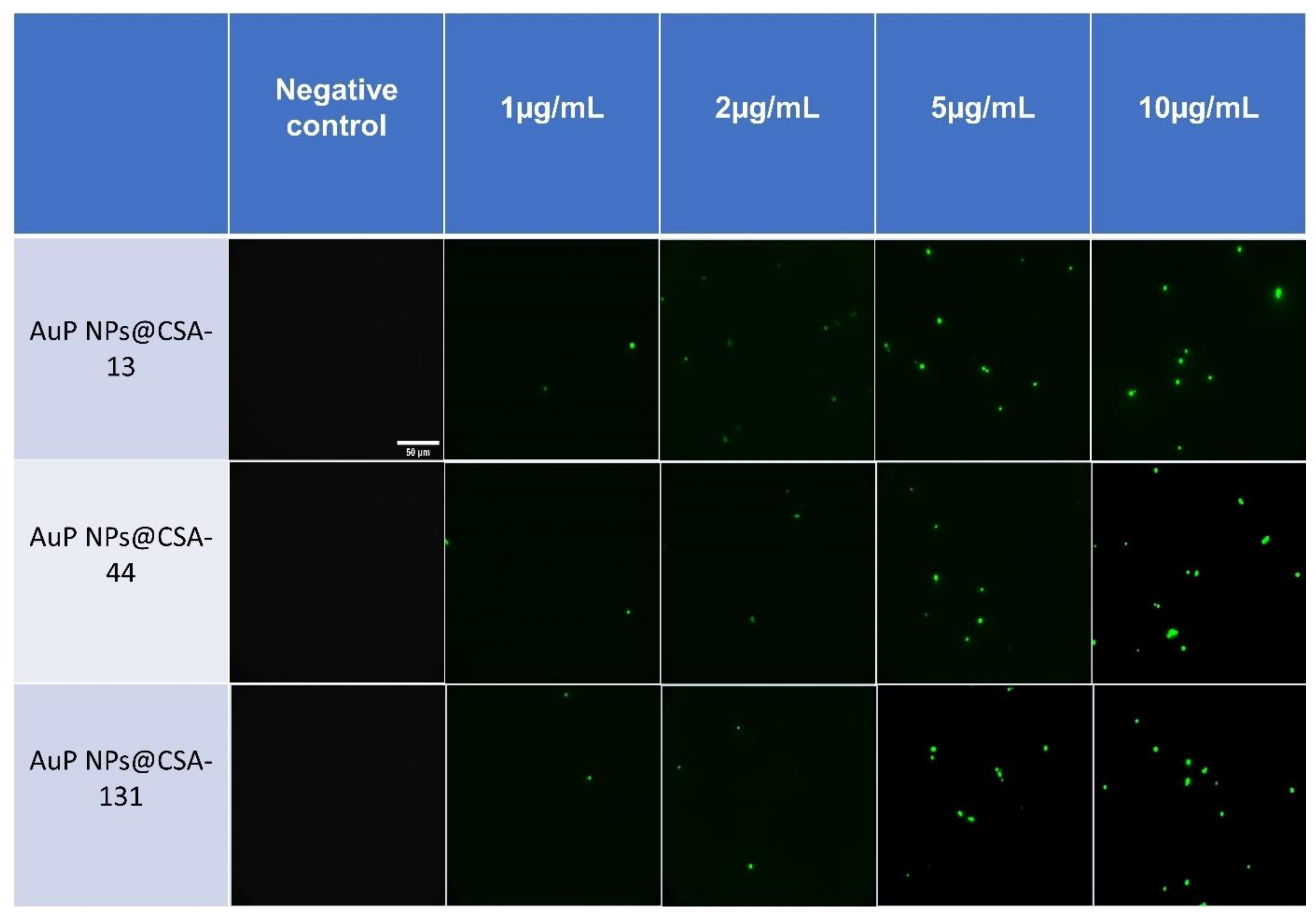
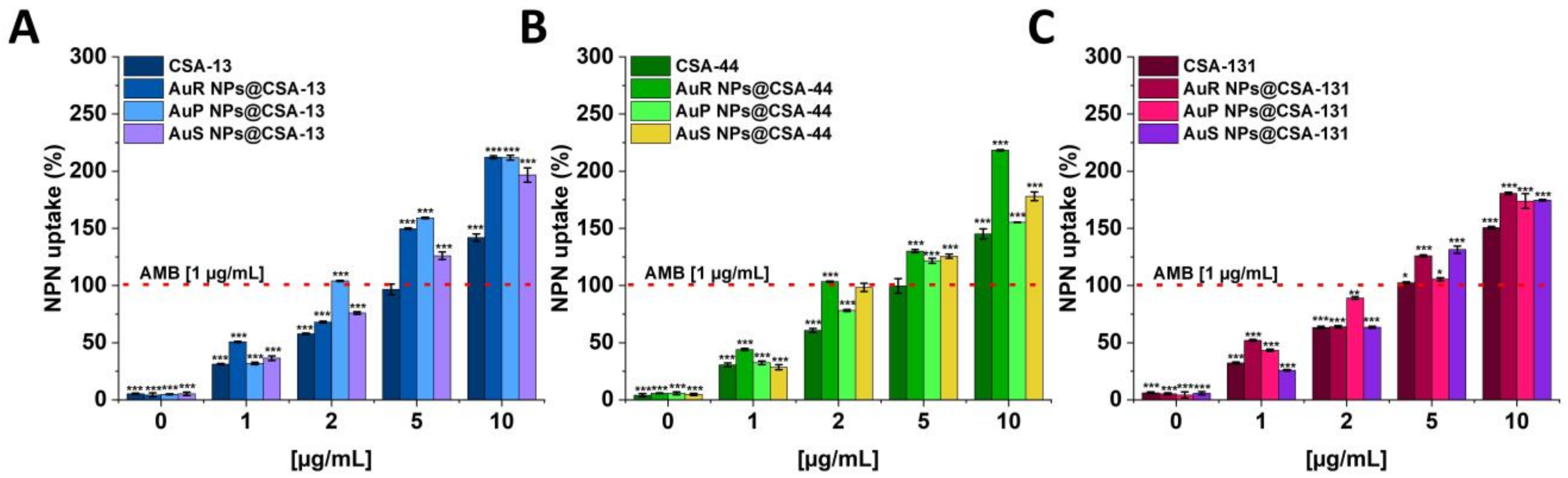
2.3.4. Membrane Permeabilization Assay
2.3.5. diSC(3) Assay
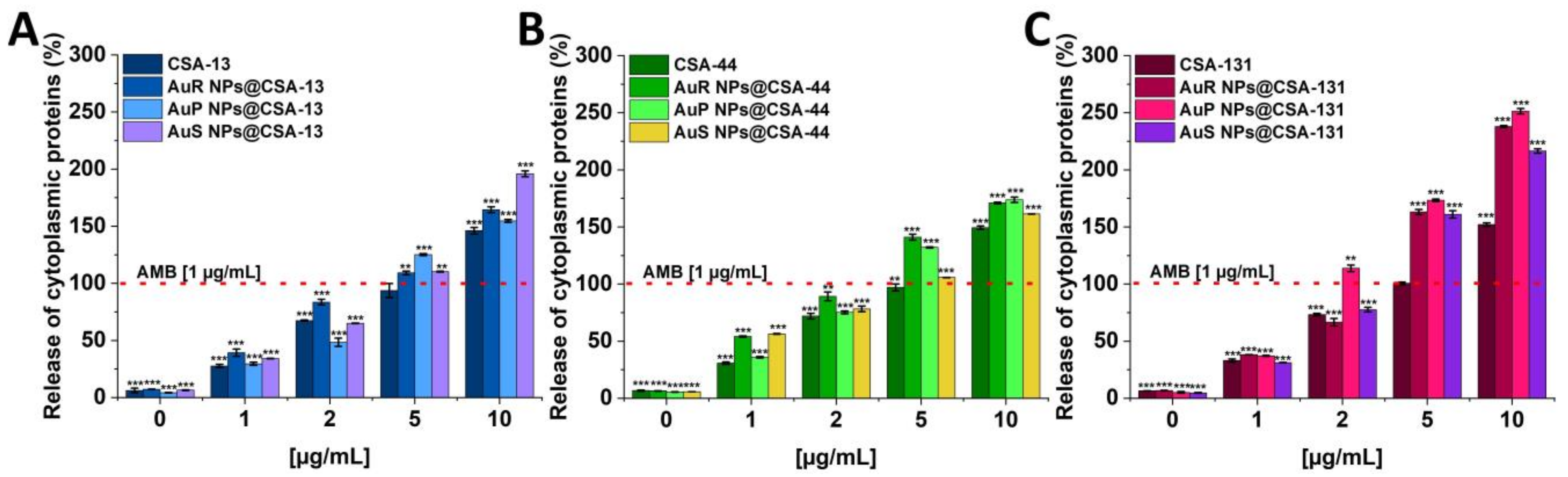
2.3.6. Assessment of Protein Leakage
2.3.7. Haemolytic Activity Assessment
2.3.8. Statistical Analysis
3. Results
3.1. Physicochemical Nature of Rod-, Peanut-, and Star-Shaped Au NPs
3.2. Susceptibility of Tested Candida Strains to Antimycotics and Developed Nanoparticles
3.3. Serial Passaging Experiment to Induce Development of Resistance towards AuR NPs@CSA-13, AuR NPs@CSA-44, and AuR NPs@CSA-131 by Candida Cells
3.4. Antifungal Activity of Nanosystems Involves Generation of Reactive Oxygen Species, Depolarization, and Disruption of the Cell Membrane as Well as Protein Leakage
3.5. Ceragenin-Based Nanosystems Exhibit Biocompatibility at Fungicidal Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwab, F.; Geffers, C.; Behnke, M.; Gastmeier, P. ICU mortality following ICU-acquired primary bloodstream infections according to the type of pathogen: A prospective cohort study in 937 Germany ICUs (2006–2015). PLoS ONE 2018, 13, e0194210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem-Zoubi, N.; Zorbavel, D.; Khoury, J.; Geffen, Y.; Qasum, M.; Predescu, S.; Paul, M. The association between treatment appropriateness according to EUCAST and CLSI breakpoints and mortality among patients with candidemia: A retrospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzzati, R.; Merelli, M.; Ansaldi, F.; Rosin, C.; Azzini, A.; Cavinato, S.; Brugnaro, P.; Vedovelli, C.; Cattelan, A.; Marina, B.; et al. Nosocomial candidemia in patients admitted to medicine wards compared to other wards: A multicentre study. Infection 2016, 44, 747–755. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- CDC Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 15 November 2021).
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.V.; Prado, C.G.; Carvalho, R.R.; Dias, K.S.; Dias, A.L. Candida albicans and non-C. albicans Candida species: Comparison of biofilm production and metabolic activity in biofilms, and putative virulence properties of isolates from hospital environments and infections. Mycopathologia 2013, 175, 265–272. [Google Scholar] [CrossRef]
- Brunke, S.; Hube, B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol. 2013, 15, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [Green Version]
- Maccallum, D.M. Hosting infection: Experimental models to assay Candida virulence. Int. J. Microbiol. 2012, 2012, 363764. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [Green Version]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [Green Version]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Perlin, D.S. Antifungal drug resistance: Do molecular methods provide a way forward? Curr. Opin. Infect. Dis. 2009, 22, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Pagano, L.; Mayor, S. Invasive fungal infections in high-risk patients: Report from TIMM-8 2017. Future Sci. OA 2018, 4, FSO307. [Google Scholar] [CrossRef] [Green Version]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front. Cell. Infect. Microbiol. 2020, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.; Del Fiol, F.S.; Balcão, V.M. Prospects for the Use of New Technologies to Combat Multidrug-Resistant Bacteria. Front. Pharmacol. 2019, 10, 692. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. Biomed. Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Durnas, B.; Piktel, E.; Watek, M.; Wollny, T.; Gozdz, S.; Smok-Kalwat, J.; Niemirowicz, K.; Savage, P.B.; Bucki, R. Anaerobic bacteria growth in the presence of cathelicidin LL-37 and selected ceragenins delivered as magnetic nanoparticles cargo. BMC Microbiol. 2017, 17, 167. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. Ayu 2018, 39, 182–186. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of action and anti-Candida activity of Artemisia annua mediated-synthesized silver nanoparticles. J. Mycol. Med. 2019, 29, 201–209. [Google Scholar] [CrossRef]
- Edis, Z.; Haj Bloukh, S.; Ibrahim, M.R.; Abu Sara, H. “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI). Pharmaceutics 2020, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Chmielewska, S.J.; Skłodowski, K.; Depciuch, J.; Deptuła, P.; Piktel, E.; Fiedoruk, K.; Kot, P.; Paprocka, P.; Fortunka, K.; Wollny, T.; et al. Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains. Pharmaceutics 2021, 13, 425. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Cieśluk, M.; Chmielewska, S.; Durnaś, B.; Król, G.; Wollny, T.; Deptuła, P.; Kochanowicz, J. Rod-shaped gold nanoparticles exert potent candidacidal activity and decrease the adhesion of fungal cells. Nanomedicine 2020, 15, 2733–2752. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [Green Version]
- Jebali, A.; Hajjar, F.H.; Pourdanesh, F.; Hekmatimoghaddam, S.; Kazemi, B.; Masoudi, A.; Daliri, K.; Sedighi, N. Silver and gold nanostructures: Antifungal property of different shapes of these nanostructures on Candida species. Med. Mycol. 2014, 52, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.; Taotofa, U.; Orsak, T.; Chadwell, M.; Savage, P.B. Synthesis and characterization of peptide-cationic steroid antibiotic conjugates. Org. Lett. 2004, 6, 3433–3436. [Google Scholar] [CrossRef]
- Lai, X.Z.; Feng, Y.; Pollard, J.; Chin, J.N.; Rybak, M.J.; Bucki, R.; Epand, R.F.; Epand, R.M.; Savage, P.B. Ceragenins: Cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 2008, 41, 1233–1240. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Skłodowski, K.; Piktel, E.; Suprewicz, Ł.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef]
- Savage, P.B.; Li, C.; Taotafa, U.; Ding, B.; Guan, Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef]
- Wnorowska, U.; Niemirowicz, K.; Myint, M.; Diamond, S.L.; Wróblewska, M.; Savage, P.B.; Janmey, P.A.; Bucki, R. Bactericidal activity of cathelicidin LL-37 and select cationic lipids against the hypervirulent P. aeruginosa strain LESB58. Antimicrob. Agents Chemother. 2015, 59, 3808–3815. [Google Scholar] [CrossRef] [Green Version]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal activity of ceragenin CSA-13 in cell culture and an animal model of peritoneal infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef] [Green Version]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Luca, V.; McDermott, A.M. Fighting microbial infections: A lesson from amphibian skin-derived esculentin-1 peptides. Peptides 2015, 71, 286–295. [Google Scholar] [CrossRef]
- Kodedová, M.; Sychrová, H. Synthetic antimicrobial peptides of the halictines family disturb the membrane integrity of Candida cells. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Wnorowska, U.; Pogoda, K.; Deptuła, P.; Wątek, M.; Piktel, E.; Głuszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Piktel, E.; Durnaś, B.; Fiedoruk, K.; Savage, P.B.; Bucki, R. Use of ceragenins as a potential treatment for urinary tract infections. BMC Infect. Dis. 2019, 19, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Guan, Q.; Walsh, J.P.; Boswell, J.S.; Winter, T.W.; Winter, E.S.; Boyd, S.S.; Li, C.; Savage, P.B. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 2002, 45, 663–669. [Google Scholar] [CrossRef]
- Levin, C.S.; Janesko, B.G.; Bardhan, R.; Scuseria, G.E.; Hartgerink, J.D.; Halas, N.J. Chain-length-dependent vibrational resonances in alkanethiol self-assembled monolayers observed on plasmonic nanoparticle substrates. Nano Lett. 2006, 6, 2617–2621. [Google Scholar] [CrossRef]
- Bazylewski, P.; Divigalpitiya, R.; Fanchini, G. In situ Raman spectroscopy distinguishes between reversible and irreversible thiol modifications in L-cysteine. RSC Adv. 2017, 7, 2964–2970. [Google Scholar] [CrossRef] [Green Version]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Kapoor, K. Multidrug resistance in yeast Candida. Int. Rev. Cytol. 2005, 242, 215–248. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [Green Version]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar]
- Canela, H.M.S.; Cardoso, B.; Vitali, L.H.; Coelho, H.C.; Martinez, R.; Ferreira, M.E.D.S. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses 2018, 61, 11–21. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef]
- Gauthier, G.M. Fungal Dimorphism and Virulence: Molecular Mechanisms for Temperature Adaptation, Immune Evasion, and In Vivo Survival. Mediat. Inflamm. 2017, 2017, 8491383. [Google Scholar] [CrossRef] [Green Version]
- Modrzewska, B.; Kurnatowski, P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann. Parasitol. 2015, 61, 3–9. [Google Scholar]
- Pal, I.; Brahmkhatri, V.P.; Bera, S.; Bhattacharyya, D.; Quirishi, Y.; Bhunia, A.; Atreya, H.S. Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J. Colloid Interface Sci. 2016, 483, 385–393. [Google Scholar] [CrossRef]
- Jones, L.; Hobden, C.; Oshea, P. Use of a Real-Time Fluorescent-Probe to Study the Electrostatic Properties of the Cell-Surface of Candida albicans. Mycol. Res. 1995, 99, 969–976. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, R.J.; Jin, K.; Hernandez-Chavez, M.J.; Diaz-Jimenez, D.F.; Trujillo-Esquivel, E.; Clavijo-Giraldo, D.M.; Tamez-Castrellon, A.K.; Franco, B.; Gow, N.A.R.; Mora-Montes, H.M. Phosphomannosylation and the Functional Analysis of the Extended Candida albicans MNN4-Like Gene Family. Front. Microbiol. 2017, 8, 2156. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, H.; Roudbarmohammadi, S.; Delavari, H.H.; Roudbary, M. Antifungal effects of indolicidin-conjugated gold nanoparticles against fluconazole-resistant strains of. Int. J. Nanomed. 2019, 14, 5323–5338. [Google Scholar] [CrossRef] [Green Version]
- de Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of. Infect. Drug Resist. 2018, 11, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef] [Green Version]
- Jebali, A.; Hajjar, F.H.; Hekmatimoghaddam, S.; Kazemi, B.; De La Fuente, J.M.; Rashidi, M. Triangular gold nanoparticles conjugated with peptide ligands: A new class of inhibitor for Candida albicans secreted aspartyl proteinase. Biochem. Pharmacol. 2014, 90, 349–355. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T.; Manzoor, N. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold Nanoclusters for Targeting Methicillin-Resistant Staphylococcus aureus In Vivo. Angew Chem. Int. Ed. Engl. 2018, 57, 3958–3962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.K.; Liu, W.W.; Qin, Z.J.; Chen, Y.; Jiang, H.; Wang, X.M. Mercaptopyrimidine-Conjugated Gold Nanoclusters as Nanoantibiotics for Combating Multidrug-Resistant Superbugs. Bioconjugate Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. Microbiologyopen 2019, 8, e00698. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef]
- Poli, G.; Leonarduzzi, G.; Biasi, F.; Chiarpotto, E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004, 11, 1163–1182. [Google Scholar] [CrossRef]
- Shi, H.; Hudson, L.G.; Liu, K.J. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic. Biol. Med. 2004, 37, 582–593. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef]
- Sun, N.; Parrish, R.S.; Calderone, R.A.; Fonzi, W.A. Unique, Diverged, and Conserved Mitochondrial Functions Influencing Candida albicans Respiration. mBio 2019, 10, e00300-19. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold nanoparticles: A paradigm shift in biomedical applications. Adv. Colloid Interface Sci. 2013, 199, 44–58. [Google Scholar] [CrossRef]
- Raja, A.; Salique, S.M.; Gajalakshmi, P.; James, A. Antibacterial and Hemolytic Activity of Green Silver Nanoparticles from Catharanthus roseus. Int. J. Pharm. Sci. Nanotechnol. 1970, 9, 3112–3117. [Google Scholar] [CrossRef]

| Fungal Strain | Antimycotic Susceptibility ([μg/mL]/Interpretation) | |||||
|---|---|---|---|---|---|---|
| AMB | FLU | VOR | CSF | MCF | FC | |
| C. albicans 138 | 0.25/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans 166 | 0.5/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans 177 | 1/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans 185 | 0.25/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans 197 | 0.5/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans 1408 | 1/S | 1/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. albicans ATCC 26790 | 1/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. glabrata 132 | 1/S | -/R | 0.25/I | 0.25/I | 0.06/S | 1/S |
| C. glabrata 145 | 1/S | -/R | 0.25/I | 0.25/I | 0.06/S | 1/S |
| C. glabrata 154 | 0.5/S | -/R | 0.12/S | 0.25/I | 0.06/S | 1/S |
| C. krusei 137 | 0.5/S | -/R | 0.12/S | 0.5/I | 0.12/S | 16/I |
| C. krusei 148 | 0.5/S | -/R | 0.12/S | 0.5/S | 0.12/S | 16/I |
| C. krusei 156 | 0.5/S | -R | 0.12/S | 0.5/I | 0.12/S | 16/I |
| C. krusei 163 | 0.5/S | -/R | 0.12/S | 0.5/I | 0.12/S | 16/I |
| C. krusei 176 | 0.5/S | -/R | 0.12/S | 0.5/I | 0.12/S | 16/I |
| C. krusei 184 | 0.5/S | -/R | 0.12/S | 0.5/I | 0.12/S | 16/I |
| C. tropicalis 133 | 0.25/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. tropicalis 157 | 0.5/S | 1/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. tropicalis 165 | 0.25/S | 1/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. tropicalis 178 | 0.5/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
| C. tropicalis 191 | 0.25/S | 0.5/S | 0.12/S | 0.12/S | 0.06/S | 1/S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skłodowski, K.; Chmielewska, S.J.; Depciuch, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Zakrzewska, M.; Czarnowski, M.; Cieśluk, M.; Durnaś, B.; et al. Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics 2021, 13, 1940. https://doi.org/10.3390/pharmaceutics13111940
Skłodowski K, Chmielewska SJ, Depciuch J, Deptuła P, Piktel E, Daniluk T, Zakrzewska M, Czarnowski M, Cieśluk M, Durnaś B, et al. Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics. 2021; 13(11):1940. https://doi.org/10.3390/pharmaceutics13111940
Chicago/Turabian StyleSkłodowski, Karol, Sylwia Joanna Chmielewska, Joanna Depciuch, Piotr Deptuła, Ewelina Piktel, Tamara Daniluk, Magdalena Zakrzewska, Michał Czarnowski, Mateusz Cieśluk, Bonita Durnaś, and et al. 2021. "Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents" Pharmaceutics 13, no. 11: 1940. https://doi.org/10.3390/pharmaceutics13111940
APA StyleSkłodowski, K., Chmielewska, S. J., Depciuch, J., Deptuła, P., Piktel, E., Daniluk, T., Zakrzewska, M., Czarnowski, M., Cieśluk, M., Durnaś, B., Parlińska-Wojtan, M., Savage, P. B., & Bucki, R. (2021). Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics, 13(11), 1940. https://doi.org/10.3390/pharmaceutics13111940







