Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy
Abstract
:1. Introduction of Photodynamic Therapy and Photosensitizers
1.1. Photodynamic Therapy
1.2. Photosensitizers
2. Nanotechniques to Improve Photodynamic Therapy
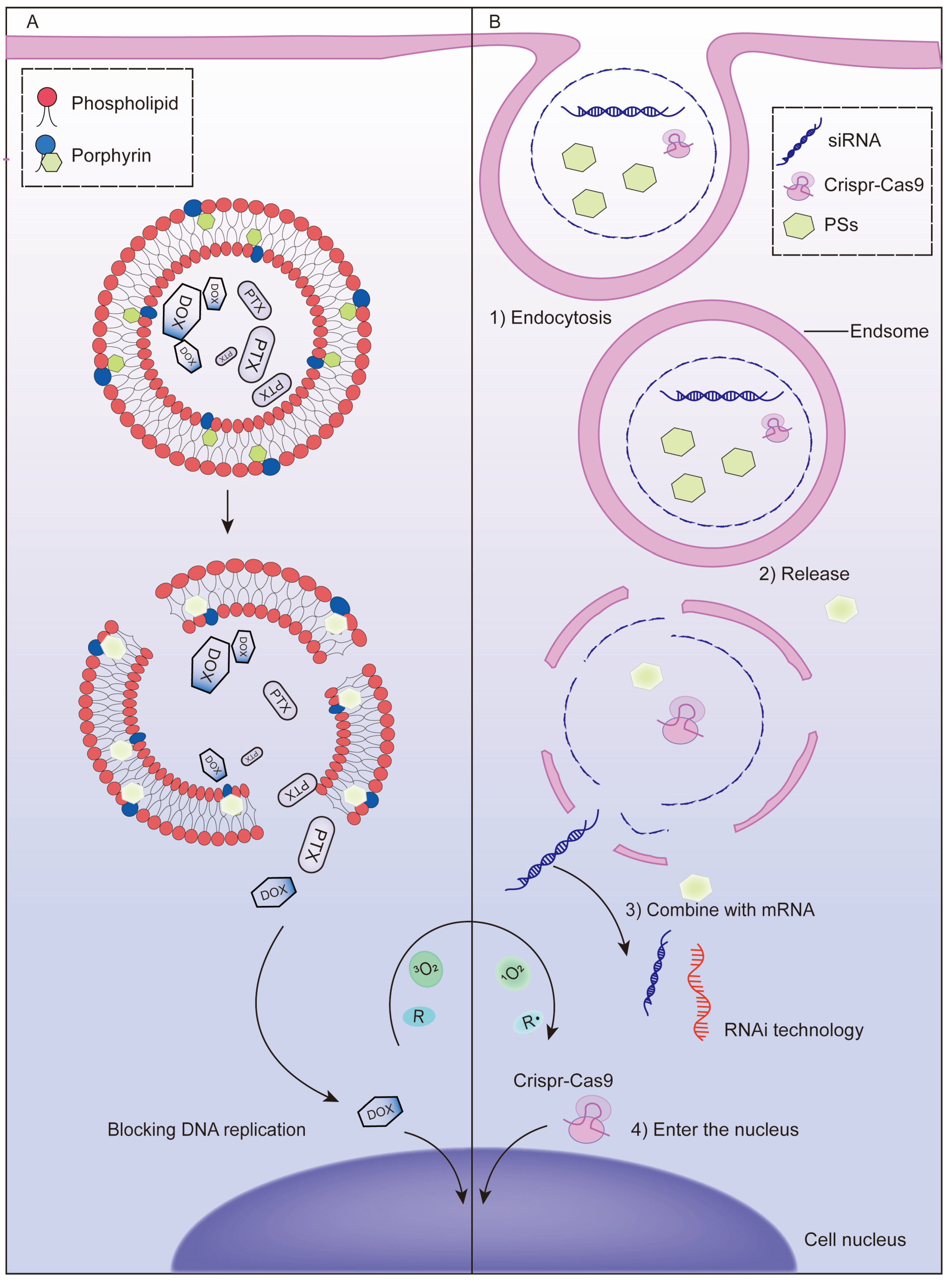
3. Codelivery of PSs and Anticancer Drugs with Nanoparticles
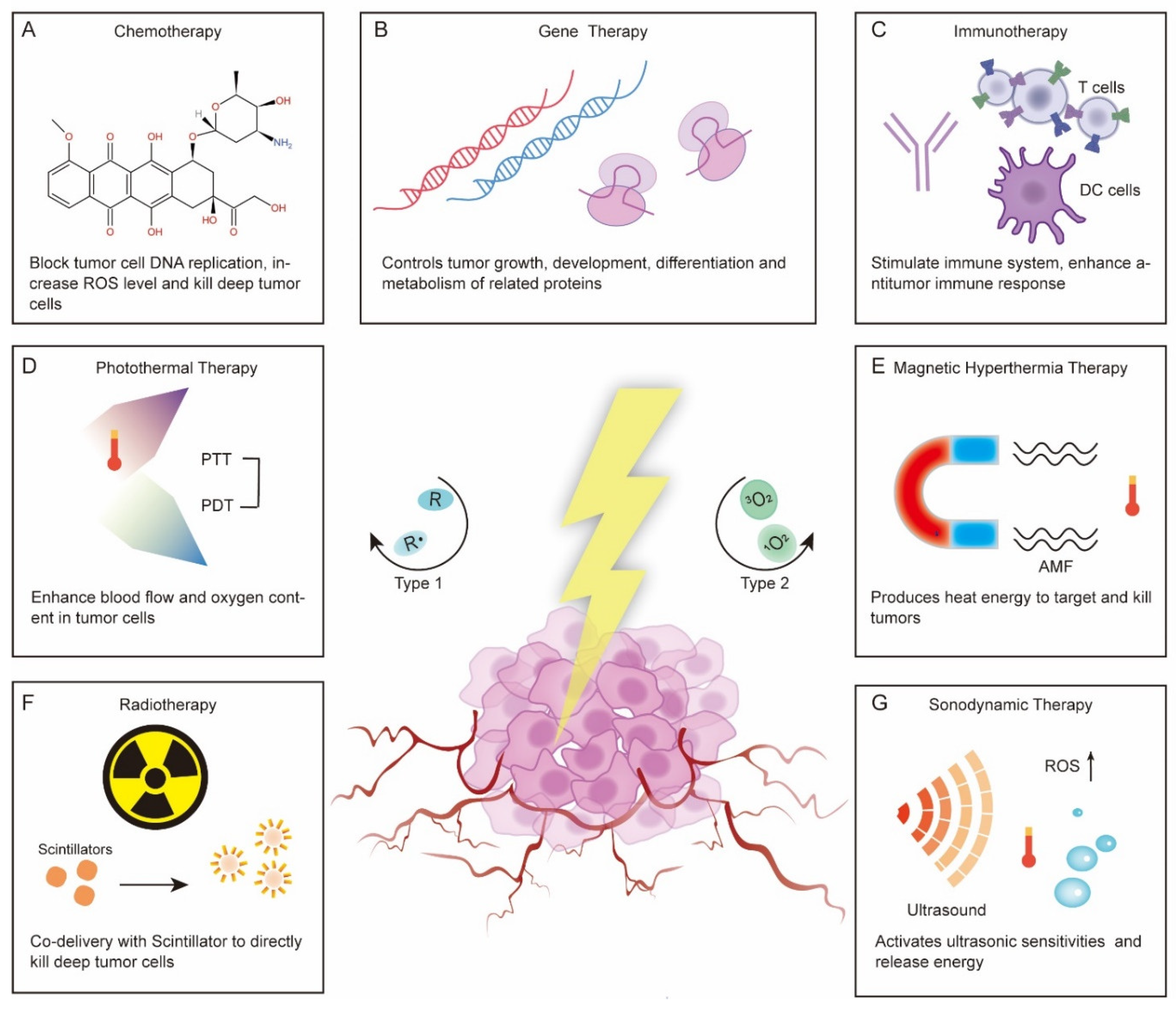
3.1. Chemotherapy
3.2. Gene Therapy
3.3. Immunotherapy
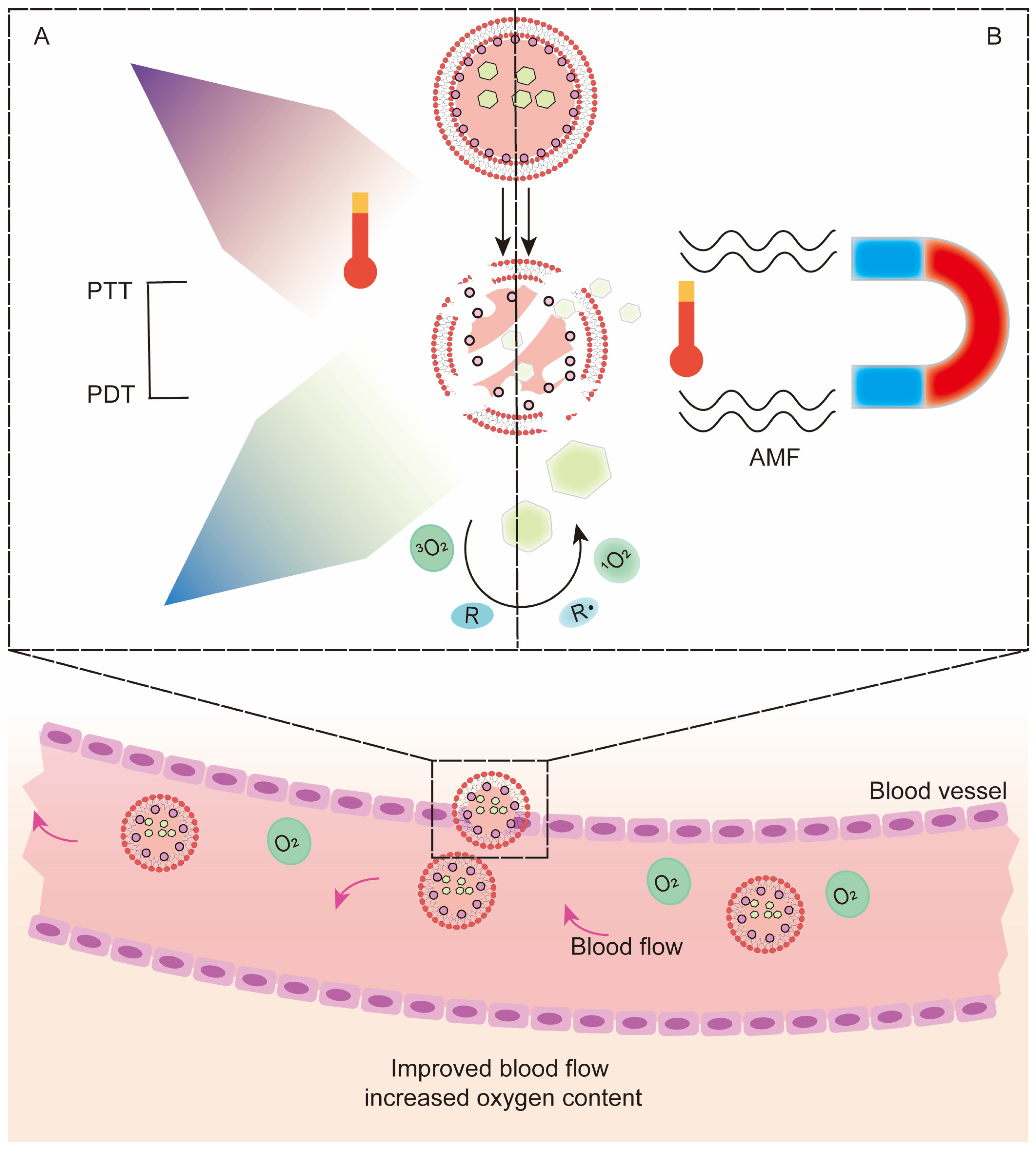
3.4. Photothermal Therapy (PTT)
3.5. Hyperthermia Therapy (HT) and Magnetic Hyperthermia Therapy (MH)
3.6. Radiotherapy
3.7. Sonodynamic Therapy (SDT)
3.8. Multidrug Codelivery
4. Outlook and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
| HT | hyperthermia therapy |
| MT | magnetic hyperthermia therapy |
| MNPs | magnetic nanoparticles |
| AMF | alternating magnetic field |
| ROS | reactive oxygen |
| ICD | immunogenic cell death |
| PFC | perfluorocarbon |
| CS | chitosan |
| NPs | nanoparticles |
| OVA | ovalbumin |
| DOX | doxorubicin |
| PS | photosensitisers |
| PoP | Porphyrin-phospholipid |
| Qu | quercetin |
| Pt | platinum |
| CRT | calreticulin |
| DAMPs | damage-associated molecular patterns |
| TLR | toll-like receptor |
| APC | antigen-presenting cell |
| nMOF | nano-metal organic framework |
| TME | tumor microenvironment |
| PEG | polyethylene glycol |
| PLGA | poly(lactide-co-glycolic) |
| GO | graphene oxide |
| PpIX | protoporphyrin IX |
| PTX | paclitaxel |
| SDT | sonodynamic therapy |
| SPDT | sono-photodynamic therapy |
| IgG | immunoglobulin G |
| HIF1α | hypoxia-inducible factor α |
| VEGF | vascular growth factor |
| PCI | photochemical Internalization |
| IDO1 | indoleamine 2, 3-dioxygenase 1 |
| CLTA-4 | cytotoxic T-lymphocyte-associated antigen 4 |
| PD-L1 | programmed death-ligand 1 |
| Ce6 | chlorin e6 |
| PEI | polythylenimide |
| ERP | enhanced permeability and retention effects |
| XPDT | X-ray photodynamic therapy |
| LDL | low-density lipoprotein |
| 1MT | dextro-1-methyltryptophan |
| LCP | lipid-calcium-phosphate |
| EMT | epithelial mesenchymal transition |
| HPD | hematoporphyrin |
| MB | methylene blue |
| NIR | near infrared |
| ZnPc | zinc phthalocyanine |
| ZnF16Pc | zinc hexadecafluorophthalocyanine |
| ClAlPc | aluminum chloride phthalocyanine |
| HYP | hypericin |
| LAHP | linoleic acid peroxide |
| CRT | calreticulin |
| AXT | axitinib |
| 5-ALA | 5-aminolevulinic acid |
| PAH | polyacrylamide |
| PAA | polyacrylic acid |
| H2TCPP | tetrakis (4-carboxyphenyl) porphyrin |
| RNP | ribonucleoprotein |
| EGFR | epidermal growth factor receptor |
| HER-2 | human epidermal growth factor receptor 2 |
| TfR | transferrin receptor |
| PIN | photoimmune nanoconjugated platform |
References
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Demkow, U. Immunological aspects of antitumor photodynamic therapy outcome. Central-Eur. J. Immunol. 2015, 40, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Y.; Sun, Y.; Wan, C.; Zhang, Z.; Dai, X.; Lin, Z.; He, Q.; Yang, Z.; Huang, P.; et al. Co-delivery of Bee Venom Melittin and a Photosensitizer with an Organic-Inorganic Hybrid Nanocarrier for Photodynamic Therapy and Immunotherapy. ACS Nano 2019, 13, 12638–12652. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fatima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Junqueira, H.C.; Severino, D.; Dias, L.G.; Gugliotti, M.S.; Baptista, M.S. Modulation of methylene blue photochemical properties based on adsorption at aqueous micelle interfaces. Phys. Chem. Chem. Phys. 2002, 4, 2320–2328. [Google Scholar] [CrossRef]
- Ma, M.; Cheng, L.; Zhao, A.; Zhang, H.; Zhang, A. Pluronic-based graphene oxide-methylene blue nanocomposite for photodynamic/photothermal combined therapy of cancer cells. Photodiagn. Photodyn. Ther. 2020, 29, 101640. [Google Scholar] [CrossRef]
- Vallejo, M.C.S.; Moura, N.M.M.; Ferreira Faustino, M.A.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing. Int. J. Mol. Sci. 2020, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.S.; Moura, N.M.M.; Gomes, A.T.P.C.; Joaquinito, A.S.M.; Faustino, M.A.F.; Almeida, A.; Goncalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The Role of Porphyrinoid Photosensitizers for Skin Wound Healing. Int. J. Mol. Sci. 2021, 22, 4121. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Eskandani, M.; Barar, J.; Omidi, Y. Recent trends in targeted therapy of cancer using graphene oxide-modified multifunctional nanomedicines. J. Drug Target. 2017, 25, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Bekmukhametova, A.; Ruprai, H.; Hook, J.M.; Mawad, D.; Houang, J.; Lauto, A. Photodynamic therapy with nanoparticles to combat microbial infection and resistance. Nanoscale 2020, 12, 21034–21059. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jiang, S.; He, H.; Ling, G.; Zhang, P. Functional black phosphorus nanosheets for cancer therapy. J. Control Release 2020, 318, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Sharma, S.K.; Yin, R.; Agrawal, T.; Chiang, L.Y.; Hamblin, M.R. Functionalized fullerenes in photodynamic therapy. J. Biomed. Nanotechnol. 2014, 10, 1918–1936. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Kang, L.; Chen, Z.; Wang, H.; Qiu, H.; Ren, J.; Qu, X. Graphitic carbon nitride nanosheets as a multifunctional nanoplatform for photochemical internalization-enhanced photodynamic therapy. J. Mater. Chem. B 2018, 6, 7908–7915. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Zhang, Y.; Wang, J.; Sui, A.; Xiu, L.; Zhu, X. The study of effect and mechanism of 630-nm laser on human lung adenocarcinoma cell xenograft model in nude mice mediated by hematoporphyrin derivatives. Lasers Med. Sci. 2020, 35, 1085–1094. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Lawrence, G.; Kaufman, J.H.; Boyle, D.; Weishaupt, K.R.; Goldfarb, A. Photoradiation in the treatment of recurrent breast carcinoma. J. Natl. Cancer Inst. 1979, 62, 231–237. [Google Scholar] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, Z.; Luo, R.; Sang, M.; Liu, B.; Sun, M.; Qu, W.; Feng, F.; Liu, W. Tumor-specific activated photodynamic therapy with an oxidation-regulated strategy for enhancing anti-tumor efficacy. Theranostics 2018, 8, 5059–5071. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.I.; Cha, B.; Lee, H.L.; Song, Y.H.; Jung, Y.H.; Kwak, T.W.; Choi, C.; Jeong, G.W.; Nah, J.W.; Kang, D.H. Simple nanophotosensitizer fabrication using water-soluble chitosan for photodynamic therapy in gastrointestinal cancer cells. Int. J. Pharm. 2017, 532, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Yi, G.; Kwak, M.H.; Yang, S.M.; Park, J.M.; Lee, B.I.; Choi, M.G.; Koo, H. Gelatin-chlorin e6 conjugate for in vivo photodynamic therapy. J. Nanobiotechnol. 2019, 17, 50. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, L.Z.; Xavierselvan, M.; Kuriakose, M.; Kennedy, M.D.; Nguyen, C.D.; Batt, J.J.; Detels, K.B.; Mallidi, S. Mutual impact of clinically translatable near-infrared dyes on photoacoustic image contrast and in vitro photodynamic therapy efficacy. J. Biomed. Opt. 2020, 25, 063808. [Google Scholar]
- Yan, L.; Luo, L.; Amirshaghaghi, A.; Miller, J.; Meng, C.; You, T.; Busch, T.M.; Tsourkas, A.; Cheng, Z. Dextran-Benzoporphyrin Derivative (BPD) Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Micelles for T2-Weighted Magnetic Resonance Imaging and Photodynamic Therapy. Bioconjug. Chem. 2019, 30, 2974–2981. [Google Scholar] [CrossRef]
- Rizvi, I.; Nath, S.; Obaid, G.; Ruhi, M.K.; Moore, K.; Bano, S.; Kessel, D.; Hasan, T. A Combination of Visudyne and a Lipid-anchored Liposomal Formulation of Benzoporphyrin Derivative Enhances Photodynamic Therapy Efficacy in a 3D Model for Ovarian Cancer. Photochem. Photobiol. 2019, 95, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Roguin, L.P.; Chiarante, N.; García Vior, M.C.; Marino, J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef]
- Santos, K.L.M.; Barros, R.M.; da Silva Lima, D.P.; Nunes, A.M.A.; Sato, M.R.; Faccio, R.; de Lima Damasceno, B.P.G.; Oshiro-Junior, J.A. Prospective application of phthalocyanines in the photodynamic therapy against microorganisms and tumor cells: A mini-review. Photodiagn. Photodyn. Ther. 2020, 32, 102032. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Callewaert, G.; Hendrickx, N.; Scorrano, L.; Hartmann, D.; Missiaen, L.; Vandenheede, J.R.; Heirman, I.; Grooten, J.; Agostinis, P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006, 20, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schafer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Machado, F.C.; Adum de Matos, R.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef]
- de Morais, F.A.P.; Goncalves, R.S.; Vilsinski, B.H.; Lazarin-Bidoia, D.; Balbinot, R.B.; Tsubone, T.M.; Brunaldi, K.; Nakamura, C.V.; Hioka, N.; Caetano, W. Hypericin photodynamic activity in DPPC liposomes-part II: Stability and application in melanoma B16-F10 cancer cells. Photochem. Photobiol. Sci. 2020, 19, 620–630. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Jiao, T.; Xing, R.; Shen, G.; Yan, X. Water-Insoluble Photosensitizer Nanocolloids Stabilized by Supramolecular Interfacial Assembly towards Photodynamic Therapy. Sci. Rep. 2017, 7, 42978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanda Pedroso de Morais, F.; Sonchini Goncalves, R.; Souza Campanholi, K.; Martins de Franca, B.; Augusto Capeloto, O.; Lazarin-Bidoia, D.; Bento Balbinot, R.; Vataru Nakamura, C.; Carlos Malacarne, L.; Caetano, W.; et al. Photophysical characterization of Hypericin-loaded in micellar, liposomal and copolymer-lipid nanostructures based F127 and DPPC liposomes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119173. [Google Scholar] [CrossRef]
- Wu, P.T.; Lin, C.L.; Lin, C.W.; Chang, N.C.; Tsai, W.B.; Yu, J. Methylene-Blue-Encapsulated Liposomes as Photodynamic Therapy Nano Agents for Breast Cancer Cells. Nanomaterials 2018, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Khanal, A.; Bui, M.P.; Seo, S.S. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J. Breast Cancer 2014, 17, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, Z.; Chen, H.; Gao, Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur. J. Pharm. Sci. 2020, 144, 105213. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Tan, Q.; He, D.; Wu, M.; Fan, J.; Yang, J.; Zhong, C.; Li, K.; Zhang, J. Smart Stimuli-Responsive and Mitochondria Targeting Delivery in Cancer Therapy. Int. J. Nanomed. 2021, 16, 4117–4146. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Y.; Li, Y.; Duan, Q. Preparation of a star-shaped copolymer with porphyrin core and four PNIPAM-b-POEGMA arms for photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 74–82. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Dabrzalska, M.; Janaszewska, A.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Complexing Methylene Blue with Phosphorus Dendrimers to Increase Photodynamic Activity. Molecules 2017, 22, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Tang, H.; Zhang, P. Plasmonic Nanoparticle-based Hybrid Photosensitizers with Broadened Excitation Profile for Photodynamic Therapy of Cancer Cells. Sci. Rep. 2016, 6, 34981. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, X.; Chen, M.; Sheng, J.; Ren, J.; Jiang, Z.; Cai, J.; Hu, Y. Fluorescence guided photothermal/photodynamic ablation of tumours using pH-responsive chlorin e6-conjugated gold nanorods. Colloids Surf. B Biointerfaces 2017, 160, 345–354. [Google Scholar] [CrossRef]
- Amanda Pedroso de Morais, F.; Sonchini Gonçalves, R.; Souza Campanholi, K.; Martins de França, B.; Augusto Capeloto, O.; Lazarin-Bidoia, D.; Bento Balbinot, R.; Vataru Nakamura, C.; Carlos Malacarne, L.; Caetano, W.; et al. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 9. [Google Scholar]
- Shi, X.; Zhan, Q.; Yan, X.; Zhou, J.; Zhou, L.; Wei, S. Oxyhemoglobin nano-recruiter preparation and its application in biomimetic red blood cells to relieve tumor hypoxia and enhance photodynamic therapy activity. J. Mater. Chem. B 2020, 8, 534–545. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, H.; Liu, L.; Chen, Z.; Liang, R.; Chen, Z.; Wu, Z.; Ma, A.; Zheng, M.; Cai, L. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584–3596. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Gai, Y.; Wang, S.; Liu, Q.; Zhang, X.; Ye, M.; Tan, J.; Long, Y.; Wang, K.; Zhang, Y.; et al. Biomimetic oxygen delivery nanoparticles for enhancing photodynamic therapy in triple-negative breast cancer. J. Nanobiotechnol. 2021, 19, 81. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 6492–6496. [Google Scholar] [CrossRef]
- Hu, D.; Chen, L.; Qu, Y.; Peng, J.; Chu, B.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.; Qian, Z. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.; Swaminathan, S.K.; Kirtane, A.R.; Panyam, J. Enhanced photodynamic therapy and effective elimination of cancer stem cells using surfactant-polymer nanoparticles. Mol. Pharm. 2014, 11, 3186–3195. [Google Scholar] [CrossRef]
- Yue, D.; Cai, X.; Fan, M.; Zhu, J.; Tian, J.; Wu, L.; Jiang, Q.; Gu, Z. An Alternating Irradiation Strategy-Driven Combination Therapy of PDT and RNAi for Highly Efficient Inhibition of Tumor Growth and Metastasis. Adv. Healthc. Mater. 2021, 10, e2001850. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Fahey, J.M.; Korytowski, W. Multiple Means by Which Nitric Oxide can Antagonize Photodynamic Therapy. Curr. Med. Chem. 2016, 23, 2754–2769. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.M.; Korytowski, W. Upregulation of nitric oxide in tumor cells as a negative adaptation to photodynamic therapy. Lasers Surg. Med. 2018, 50, 590–598. [Google Scholar] [CrossRef]
- Fahey, J.M.; Girotti, A.W. Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: Improved outcome with NOS2 inhibitors. Nitric Oxide 2017, 62, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Carter, K.A.; Luo, D.; Razi, A.; Geng, J.; Shao, S.; Ortega, J.; Lovell, J.F. Sphingomyelin Liposomes Containing Porphyrin-phospholipid for Irinotecan Chemophototherapy. Theranostics 2016, 6, 2329–2336. [Google Scholar] [CrossRef]
- Cramer, G.M.; Moon, E.K.; Cengel, K.A.; Busch, T.M. Photodynamic Therapy and Immune Checkpoint Blockade†. Photochem. Photobiol. 2020, 96, 954–961. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Abba, M.L.; Gamal-Eldeen, A.M. The role of microRNAs in photodynamic therapy of cancer. Eur. J. Med. Chem. 2017, 142, 550–555. [Google Scholar] [CrossRef]
- Liu, X.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Photosensitizer cross-linked nano-micelle platform for multimodal imaging guided synergistic photothermal/photodynamic therapy. Nanoscale 2016, 8, 15323–15339. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xu, K.; Yang, Z.; Chang, C.K.; Xu, B. Synthesis and cellular uptake of porphyrin decorated iron oxide nanoparticles-a potential candidate for bimodal anticancer therapy. Chem. Commun. 2005, 4270–4272. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.; Procházková, L.; Pelikánová, I.T.; Vlk, M.; Palkovský, M.; Jarý, V.; Nikl, M.; Múčka, V.; Mihóková, E.; Čuba, V. Preliminary study on singlet oxygen production using CeF3:Tb3+@SiO2-PpIX. Radiat. Meas. 2016, 90, 325–328. [Google Scholar] [CrossRef]
- Hong, L.; Pliss, A.M.; Zhan, Y.; Zheng, W.; Xia, J.; Liu, L.; Qu, J.; Prasad, P.N. Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor. Nanomaterials 2020, 10, 2058. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Wang, Q.; Tong, R.; Lin, H.; Qu, F. Near-infrared light-mediated LA-UCNPs@SiO2-C/HA@mSiO2-DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalton Trans. 2017, 46, 14293–14300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Guo, H.L.; Xie, A.J.; Shen, Y.H.; Zhu, M.Z. 4-in-1 Fe3O4/g-C3N4@PPy-DOX nanocomposites: Magnetic targeting guided trimode combinatorial chemotherapy/PDT/PTT for cancer. J. Inorg. Biochem. 2021, 215, 111329. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Cheng, Y.J.; Lei, Q.; Zhang, A.Q.; Zhang, X.Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. Nanocarriers in Improving Chemotherapy of Multidrug Resistant Tumors: Key Developments and Perspectives. Curr. Pharm. Des. 2017, 23, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Schreiber, S.; Gross, S.; Brandis, A.; Harmelin, A.; Rosenbach-Belkin, V.; Scherz, A.; Salomon, Y. Local photodynamic therapy (PDT) of rat C6 glioma xenografts with Pd-bacteriopheophorbide leads to decreased metastases and increase of animal cure compared with surgery. Int. J. Cancer 2002, 99, 279–285. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef] [Green Version]
- Lila, A.S.; Ishida, T.; Kiwada, H. Targeting anticancer drugs to tumor vasculature using cationic liposomes. Pharm. Res. 2010, 27, 1171–1183. [Google Scholar] [CrossRef]
- Luo, D.; Li, N.; Carter, K.A.; Lin, C.; Geng, J.; Shao, S.; Huang, W.C.; Qin, Y.; Atilla-Gokcumen, G.E.; Lovell, J.F. Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin-Phospholipids. Small 2016, 12, 3039–3047. [Google Scholar] [CrossRef] [Green Version]
- Miranda, D.; Lovell, J.F. Mechanisms of Light-induced Liposome Permeabilization. Bioeng. Transl. Med. 2016, 1, 267–276. [Google Scholar] [CrossRef]
- Luo, D.; Geng, J.; Li, N.; Carter, K.A.; Shao, S.; Atilla-Gokcumen, G.E.; Lovell, J.F. Vessel-Targeted Chemophototherapy with Cationic Porphyrin-Phospholipid Liposomes. Mol. Cancer Ther. 2017, 16, 2452–2461. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Carter, K.A.; Molins, E.A.G.; Straubinger, N.L.; Geng, J.; Shao, S.; Jusko, W.J.; Straubinger, R.M.; Lovell, J.F. Pharmacokinetics and pharmacodynamics of liposomal chemophototherapy with short drug-light intervals. J. Control Release 2019, 297, 39–47. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Giraldo, D.; Sunar, U.; Ortega, J.; Lovell, J.F. Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials 2016, 75, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Goel, S.; Liu, H.J.; Carter, K.A.; Jiang, D.; Geng, J.; Kutyreff, C.J.; Engle, J.W.; Huang, W.C.; Shao, S.; et al. Intrabilayer (64)Cu Labeling of Photoactivatable, Doxorubicin-Loaded Stealth Liposomes. ACS Nano 2017, 11, 12482–12491. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Geng, J.; He, X.; Lovell, J.F. Short Drug-Light Intervals Improve Liposomal Chemophototherapy in Mice Bearing MIA PaCa-2 Xenografts. Mol. Pharm. 2018, 15, 3682–3689. [Google Scholar] [CrossRef]
- Jiang, W.; Delahunty, I.M.; Xie, J. Oxygenating the way for enhanced chemophototherapy. Theranostics 2018, 8, 3870–3871. [Google Scholar] [CrossRef] [PubMed]
- Sunil, V.; Mozhi, A.; Zhan, W.; Teoh, J.H.; Wang, C.H. Convection enhanced delivery of light responsive antigen capturing oxygen generators for chemo-phototherapy triggered adaptive immunity. Biomaterials 2021, 275, 120974. [Google Scholar] [CrossRef]
- Liu, X.L.; Dong, X.; Yang, S.C.; Lai, X.; Liu, H.J.; Gao, Y.; Feng, H.Y.; Zhu, M.H.; Yuan, Y.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, J.; Zhang, H.; Jiang, Y.; Song, A.; Luan, Y. Low side-effect and heat-shock protein-inhibited chemo-phototherapy nanoplatform via co-assembling strategy of biotin-tailored IR780 and quercetin. Chem. Eng. J. 2020, 382, 123043. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, M.; Li, J.; Lan, S.; Zeng, Y.; Liu, X.; Liu, J. Light-Enhanced Hypoxia-Response of Conjugated Polymer Nanocarrier for Successive Synergistic Photodynamic and Chemo-Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21909–21919. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Liu, C.; Zheng, W.; Li, X.; Ge, R.; Shen, H.; Guo, X.; Lian, Q.; Shen, X.; Li, C. Cyclic cRGDfk peptide and Chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials 2018, 161, 306–320. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Xue, L.; Zhong, L.; Ge, W.; Song, X.; Zhao, Y.; Wang, W.; Dong, X. On-demand drug release nanoplatform based on fluorinated aza-BODIPY for imaging-guided chemo-phototherapy. Biomaterials 2020, 256, 120211. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Peng, H.; Qing, X.; Fan, Z.; Xu, W.; Du, X.; Shi, R.; Zhang, Y. pH-Responsive Pluronic F127-Lenvatinib-Encapsulated Halogenated Boron-Dipyrromethene Nanoparticles for Combined Photodynamic Therapy and Chemotherapy of Liver Cancer. ACS Omega 2021, 6, 12331–12342. [Google Scholar] [CrossRef]
- Cheng, X.; He, L.; Xu, J.; Fang, Q.; Yang, L.; Xue, Y.; Wang, X.; Tang, R. Oxygen-producing catalase-based prodrug nanoparticles overcoming resistance in hypoxia-mediated chemo-photodynamic therapy. Acta Biomater. 2020, 112, 234–249. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Y.; Yu, H.; Wang, C.; Zhang, X.; Zhao, H.; Chen, Q.; He, Z.; Luo, C.; Sun, J. Photodynamic PEG-coated ROS-sensitive prodrug nanoassemblies for core-shell synergistic chemo-photodynamic therapy. Acta Biomater. 2019, 92, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Cai, H.; Wei, Q.; Tang, X.; Zhang, Q.; Kopytynski, M.; Yang, J.; Yi, Y.; Zhang, H.; Gong, Q.; et al. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials 2021, 277, 121061. [Google Scholar] [CrossRef]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Yang, J.; Gong, Q.; Luo, K.; Gu, Z. Redox dual-responsive dendrimeric nanoparticles for mutually synergistic chemo-photodynamic therapy to overcome drug resistance. J. Control Release 2021, 329, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, J.J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-Guiding Polymeric Prodrug Micelles with Two Aggregation-Induced Emission Photosensitizers for Enhanced Chemo-Photodynamic Therapy. ACS Nano 2021, 15, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, H.J.; Green, J.J.; Tzeng, S.Y. Cancer-Targeting Nanoparticles for Combinatorial Nucleic Acid Delivery. Adv. Mater. 2020, 32, e1901081. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Hogan, N.J.; Gottheim, S.; Li, C.; Clare, S.E.; Halas, N.J. Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano 2017, 11, 171–179. [Google Scholar] [CrossRef]
- Matsushita-Ishiodori, Y.; Ohtsuki, T. Photoinduced RNA interference. Acc. Chem. Res. 2012, 45, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, H.; Xu, X.; Wang, X.; Chen, Y.; Yin, L. Far-red light-mediated programmable anti-cancer gene delivery in cooperation with photodynamic therapy. Biomaterials 2018, 171, 72–82. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Chen, W.; Li, K.; Chen, X.; Tang, X.; Xie, G.; Luo, X.; Wang, X.; Liang, H.; et al. Biodegradable Black Phosphorus Nanosheets Mediate Specific Delivery of hTERT siRNA for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21137–21148. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Chen, W.H.; Lecaros, R.L.; Tseng, Y.C.; Huang, L.; Hsu, Y.C. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett. 2015, 359, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Li, X.; Liu, S.; Chen, J.; Li, M.; Chew, S.Y.; Leong, K.W.; Cheng, D. Codelivery of CRISPR-Cas9 and chlorin e6 for spatially controlled tumor-specific gene editing with synergistic drug effects. Sci. Adv. 2020, 6, eabb4005. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, D.; Lee, H.; Jang, H.; Park, S.; Kim, G.E.; Lee, H.J.; Kim, H.J.; Kim, H. Development of an ultrasound triggered nanomedicine-microbubble complex for chemo-photodynamic-gene therapy. Nanomedicine 2020, 27, 102194. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Meng, X.; Wang, D.; Zhang, K.; Dai, W.; Dong, H.; Zhang, X. Intelligent MnO2/Cu2−xS for Multimode Imaging Diagnostic and Advanced Single-Laser Irradiated Photothermal/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 17732–17741. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, L.; Huang, Y.; Shen, L.; Peng, H.; Zhu, X.; Zhou, G. Nanoparticle delivery of Wnt-1 siRNA enhances photodynamic therapy by inhibiting epithelial-mesenchymal transition for oral cancer. Biomater. Sci. 2017, 28, 494–501. [Google Scholar] [CrossRef]
- Liu, S.Y.; Xu, Y.; Yang, H.; Liu, L.; Zhao, M.; Yin, W.; Xu, Y.T.; Huang, Y.; Tan, C.; Dai, Z.; et al. Ultrathin 2D Copper(I) 1,2,4-Triazolate Coordination Polymer Nanosheets for Efficient and Selective Gene Silencing and Photodynamic Therapy. Adv. Mater. 2021, 33, e2100849. [Google Scholar] [CrossRef]
- Laroui, N.; Coste, M.; Lichon, L.; Bessin, Y.; Gary-Bobo, M.; Pratviel, G.; Bonduelle, C.; Bettache, N.; Ulrich, S. Combination of photodynamic therapy and gene silencing achieved through the hierarchical self-assembly of porphyrin-siRNA complexes. Int. J. Pharm. 2019, 659, 118585. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Cao, W.; Xia, F.; Liu, B.; Niu, J.; Alfranca, G.; Sun, X.; Ma, L.; de la Fuente, J.M.; et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2-based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics 2019, 9, 6867–6884. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, X.; Zhao, B.; Chen, M.; Liu, R.; Sun, S.; Yue, X.; Wang, S. Ultrasound assisted gene and photodynamic synergistic therapy with multifunctional FOXA1-siRNA loaded porphyrin microbubbles for enhancing therapeutic efficacy for breast cancer. Biomaterials 2018, 173, 58–70. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Li, X.; Zhu, H.; Sun, D.; Sun, X.; Liu, H.; Zhang, Z.; Cao, L.; Gao, C.; et al. Multifunctional DNA Polymer-Assisted Upconversion Therapeutic Nanoplatform for Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 26832–26841. [Google Scholar] [CrossRef]
- Lichon, L.; Kotras, C.; Myrzakhmetov, B.; Arnoux, P.; Daurat, M.; Nguyen, C.; Durand, D.; Bouchmella, K.; Ali, L.M.A.; Durand, J.O.; et al. Polythiophenes with Cationic Phosphonium Groups as Vectors for Imaging, siRNA Delivery, and Photodynamic Therapy. Nanomaterials 2020, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Luo, X.; Zhang, Z.; Wang, A.; Song, W. Cationic Polyporphyrins as siRNA Delivery Vectors for Photodynamic and Gene Synergistic Anticancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 27513–27521. [Google Scholar] [CrossRef] [PubMed]
- Mezghrani, B.; Ali, L.M.A.; Richeter, S.; Durand, J.O.; Hesemann, P.; Bettache, N. Periodic Mesoporous Ionosilica Nanoparticles for Green Light Photodynamic Therapy and Photochemical Internalization of siRNA. ACS Appl. Mater. Interfaces 2021, 13, 29325–29339. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef] [Green Version]
- Banstola, A.; Jeong, J.H.; Yook, S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020, 114, 16–30. [Google Scholar] [CrossRef]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale Metal-Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv. Mater. 2018, 30, e1707634. [Google Scholar] [CrossRef]
- Cai, Z.; Xin, F.; Wei, Z.; Wu, M.; Lin, X.; Du, X.; Chen, G.; Zhang, D.; Zhang, Z.; Liu, X.; et al. Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal-Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv. Healthc. Mater. 2020, 9, e1900996. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Lan, G.; Culbert, A.; Song, Y.; Wu, T.; Jiang, X.; Lin, W. A Nanoscale Metal-Organic Framework to Mediate Photodynamic Therapy and Deliver CpG Oligodeoxynucleotides to Enhance Antigen Presentation and Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 1108–1112. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophotonics 2014, 7, 897–905. [Google Scholar] [CrossRef]
- Huang, R.; Ding, Z.; Jiang, B.P.; Luo, Z.; Chen, T.; Guo, Z.; Ji, S.C.; Liang, H.; Shen, X.C. Artificial Metalloprotein Nanoanalogues: In Situ Catalytic Production of Oxygen to Enhance Photoimmunotherapeutic Inhibition of Primary and Abscopal Tumor Growth. Small 2020, 16, e2004345. [Google Scholar] [CrossRef]
- Ding, B.; Shao, S.; Yu, C.; Teng, B.; Wang, M.; Cheng, Z.; Wong, K.L.; Ma, P.; Lin, J. Large-Pore Mesoporous-Silica-Coated Upconversion Nanoparticles as Multifunctional Immunoadjuvants with Ultrahigh Photosensitizer and Antigen Loading Efficiency for Improved Cancer Photodynamic Immunotherapy. Adv. Mater. 2018, 30, e1802479. [Google Scholar] [CrossRef]
- Xu, C.; Nam, J.; Hong, H.; Xu, Y.; Moon, J.J. Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy. ACS Nano 2019, 13, 12148–12161. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, W.; Jiang, W.; Kumar, A.; Zhou, S.; Cao, Z.; Zhan, S.; Yang, W.; Liu, R.; Teng, Y.; et al. Nanoconjugates to enhance PDT-mediated cancer immunotherapy by targeting the indoleamine-2,3-dioxygenase pathway. J. Nanobiotechnol. 2021, 19, 182. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, X.; Hou, Z.; Wang, N.; Jiang, Y.; Luan, Y. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, S.; Wang, X.; Qian, Y.; Wu, W.; Zhang, S.; Zheng, B.; Wei, G.; Gao, S.; Cao, Z.; et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control Release 2020, 324, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Tian, Y.; Guan, X.; Liu, Q.; Li, S.; Qin, X.; Yang, H.; Liu, Y. “Triple-Punch” Anticancer Strategy Mediated by Near-Infrared Photosensitizer/CpG Oligonucleotides Dual-Dressed and Mitochondria-Targeted Nanographene. ACS Appl. Mater. Interfaces 2018, 10, 6942–6955. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [Green Version]
- Obaid, G.; Bano, S.; Mallidi, S.; Broekgaarden, M.; Kuriakose, J.; Silber, Z.; Bulin, A.L.; Wang, Y.; Mai, Z.; Jin, W.; et al. Impacting Pancreatic Cancer Therapy in Heterotypic in Vitro Organoids and in Vivo Tumors with Specificity-Tuned, NIR-Activable Photoimmunonanoconjugates: Towards Conquering Desmoplasia? Nano Lett. 2019, 19, 7573–7587. [Google Scholar] [CrossRef]
- Bano, S.; Obaid, G.; Swain, J.W.R.; Yamada, M.; Pogue, B.W.; Wang, K.; Hasan, T. NIR Photodynamic Destruction of PDAC and HNSCC Nodules Using Triple-Receptor-Targeted Photoimmuno-Nanoconjugates: Targeting Heterogeneity in Cancer. J. Clin. Med. 2020, 9, 2390. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Liu, B.; Cao, W.; Zhang, J.; Yang, Y.; Ma, L.; de la Fuente, J.M.; Song, J.; Ni, J.; et al. Human iPS Cells Loaded with MnO2-Based Nanoprobes for Photodynamic and Simultaneous Enhanced Immunotherapy Against Cancer. Nano-Micro Lett. 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, Z.; Huang, J.; Wang, Y.; An, Y.; Xiao, H.; Peng, Y.; Pang, P.; Han, S.; Zhu, K.; et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 12845–12856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Gao, L.; Fei, J.; Zhao, J.; Li, H.; Cui, Y.; Li, J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012, 6, 8030–8040. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef]
- Liu, X.; Su, H.; Shi, W.; Liu, Y.; Sun, Y.; Ge, D. Functionalized poly(pyrrole-3-carboxylic acid) nanoneedles for dual-imaging guided PDT/PTT combination therapy. Biomaterials 2018, 167, 177–190. [Google Scholar] [CrossRef]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef] [Green Version]
- Kalluru, P.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.N.; Lu, J.; Zubair Iqbal, M.; Zhang, Q.; Kong, X. Synergistic photodynamic and photothermal therapy of BODIPY-conjugated hyaluronic acid nanoparticles. J. Biomater. Sci. Polym. Ed. 2021, 32, 2028–2045. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, A.; Li, D.; Wang, L.; Yang, H.; Zeng, H.; Liu, Y. Photosensitizer-assembled PEGylated graphene-copper sulfide nanohybrids as a synergistic near-infrared phototherapeutic agent. Expert Opin. Drug Deliv. 2016, 13, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Manivasagan, P.; Santha Moorthy, M.; Bui, N.Q.; Jang, B.; Phan, T.; Jung, W.K.; Kim, Y.M.; Lee, K.D.; Oh, J. Photo-based PDT/PTT dual model killing and imaging of cancer cells using phycocyanin-polypyrrole nanoparticles. Eur. J. Pharm. Biopharm. 2018, 123, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Dong, P.; Lin, Z.; Guo, X.; Jiang, B.P.; Ji, S.; Liang, H.; Shen, X.C. Near-Infrared Light Responsive Imaging-Guided Photothermal and Photodynamic Synergistic Therapy Nanoplatform Based on Carbon Nanohorns for Efficient Cancer Treatment. Chemistry 2018, 24, 12827–12837. [Google Scholar] [CrossRef]
- Wu, J.; Williams, G.R.; Niu, S.; Yang, Y.; Li, Y.; Zhang, X.; Zhu, L.M. Biomineralized Bimetallic Oxide Nanotheranostics for Multimodal Imaging-Guided Combination Therapy. Theranostics 2020, 10, 841–855. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N.T.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Six Birds with One Stone: Versatile Nanoporphyrin for Single-Laser-Triggered Synergistic Phototheranostics and Robust Immune Activation. Adv. Mater. 2020, 32, e2004481. [Google Scholar] [CrossRef] [PubMed]
- Jana, D.; Jia, S.; Bindra, A.K.; Xing, P.; Ding, D.; Zhao, Y. Clearable Black Phosphorus Nanoconjugate for Targeted Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 18342–18351. [Google Scholar] [CrossRef]
- Chen, S.; Huang, B.; Pei, W.; Wang, L.; Xu, Y.; Niu, C. Mitochondria-Targeting Oxygen-Sufficient Perfluorocarbon Nanoparticles for Imaging-Guided Tumor Phototherapy. Int. J. Nanomed. 2020, 15, 8641–8658. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Lu, Q.; Yang, S.; Yang, L.; Cheng, Y.; Wang, Y.; Wang, S.; Song, Y.; Tan, F.; et al. Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 5771–5781. [Google Scholar] [CrossRef]
- Shao, W.; Yang, C.; Li, F.; Wu, J.; Wang, N.; Ding, Q.; Gao, J.; Ling, D. Molecular Design of Conjugated Small Molecule Nanoparticles for Synergistically Enhanced PTT/PDT. Nano-Micro Lett. 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Chen, Z.; Zhang, F.; Wang, Q.; Guo, W.; Wang, K.; Lin, H.; Qu, F. Construct of MoSe2/Bi2Se3 nanoheterostructure: Multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials 2019, 217, 119282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gou, H.; Liu, Y.; Xi, K.; Jiang, D.; Jia, X. pH-responsive PEG-chitosan/iron oxide hybrid nanoassemblies for low-power assisted PDT/PTT combination therapy. Nanomedicine 2020, 15, 1097–1112. [Google Scholar] [CrossRef]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-loaded gold nano-bipyramids with NIR activatable dual PTT-PDT therapeutic potential in melanoma cells. Colloids Surf. B Biointerfaces 2020, 194, 111213. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, M.; Sun, W.; Wu, Q.; Xu, J.; Xiong, L.; Chai, Y.; Liu, Y.; Yu, M.; Wang, H.; et al. Sequential PDT and PTT Using Dual-Modal Single-Walled Carbon Nanohorns Synergistically Promote Systemic Immune Responses against Tumor Metastasis and Relapse. Adv. Sci. 2020, 7, 2001088. [Google Scholar] [CrossRef]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef]
- Streffer, C. Biological Basis of Thermotherapy (With Special Reference to Oncology). In Biological Basis of Oncologic Thermotherapy; Gautherie, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 1–71. [Google Scholar]
- Van Rhoon, G.C.; Franckena, M.; Ten Hagen, T.L. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Fahling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Huang, W.C.; Shen, M.Y.; Chen, H.H.; Lin, S.C.; Chiang, W.H.; Wu, P.H.; Chang, C.W.; Chiang, C.S.; Chiu, H.C. Monocytic delivery of therapeutic oxygen bubbles for dual-modality treatment of tumor hypoxia. J. Control Release 2015, 220, 738–750. [Google Scholar] [CrossRef]
- Yanase, S.; Nomura, J.; Matsumura, Y.; Nagata, T.; Fujii, T.; Tagawa, T. Synergistic interaction of 5-aminolevulinic acid-based photodynamic therapy with simultaneous hyperthermia in an osteosarcoma tumor model. Int. J. Oncol. 2006, 29, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, F.; Shao, D.; Chang, Z.; Wang, L.; Hu, H.; Zheng, X.; Li, X.; Chen, F.; Tu, Z.; et al. Janus Nanobullets Combine Photodynamic Therapy and Magnetic Hyperthermia to Potentiate Synergetic Anti-Metastatic Immunotherapy. Adv. Sci. 2019, 6, 1901690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Corato, R.; Bealle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clement, O.; Silva, A.K.; Menager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef]
- Pellosi, D.S.; Macaroff, P.P.; Morais, P.C.; Tedesco, A.C. Magneto low-density nanoemulsion (MLDE): A potential vehicle for combined hyperthermia and photodynamic therapy to treat cancer selectively. Mater. Sci. Eng. C 2018, 92, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef]
- Rimoldi, T.; Orsi, D.; Lagonegro, P.; Ghezzi, B.; Galli, C.; Rossi, F.; Salviati, G.; Cristofolini, L. CeF3-ZnO scintillating nanocomposite for self-lighted photodynamic therapy of cancer. J. Mater. Sci. Mater. Med. 2016, 27, 159. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Jenh, Y.J.; Wu, S.K.; Chen, Y.S.; Hanagata, N.; Lin, F.H. Non-invasive Photodynamic Therapy in Brain Cancer by Use of Tb3+-Doped LaF3 Nanoparticles in Combination with Photosensitizer Through X-ray Irradiation: A Proof-of-Concept Study. Nanoscale Res. Lett. 2017, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, X.; Wu, W.; Yang, K.; Mao, R.; Ahmad, F.; Chen, X.; Li, W. CT/MRI-Guided Synergistic Radiotherapy and X-ray Inducible Photodynamic Therapy Using Tb-Doped Gd-W-Nanoscintillators. Angew. Chem. Int. Ed. Engl. 2019, 58, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Martinčík, J.; Nikl, M.; Ruml, T.; Lang, K. Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. J. Mater. Chem. B 2018, 6, 4301–4307. [Google Scholar] [CrossRef]
- Rossi, F.; Bedogni, E.; Bigi, F.; Rimoldi, T.; Cristofolini, L.; Pinelli, S.; Alinovi, R.; Negri, M.; Dhanabalan, S.C.; Attolini, G.; et al. Porphyrin conjugated SiC/SiOx nanowires for X-ray-excited photodynamic therapy. Sci. Rep. 2015, 5, 7606. [Google Scholar] [CrossRef]
- Juzenas, P.; Chen, W.; Sun, Y.P.; Coelho, M.A.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Wu, Y.; Wang, J.; Lu, D.; Zhao, Z.; Liu, T.; Zhang, X.; Tan, W. Targeted bioimaging and photodynamic therapy nanoplatform using an aptamer-guided G-quadruplex DNA carrier and near-infrared light. Angew. Chem. Int. Ed. Engl. 2013, 52, 13965–13969. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, Q.; Pan, W.; Li, N.; Tang, B. A Near-Infrared Triggered Nanophotosensitizer Inducing Domino Effect on Mitochondrial Reactive Oxygen Species Burst for Cancer Therapy. ACS Nano 2015, 9, 11064–11074. [Google Scholar] [CrossRef]
- Dinakaran, D.; Sengupta, J.; Pink, D.; Raturi, A.; Chen, H.; Usmani, N.; Kumar, P.; Lewis, J.D.; Narain, R.; Moore, R.B. PEG-PLGA nanospheres loaded with nanoscintillators and photosensitizers for radiation-activated photodynamic therapy. Acta Biomater. 2020, 117, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.D.; Chuang, Y.J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. Engl. 2015, 54, 1770–1774. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chu, C.H.; Cheng, S.H.; Liao, L.D.; Chu, T.S.; Chen, N.T.; Paldino, A.; Hsia, Y.; Chen, C.T.; Lo, L.W. Annealing-modulated nanoscintillators for nonconventional X-ray activation of comprehensive photodynamic effects in deep cancer theranostics. Theranostics 2020, 10, 6758–6773. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, N.; Hou, Z.; Shi, J.; Su, X.; Sun, X. Radioiodinated Persistent Luminescence Nanoplatform for Radiation-Induced Photodynamic Therapy and Radiotherapy. Adv. Healthc. Mater. 2021, 10, e2000802. [Google Scholar] [CrossRef]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef] [Green Version]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO2 Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lv, B.; Tang, Z.; Zhang, M.; Ge, W.; Liu, Y.; He, X.; Zhao, K.; Zheng, X.; He, M.; et al. Scintillator-Based Nanohybrids with Sacrificial Electron Prodrug for Enhanced X-ray-Induced Photodynamic Therapy. Nano Lett. 2018, 18, 5768–5774. [Google Scholar] [CrossRef]
- Park, W.; Cho, S.; Kang, D.; Han, J.H.; Park, J.H.; Lee, B.; Lee, J.; Kim, D.H. Tumor Microenvironment Targeting Nano-Bio Emulsion for Synergistic Combinational X-Ray PDT with Oncolytic Bacteria Therap. Adv. Healthc. Mater. 2020, 9, e1901812. [Google Scholar] [CrossRef]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, B. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv. Exp. Med. Biol. 2016, 880, 429–450. [Google Scholar]
- Ashush, H.; Rozenszajn, L.A.; Blass, M.; Barda-Saad, M.; Azimov, D.; Radnay, J.; Zipori, D.; Rosenschein, U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000, 60, 1014–1020. [Google Scholar]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966. [Google Scholar] [CrossRef]
- Ma, A.; Chen, H.; Cui, Y.; Luo, Z.; Liang, R.; Wu, Z.; Chen, Z.; Yin, T.; Ni, J.; Zheng, M.; et al. Metalloporphyrin Complex-Based Nanosonosensitizers for Deep-Tissue Tumor Theranostics by Noninvasive Sonodynamic Therapy. Small 2019, 15, e1804028. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.G.; Wang, Y.Y.; Yang, Y.D.; Zhang, X.C.; Gao, Y.; Yang, Y.; Zhang, J.B.; Li, G.L. Efficacy of topical ALA-PDT combined with excision in the treatment of skin malignant tumor. Photodiagn. Photodyn. Ther. 2014, 11, 122–126. [Google Scholar] [CrossRef]
- Yano, T.; Muto, M.; Minashi, K.; Iwasaki, J.; Kojima, T.; Fuse, N.; Doi, T.; Kaneko, K.; Ohtsu, A. Photodynamic therapy as salvage treatment for local failure after chemoradiotherapy in patients with esophageal squamous cell carcinoma: A phase II study. Int. J. Cancer 2012, 131, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Tan, L.C.; Dong, L.W.; Zhang, W.Q.; Shen, X.X.; Lu, X.; Zheng, H.; Lu, Y.G. Susceptibility and Resistance Mechanisms During Photodynamic Therapy of Melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar] [CrossRef]
- McEwan, C.; Nesbitt, H.; Nicholas, D.; Kavanagh, O.N.; McKenna, K.; Loan, P.; Jack, I.G.; McHale, A.P.; Callan, J.F. Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer. Bioorg. Med. Chem. 2016, 24, 3023–3028. [Google Scholar] [CrossRef]
- An, Y.W.; Liu, H.Q.; Zhou, Z.Q.; Wang, J.C.; Jiang, G.Y.; Li, Z.W.; Wang, F.; Jin, H.T. Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma. Oncol. Rep. 2020, 44, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, P.; Hu, J.; Jia, Y.; Wu, L.; Chen, X.; Liu, Q.; Wang, X. A new sensitizer DVDMS combined with multiple focused ultrasound treatments: An effective antitumor strategy. Sci. Rep. 2015, 5, 17485. [Google Scholar] [CrossRef] [Green Version]
- Sadanala, K.C.; Chaturvedi, P.K.; Seo, Y.M.; Kim, J.M.; Jo, Y.S.; Lee, Y.K.; Ahn, W.S. Sono-photodynamic combination therapy: A review on sensitizers. Anticancer Res. 2014, 34, 4657–4664. [Google Scholar]
- Chen, H.J.; Zhou, X.B.; Wang, A.L.; Zheng, B.Y.; Yeh, C.K.; Huang, J.D. Synthesis and biological characterization of novel rose bengal derivatives with improved amphiphilicity for sono-photodynamic therapy. Eur. J. Med. Chem. 2018, 145, 86–95. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, Z.; Chen, M.; Zhang, Y.; Amagat, J.; Kang, S.; Zheng, Y.; Hu, B.; Chen, M. Co-Immobilization of Ce6 Sono/Photosensitizer and Protonated Graphitic Carbon Nitride on PCL/Gelation Fibrous Scaffolds for Combined Sono-Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 40728–40739. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Yang, P.; Liu, Q.; Hu, J.; Yang, W.; Liu, P.; He, F.; Bai, Y.; Gai, S.; et al. GPC3-targeted and curcumin-loaded phospholipid microbubbles for sono-photodynamic therapy in liver cancer cells. Colloids Surf. B Biointerfaces 2021, 197, 111358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Zhu, W.T.; Hu, J.H.; Yang, W.; Liu, P.; Liu, Q.H.; Bai, Y.X.; Xie, R. Curcumin-Loaded Poly(L-lactide-co-glycolide) Microbubble-Mediated Sono-photodynamic Therapy in Liver Cancer Cells. Ultrasound Med. Biol. 2020, 46, 2030–2043. [Google Scholar] [CrossRef]
- Miyoshi, N.; Kundu, S.K.; Tuziuti, T.; Yasui, K.; Shimada, I.; Ito, Y. Combination of Sonodynamic and Photodynamic Therapy against Cancer Would Be Effective through Using a Regulated Size of Nanoparticles. Nanosci. Nanoeng. 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Rashidi, L.H.; Yao, M.; Ma, L.; Chen, L.; Zhang, J.; Zhang, Y.; Chen, W. CuS nanoagents for photodynamic and photothermal therapies: Phenomena and possible mechanisms. Photodiagn. Photodyn. Ther. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Luo, L.; Sun, W.; Feng, Y.; Qin, R.; Zhang, J.; Ding, D.; Shi, T.; Liu, X.; Chen, X.; Chen, H. Conjugation of a Scintillator Complex and Gold Nanorods for Dual-Modal Image-Guided Photothermal and X-ray-Induced Photodynamic Therapy of Tumors. ACS Appl. Mater. Interfaces 2020, 12, 12591–12599. [Google Scholar] [CrossRef] [PubMed]
- Kodumudi, K.N.; Woan, K.; Gilvary, D.L.; Sahakian, E.; Wei, S.; Djeu, J.Y. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 2010, 16, 4583–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhou, L.; Wang, C.; Han, Y.; Lu, Y.; Liu, J.; Hu, X.; Yao, T.; Lin, Y.; Liang, S.; et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019, 31, e1904997. [Google Scholar] [CrossRef]
- Yang, J.C.; Shang, Y.; Li, Y.H.; Cui, Y.; Yin, X.B. An “all-in-one” antitumor and anti-recurrence/metastasis nanomedicine with multi-drug co-loading and burst drug release for multi-modality therapy. Chem. Sci. 2018, 9, 7210–7217. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wan, Y.; Chen, Y.; Blum, N.T.; Lin, J.; Huang, P. Ultrasound-Enhanced Chemo-Photodynamic Combination Therapy by Using Albumin “Nanoglue”-Based Nanotheranostics. ACS Nano 2020, 14, 5560–5569. [Google Scholar] [CrossRef]
- Feng, L.; Gai, S.; He, F.; Dai, Y.; Zhong, C.; Yang, P.; Lin, J. Multifunctional mesoporous ZrO2 encapsulated upconversion nanoparticles for mild NIR light activated synergistic cancer therapy. Biomaterials 2017, 147, 39–52. [Google Scholar] [CrossRef]
- Hou, L.; Shan, X.; Hao, L.; Feng, Q.; Zhang, Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017, 54, 307–320. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Hou, M.; Chen, Q.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Phase-Change Material Packaged within Hollow Copper Sulfide Nanoparticles Carrying Doxorubicin and Chlorin e6 for Fluorescence-Guided Trimodal Therapy of Cancer. ACS Appl. Mater. Interfaces 2019, 11, 417–429. [Google Scholar] [CrossRef]
- Xu, X.; Han, C.; Zhang, C.; Yan, D.; Ren, C.; Kong, L. Intelligent phototriggered nanoparticles induce a domino effect for multimodal tumor therapy. Theranostics 2021, 11, 6477–6490. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Tang, K.; Hou, Y.; Yu, J.; Wang, C.; Wang, Y. Ultralow-intensity near infrared light synchronously activated collaborative chemo/photothermal/photodynamic therapy. Biomater. Sci. 2020, 8, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomater. 2018, 82, 171–183. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Ding, X.; Li, H.; Xu, Y.; Liu, F.; Sun, S. In Situ Formation of Homogeneous Tellurium Nanodots in Paclitaxel-Loaded MgAl Layered Double Hydroxide Gated Mesoporous Silica Nanoparticles for Synergistic Chemo/PDT/PTT Trimode Combinatorial Therapy. Inorg. Chem. 2019, 58, 2987–2996. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.L.; Huang, H.C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Mroz, P.; Hamblin, M.R. The immunosuppressive side of PDT. Photochem. Photobiol. Sci. 2011, 10, 751–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nanoparticle | Photosensitizers | Chemotherapy Drugs | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Azobenzene-containing conjugated polymers-camptothecin-chlorin e6 NPs (CPs-CPT-Ce6) | Chlorin e6 (Ce6) | Camptothecin (CPT) | HeLa | In vitro, Animals |
| [92] 2018 |
| Cyclic pentapeptide cRGDfk and Chlorin e6 conjugated silk fibroin (SF)-based NPs | Chlorin e6 (Ce6) | 5-Fluorouracil (5-FU) | MGC-803 | In vitro, Animals |
| [93] 2018 |
| DOX- and perfluorocarbon (PFC)- loaded fluorinated aza-boron-dipyrromethene (PDNBF) NPs | Fluorinated aza-boron-dipyrromethene (NBF) | DOX | 4T1 | In vitro, Animals |
| [94] 2020 |
| Pluronic F127 encapsulated halogenated boron-dipyrromethene NPs (LBBr2 NPs and LBCl2 NPs) | Halogenated boron-dipyrromethene (BDPBr2 and BDPCl2) | Lenvatinib | Hep3B, Huh7 | In vitro |
| [95] 2021 |
| Lactobionic acid-catalase-cis-aconitic anhydride-linked doxorubicin @ chlorin e6 (LA-CAT-CAD@Ce6) | Chlorin e6 (Ce6) | cis-Aconitic anhydride-linked doxorubicin (DOX precursor) | EMT6 | In vitro, Animals |
| [96] 2020 |
| Pyropheophorbide a–polyethylene glycol 2000 (Ppa-PEG2k) | Pyropheophorbide a (Ppa) | ROS-responsive oleate prodrug of paclitaxel (PTX) | A549, 4T1 | In vitro, Animals |
| [97] 2019 |
| Poly (oligo (ethylene glycol) methacrylate)-Paclitaxel @Chlorin e6 NPs | Chlorin e6 (Ce6) | B-sensitive polymer-paclitaxel (PTX) | T24 | In vitro, Animals |
| [98] 2021 |
| Polyethylene glycol-peptide-poly(ω-pentadecalactone-co-N-methyldiethyleneamine-co-3,3′-thiodipropionate) (PEG-M-PPMT) nanoparticles (NPs) | Chlorin e6 (Ce6) | Sorafenib (SRF) | A549 | In vitro, Animals |
| [99] 2020 |
| (Phenylboronic acid4-E2E)2-Protoporphyrin IX-(Lipoic acid)2 | Protoporphyrin IX (PpIX) | Paclitaxel (PTX) | A549 | In vitro, Animals |
| [100] 2020 |
| Poly(ethylene glycol)-b-PMPMC-g-paclitaxel-g-PyTPE micelles (PMPT) | PyTPE, TB | Paclitaxel-SS-N3 (PTX-SS-N3) | HeLa | In vitro, Animals |
| [101] 2021 |
| Nanoparticle | Photosensitizers | Gene Therapy Drugs | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Chlorin e6-DNAzyme/Cu(I)1,2,4-tri-azolate nanoscale coordination polymers (Ce6-DNAzyme/[Cu(tz)] CPs) | Chlorin e6 (Ce6) | Early Growth Response Factor-1 (EGR-1) targeted DNAzyme | MCF-7 | In vitro, Animals |
| [116] 2021 |
| Cationic guanidylated porphyrin/siRNA complexes (H2-PG/siRNA) | Porphyrin | Inhibitor of apoptosis siRNA (siIAP) | MDA-MB-231 | In vitro |
| [117] 2019 |
| CaCO3 layer modified MnO2 NPs (Mn@CaCO3) | Indocyanine green (ICG) | Programmed death ligand 1 siRNA (siPD-L1) | Lewis | In vitro, Animals |
| [118] 2019 |
| (PGL-NH2)2 and fluorocarbon inert gas of C3F8 grafted cationic porphyrin microbubbles (CpMBs)/siRNA | Porphyrin | Pioneer transcription factor 1 siRNA (siFOXA1) | MCF-7 | In vitro, Animals |
| [119] 2018 |
| (NaYF4:Yb, Er) upconversion NPs (UCNPs) | 5,10,15,20-tetrakis (1-methyl pyridinium-4-yl) porphyrin (TMPyP4) | ssDNA with chitosan aptamer (AS1411) and chitosan-targeted DNAzyme | MCF-7 | In vitro, Animals |
| [120] 2020 |
| Versatile function of cationic phosphonium-conjugated polythiophenes | Polythiophenes | Luciferase gene siRNA | MDA-MB-231 | In vitro |
| [121] 2020 |
| Cationic polyporphyrin vectors | Porphyrin | Hypoxia-inducible factor-1α siRNA (siHIF-1α) | H22 | In vitro, Animals |
| [122] 2021 |
| Periodic mesoporous ionosilica NPs (PMINPs) | Tetrasilylated porphyrin precursor | Luciferase gene siRNA | MDA-MB-231-Luc-RFP | In vitro |
| [123] 2021 |
| Dendritic arginine-rich peptide conjugated with cystamine-modified stearic acid (ALS) | HPPH | Vascular endothelial growth factor (VEGF) siVEGF | HeLa | In vitro, Animals |
| [58] 2021 |
| Nanoparticle | Photosensitizers | Immunotherapy Drugs | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Ce6-Gold nanoclusters-PEG2000-CD3 antibody-cytokine-induced killer cell NPs | Chlorin e6 (Ce6) | CD3 antibody | MGC-803 | In vitro, Animals |
| [142] 2018 |
| Human-induced pluripotent stem cells loaded with MnO2@Chlorin e6 NPs (iPS-MnO2@Ce6) | Chlorin e6 (Ce6) | Tumor antigens of human-induced pluripotent stem cells | Lewis | In vitro, Animals |
| [143] 2020 |
| Chlorin e6- and imiquimod-loaded upconversion NPs (UCNPs-Ce6-R837 NPs) | Chlorin e6 (Ce6) | Imiquimod (R837) | CT26 | In vitro, Animals |
| [144] 2017 |
| Diblock copolymer azide-modified polyethylene glycol block polyaspartic acid(benzylamine) (Azide PEG-Pasp(Bz) micelles | Zinc phthalocyanine (ZnPc) | Mal-GGPLGVRG-Pra peptide modified aPD-L1 | B16-F10 | In vitro, Animals |
| [145] 2021 |
| Serum albumin-coated boehmite B NPs | Chlorin e6 (Ce6) | Bee Venom Melittin (MLT) | 4T1 | In vitro, Animals |
| [5] 2019 |
| Nanoparticle | Photosensitizers | Photothermal Agents | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Boron dipyrromethene conjugated hyaluronic acid polymer NPs (BODIPY-HA NPs) | Boron dipyrromethene (BODIPY) | Boron dipyrromethene (BODIPY) | 4T1 | In vitro |
| [154] 2021 |
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000]-coated nanographene oxide-copper sulfide NPs (pGO-CuS NPs) | GO, CuS | Indocyanine green (ICG), CuS | MCF-7 | In vitro |
| [155] 2015 |
| Polypyrrole NPs | Phycocyanin (Pc) | Phycocyanin (Pc) | MDA-MB-231 | In vitro |
| [156] 2017 |
| Indocyanine green-coated single-walled carbon nanohorn NPs (SWNH-ICGs) | Indocyanine green (ICG) | Indocyanine green (ICG) | 4T1 | In vitro, Animals |
| [157] 2018 |
| Iridium oxide-manganese dioxide mineralized Chlorin e6 conjugated bovine serum albumin NPs (BSA-Ce6@IrO2/MnO2) | Chlorin e6 (Ce6) | Iridium oxide (IrO2) | MDA-MB-231, 4T1, PC3 | In vitro, Animals |
| [158] 2020 |
| Lipid-purpurin 18 and pure lipid self-assembled NPs (Pp18-lipos) | Lipid-purpurin 18 (Pp18-lipids) | Lipid-purpurin 18 (Pp18-lipids) | 4T1 | In vitro, Animals |
| [159] 2020 |
| Folic acid-polyethylene glycol-coated black phosphorus nanosheets conjugated with copper sulfide (BP-CuS-FA) | Black phosphorus (BP) | Copper sulfide (CuS), Black phosphorus (BP) | 4T1 | In vitro, Animals |
| [160] 2020 |
| Poly(lactic-co-glycolic acid) (PLGA) NPs | IR780 iodide | Perfluorocarbon (PFC) | 4T1 | In vitro, Animals |
| [161] 2020 |
| Amino-modified nanomaterial based on MoS2 quantum-dot-doped disulfide-based SiO2 NPs coated with hyaluronic acid and chlorin e6 (MoS2@ss- SiO2-Ce6/HA) | Chlorin e6 (Ce6) | MoS2 quantum dots | 4T1 | In vitro, Animals |
| [162] 2019 |
| Isoindigo/triphenylamine donor-acceptor-donor conjugated small molecule NPs (IID-ThTPA NPs) | IID-ThTPA | IID-ThTPA | 4T1 | In vitro, Animals |
| [163] 2020 |
| MoSe2/Bi2Se3 nanoheterostructure | MoSe2/Bi2Se3 | MoSe2/Bi2Se3 | HepG2 | In vitro, Animals |
| [164] 2019 |
| Polyethylene glycolated triphenylphosphine modified hitosan/iron oxide NPs (PEG-CS/Fe2O3 NPs) | Methylene blue (MB) | Iron oxide | HeLa, A549, MCF-7 | In vitro, Animals |
| [165] 2020 |
| Indocyanine green-grafted gold nanobipyramids covalently conjugated with folic acid (AuBPs@FLA@ICG@FA NPs) | Indocyanine green (ICG) | Gold nanobipyramids (AuBPs) | B16-F10 | In vitro |
| [166] 2020 |
| Pardaxin peptide-modified, indocyanine green-conjugated hollow gold nanospheres (FAL-ICG-HAuNS) | Indocyanine green (ICG) | Gold nanospheres | CT26 | In vitro, Animals |
| [139] 2019 |
| Gd3+ and chlorin e6 loaded single-walled carbon nanohorns (Gd-Ce6@SWNHs) | Chlorin e6 (Ce6) | Gd3+ | 4T1 | In vitro, Animals |
| [167] 2020 |
| Nanoparticle | Photosensitizers | Hyperthermia Agents | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Cancer cell membrane-cloaked Ce6-loaded Janus magnetic mesoporous organosilica NPs (CM@M-MON@Ce6) | Chlorin e6 (Ce6) | Magnetic mesoporous silica nanoparticles (M-MSNs) | 4T1 | In vitro, Animals |
| [174] 2019 |
| Ultramagnetic photosensitive liposomes | Foscan | Iron oxide NPs | SKOV-3 | In vitro, Animals |
| [175] 2015 |
| Magneto low-density nanoemulsion (MLDE) | Chlorin e6 (Ce6) | Iron oxide NPs | MCF-7 | In vitro |
| [176] 2018 |
| Nanoparticle | Photosensitizers | Scintillators/Radionuclides | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Hollow mesoporous silica NPs (HMSNs) | Chlorin e6 (Ce6) | Zirconium-89 (89Zr) | 4T1 | In vitro, Animals |
| [194] 2016 |
| Dextran modified TiO2 NPs (D-TiO2 NPs) | TiO2 | Gallium-68 (Ga-68) | 4T1 | In vitro, Animals |
| [195] 2018 |
| Magetic NPs with 89Zr radiolabeling and porphyrin molecules surface modification (89Zr-MNPs/TCPP) | Meso-tetrakis(4-carboxyphenyl)porphyrin (TCPP) | Zirconium-89 (89Zr) | 4T1 | In vitro, Animals |
| [196] 2018 |
| Titanocene-modified, transferrin-coated TiO2 NPs (TiO2-Tf-Tc) | TiO2 | Radiolabeled 2′-deoxy-2′-(18F)fluoro-D-glucose (FDG) | HT1080 | In vitro, Animals |
| [192] 2015 |
| Folic acid-poly(lactide-co-glycolide) polymeric nanoparticles-verteporfin (VP), (FA-PLGA-VP NPs) | Verteporfin (VP) | Verteporfin (VP) | HCT116 | In vitro |
| [197] 2018 |
| 131I-labeled zinc tetra(4-carboxyphenoxy) phthalocyaninate conjugated Cr3+-doped zinc gallate NPs (131I-ZGCs-ZnPcC4) | Zinc tetra(4-carboxyphenoxy) phthalocyaninate (ZnPcC4) | Cr3+-doped zinc gallate (ZnGa2O4:Cr3+) | 4T1 | In vitro, Animals |
| [193] 2021 |
| LiLuF4:Ce@SiO2@Ag3PO4@cisplatin prodrug (Pt(IV)) NPs (LAPNP NPs) | Ag3PO4 | LiLuF4:Ce | HeLa | In vitro, Animals |
| [198] 2018 |
| NaGdF4:Tb,Ce@NaGdF4 core/shell structure NPs | Rose Bengal (RB) | NaGdF4:Tb,Ce | PC3 | In vitro, Animals |
| [199] 2020 |
| Nanoparticle | Photosensitizers | Sonophotosensitizers | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|---|
| Glypican-3-targeted, curcumin-loaded microbubbles (GPC3-CUR-MBs) | Curcumin (CUR) | Curcumin (CUR) | HepG2 | In vitro, Animals |
| [213] 2020 |
| Curcumin-loaded poly(L-lactic-co-glycolic acid) microbubbles (CUR-PLGA-MBs) | Curcumin (CUR) | Curcumin (CUR) | HepG2 | In vitro |
| [214] 2020 |
| 5-Aminolevulinic acid/titanium dioxide NPs (5-ALA/TiO2) | 5-Aminolevulinic acid (5-ALA) | Titanium dioxide (TiO2) | SCC | In vitro, Animals |
| [215] 2016 |
| Nanoparticle | Type of Combination Therapy | Tumor | Data Sources | Findings | Ref |
|---|---|---|---|---|---|
| Human serum albumin-paclitaxel-sinoporphyrin sodium nanotheranostics (HAS-PTX-DVDMS) | Photodynamic therapy/Chemotherapy/Sonodynamic therapy | 4T1 | In vitro, Animals |
| [222] 2020 |
| ZrO2-coated, doxorubicin hydrochloride-, chlorin e6- and tetradecanol-loaded upconversion NPs (UCNPs@ZrO2-Ce6/DOX/PCM) | Photodynamic therapy/Chemotherapy/Hyperthermia | U14 | In vitro, Animals |
| [223] 2017 |
| Iron-dependent artesunate-loaded, transferrin-modified, hollow mesoporous CuS NPs (AS/Tf-HMCuS NPs) | Photodynamic therapy/Photothermal therapy/Chemotherapy | MCF-7 | In vitro, Animals |
| [224] 2017 |
| 1-Tetradecanol-, doxorubicin- and chlorin e6-loaded hollow mesoporous copper sulfide NPs (H-CuS@PCM/DOX/Ce6 (HPDC) NPs) | Photodynamic therapy/Photothermal therapy/Chemotherapy | 4T1 | In vitro, Animals |
| [225] 2019 |
| (Enaminitrile molecule gel encapsulating doxorubicin core/Mesoporous silica-coated, CuS-loaded, lanthanide ion-doped upconversion shell) NPs wrapped with a cancer cell membrane | Photodynamic therapy/Photothermal therapy/Chemotherapy | MCF-7, 4T1 | In vitro, Animals |
| [226] 2021 |
| (Upconversion core/chlorin e6, doxorubicin hydrochloride coloaded mesoporous silica shell) NPs conjugated with polyethylene glycol-modified graphene (DOX-UMCG) | Photodynamic therapy/Photothermal therapy/Chemotherapy | HeLa | In vitro, Animals |
| [227] 2020 |
| Thiol-terminated monomethoxyl poly(ethylene glycol) and mercaptoropionylhydrazide-modified gold nanorods covalently conjugated with 5-aminolevulinic acid and doxorubicin (GNRs-MPH-ALA/DOX-PEG) | Photodynamic therapy/Photothermal therapy/Chemotherapy | MCF-7 | In vitro, Animals |
| [228] 2018 |
| Folic acid-functionalized, paclitaxel-loaded MgAl layered double hydroxide gated mesoporous silica NPs (MT@L-PTX@FA) | Photodynamic therapy/Photothermal therapy/Chemotherapy | HepG2 | In vitro |
| [229] 2019 |
| Folic acid-CuS/docetaxel@polyethylenimine-protoporphyrin IX-CPG (FA-CuS/DTX@PEI-PpIXCpG) | Photodynamic therapy/Photothermal therapy/Chemotherapy/Immunotherapy | 4T1 | In vitro, Animals |
| [220] 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-L.; Lin, K.; Yang, L. Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy. Pharmaceutics 2021, 13, 1951. https://doi.org/10.3390/pharmaceutics13111951
Yang Y-L, Lin K, Yang L. Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy. Pharmaceutics. 2021; 13(11):1951. https://doi.org/10.3390/pharmaceutics13111951
Chicago/Turabian StyleYang, Yu-Ling, Ke Lin, and Li Yang. 2021. "Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy" Pharmaceutics 13, no. 11: 1951. https://doi.org/10.3390/pharmaceutics13111951
APA StyleYang, Y.-L., Lin, K., & Yang, L. (2021). Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy. Pharmaceutics, 13(11), 1951. https://doi.org/10.3390/pharmaceutics13111951





