Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management
Abstract
:1. Introduction
2. Pathogenesis of Psoriasis
3. Causes and Types of Psoriasis
4. Conventional Treatment Alternatives for Psoriasis
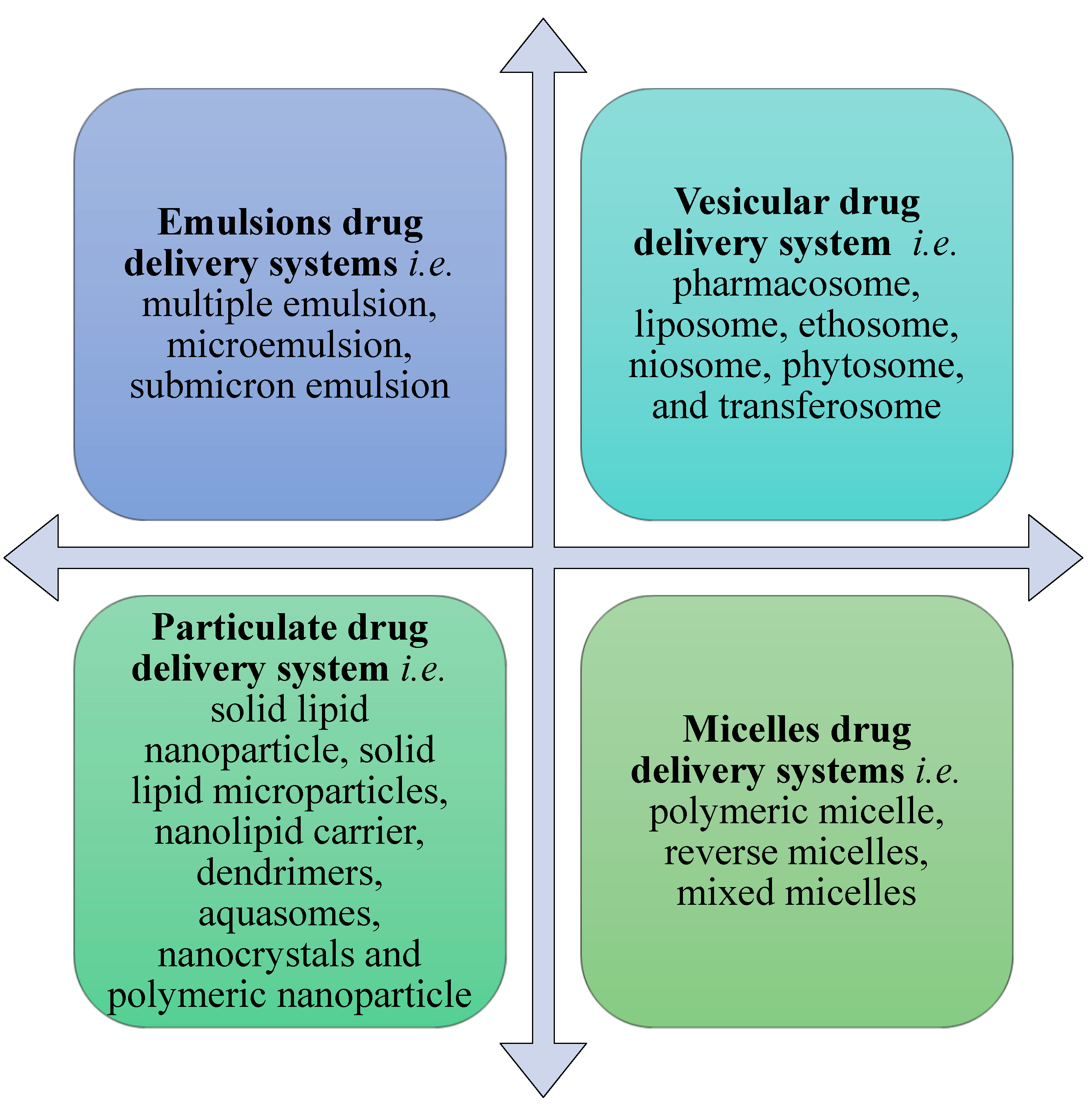
5. The Need for Colloidal Drug Delivery Systems
6. Applications of Emulsion Drug Delivery Systems in Psoriasis
6.1. Multiple Emulsions
6.2. Microemulsion
6.3. Nano-Emulsion
7. Applications of Vesicular Drug Delivery Systems in Psoriasis
7.1. Liposomes
7.2. Ethosomes and Niosomes
7.3. Transferosomes
8. Applications of Particulate Drug Delivery Systems in Psoriasis
8.1. Solid Lipid Nanoparticles (SLNs) and Solid Lipid Microparticles (SLMs)
8.2. Nano-Structured Lipid Carriers (NLCs)
8.3. Dendrimers
8.4. Nanocrystals
8.5. Polymeric and Gold Nanoparticles
9. Applications of Micelle Drug Delivery Systems in Psoriasis
10. Recent Advancements in Herbal Nano-Carriers for Psoriasis Treatment
11. Conclusions
12. Current and Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| DC | Dendritic cell |
| AMP | Antimicrobial peptide |
| pDC | Plasmacytoid dendritic cell |
| TLR | Toll-like receptor |
| mDC | myeloid dendritic cell |
| IL | Interleukin |
| IFN-α | Interferon-α |
| TPGS | Tocopherol polyethylene glycol 1000 succinate |
| DC | Dendritic cell |
| AMP | Antimicrobial peptide |
| pDC | Plasmacytoid dendritic cell |
| TLR | Toll-like receptor |
| mDC | myeloid dendritic cell |
| IL | Interleukin |
| IFN-α | Interferon-α |
| TPGS | Tocopherol polyethylene glycol 1000 succinate |
| siRNA | small interfering RNAs |
References
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S. Ajazuddin Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnostic et prise en charge du psoriasis. Can. Fam. Physician 2017, 63, e210. [Google Scholar]
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and classification of psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef]
- Sala, M.; Elaissari, A.; Fessi, H. Advances in psoriasis physiopathology and treatments: Up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J. Control. Release 2016, 239, 182–202. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.J.; Bello-Quintero, C.E. Advancements in the treatment of psoriasis: Role of biologic agents. J. Manag. Care Spec. Pharm. 2004, 10, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Mehlis, S.L.; Gordon, K.B. The immunology of psoriasis and biologic immunotherapy. J. Am. Acad. Dermatol. 2003, 49, 44–50. [Google Scholar] [CrossRef]
- Takahashi, H.; Iizuka, H. Psoriasis and metabolic syndrome. J. Dermatol. 2012, 39, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Marepally, S.; Boakye, C.H.; Patel, A.R.; Godugu, C.; Doddapaneni, R.; Desai, P.R.; Singh, M. Topical administration of dual siRNAs using fusogenic lipid nanoparticles for treating psoriatic-like plaques. Nanomedicine 2014, 9, 2157–2174. [Google Scholar] [CrossRef] [PubMed]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B 2016, 141, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Wan, T.; Pan, W.; Long, Y.; Yu, K.; Liu, S.; Ruan, W.; Pan, J.; Qin, M.; Wu, C.; Xu, Y. Effects of nanoparticles with hydrotropic nicotinamide on tacrolimus: Permeability through psoriatic skin and antipsoriatic and antiproliferative activities. Int. J. Nanomed. 2017, 12, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemati, H.; Ghahramani, M.H.; Faridi-Majidi, R.; Izadi, B.; Bahrami, G.; Madani, S.H.; Tavoosidana, G. Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release 2017, 268, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, J.; Kim, J.; Choi, M.; Jeon, H.; Choe, K.; Lee, D.Y.; Kim, P.; Jon, S. Nanoparticle-assisted transcutaneous delivery of a signal transducer and activator of transcription 3-inhibiting peptide ameliorates psoriasis-like skin inflammation. Am. Chem. Soc. Nano 2018, 12, 6904–6916. [Google Scholar] [CrossRef] [PubMed]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.; Pires, L.R. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int. J. Pharm. 2020, 591, 119942. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M. Tacrolimus-loaded chitosan nanoparticles for enhanced skin deposition and management of plaque psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Jyothi, S.L.; Krishna, K.L.; Shirin, V.A.; Sankar, R.; Pramod, K.; Gangadharappa, H.V. Drug delivery systems for the treatment of psoriasis: Current status and prospects. J. Drug Deliv. Sci. Technol. 2021, 62, 102364. [Google Scholar] [CrossRef]
- Jordan, C.T.; Cao, L.; Roberson, E.D.O.; Duan, S.; Helms, C.A.; Nair, R.P.; Duffin, K.C.; Stuart, P.E.; Goldgar, D.; Hayashi, G.; et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am. J. Hum. Genet. 2012, 90, 796–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoloff, B.J. Keratinocytes regain momentum as instigators of cutaneous inflammation. Trends Mol. Med. 2006, 12, 102–106. [Google Scholar] [CrossRef]
- Iizuka, H.; Honda, H.; Ishida-Yamamoto, A. Epidermal remodeling in psoriasis (II): A quantitative analysis of the epidermal architecture. J. Investig. Dermatol. 1997, 109, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, A.; Chrousos, G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016, 17, 295–304. [Google Scholar] [CrossRef]
- Schmid-Ott, G.; Jaeger, B.; Boehm, T.; Langer, K.; Stephan, M.; Raap, U.; Werfel, T. Immunological effects of stress in psoriasis. Br. J. Dermatol. 2009, 160, 782–785. [Google Scholar] [CrossRef] [PubMed]
- O’doherty, C.J.; Macintyre, C. Palmoplantar pustulosis and smoking. Br. Med. J. Clin. Res. Ed. 1985, 291, 861–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagami, H. Triggering factors. Clin. Dermatol. 1997, 15, 677–685. [Google Scholar] [CrossRef]
- Naldi, L.; Parazzini, P.; Brevi, A.; Peserico, A.; Veller Fornasa, C.; Grosso, G.; Rossi, E.; Marinaro, P.; Polenghi, M.M.; Finzi, A.; et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br. J. Dermatol. 1992, 127, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Powles, A.V.; Baker, B.S.; Rutman, A.J.; McFadden, J.P.; Valdimarsson, H.; Fry, L. Epidermal rupture is the initiating factor for the Koebner response in psoriasis. Acta Derm. Venereol. 1990, 70, 35–38. [Google Scholar]
- Fierlbeck, G.; Rassner, G.; Müller, C. Psoriasis Induced at the Injection Site of Recombinant Interferon Gamma: Results of Immunohistologic Investigations. Arch. Dermatol. 1990, 126, 351–355. [Google Scholar] [CrossRef]
- Langley, R.G.B.; Krueger, G.G.; Griffiths, C.E.M. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64, ii18–ii23. [Google Scholar] [CrossRef] [Green Version]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, K.; Walker, A.; Gropper, C.; Hoffman, C. To treat or not to treat? Management of guttate psoriasis and pityriasis rosea in patients with evidence of group A Streptococcal infection. J. Drugs Dermatol. 2010, 9, 241–250. [Google Scholar]
- Naldi, L.; Peli, L.; Parazzini, F.; Carrel, C.F. Family history of psoriasis, stressful life events, and recent infectious disease are risk factors for a first episode of acute guttate psoriasis: Results of a case-control study. J. Am. Acad. Dermatol. 2001, 44, 433–438. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Van Voorhees, A.S.; Lebwohl, M.G. An update on generalized pustular psoriasis. Expert Rev. Clin. Immunol. 2019, 15, 907–919. [Google Scholar] [CrossRef] [Green Version]
- Erythrodermic (Exfoliative) Psoriasis. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8453895/ (accessed on 25 February 2021).
- Balasubramaniam, P.; Berth-Jones, J. Erythroderma: 90% skin failure. Hosp. Med. 2004, 65, 100–102. [Google Scholar] [CrossRef]
- Papp, K.; Berth-Jones, J.; Kragballe, K.; Wozel, G.; de la Brassinne, M. Scalp psoriasis: A review of current topical treatment options. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoruso, G.F.; Nisticò, S.P.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab may improve renal function in psoriasis. Healthcare 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nisticò, S.P. Emerging role of anti-IL23 in the treatment of psoriasis: When humanized is very promising. Dermatol. Ther. 2020, 33, e14504. [Google Scholar] [CrossRef]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids Surf. B Biointerfaces 2009, 74, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Particle control of emulsion by membrane emulsification and its applications. Adv. Drug Deliv. Rev. 2000, 45, 47–56. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef]
- Bhatia, N.; Pandit, S.; Agrawal, S.; Gupta, D. A Review On Multiple Emulsions. Int. J. Pharm. erud. 2013, 3, 22–30. [Google Scholar]
- Prajapati, S.B.; Bhatt, H.; Koli, A.; Dharamsi, A.; Shah, S.A. An overview of Preparation, Evaluation and Applications of Multiple Emulsions. Int. J. Pharm. Res. Sch. 2013, 2, 142–150. [Google Scholar]
- Laugel, C.; Baillet, A.; Youenang Piemi, M.P.; Marty, J.P.; Ferrier, D. Oil-water-oil multiple emulsions for prolonged delivery of hydrocortisone after topical application: Comparison with simple emulsions. Int. J. Pharm. 1998, 160, 109–117. [Google Scholar] [CrossRef]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.A.; Nour, S.A.; Sakran, W.S.; El-Mancy, S.M.S. Preparation and evaluation of microemulsion systems containing salicylic acid. AAPS PharmSciTech 2009, 10, 1081–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroli, B.; López-Quintela, M.A.; Delgado-Charro, M.B.; Fadda, A.M.; Blanco-Méndez, J. Microemulsions for topical delivery of 8-methoxsalen. J. Control. Release 2000, 69, 209–218. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J. Control. Release 2013, 170, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Habib, S.; Singh, M. Recent Advances in Lipid-Based Nanosystems for Gemcitabine and Gemcitabine—Combination Therapy. Nanomaterials 2021, 11, 597. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Mittal, S.; Ali, J.; Baboota, S. Enhanced anti-psoriatic activity of tacrolimus loaded nanoemulsion gel via omega 3-Fatty acid (EPA and DHA) rich oils-fish oil and linseed oil. J. Drug Deliv. Sci. Technol. 2021, 63, 102458. [Google Scholar] [CrossRef]
- Gungor, S.; Rezigue, M. Nanocarriers mediated topical drug delivery for psoriasis treatment. Curr. Drug Metab. 2017, 18, 454–468. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of Liposomes Using Supercritical Carbon Dioxide Technology: Effects of Phospholipids and Sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Ricci, M.; Oliva, R.; Del Vecchio, P.; Paolantoni, M.; Morresi, A.; Sassi, P. DMSO-induced Perturbation of Thermotropic Properties of Cholesterol-Containing DPPC Liposomes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Saeed, R.M.; Dmour, I.; Taha, M.O. Stable chitosan-based nanoparticles using polyphosphoric acid or hexametaphosphate for tandem ionotropic/covalent crosslinking and subsequent investigation as novel vehicles for drug delivery. Front. Bioeng. Biotechnol. 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aland, R.; Ganesan, M.; Rao, P.R.; Bhikshapathi, D.V. Solid Lipid Nanoparticles for Topical Delivery of Acitretin for the Treatment of Psoriasis by Design of Experiment. Int. J. Pharm. Sci. Nanotechnol. 2019, 12, 4474–4491. [Google Scholar] [CrossRef]
- Behera, J.; Behera, J.; Keservani, R.K.; Yadav, A.; Tripathi, M.; Chadoker, A. Methoxsalen loaded chitosan coated microemulsion for effective treatment of psoriasis. Int. J. Drug Deliv. 2011, 2, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Rajitha, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1473–1482. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.-M.; Mo, F.-K.; Zhong, D.-F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int. J. Pharm. 2006, 321, 117–123. [Google Scholar] [CrossRef]
- Olsson, U.; Lindman, B. Uni- and Bicontinuous Microemulsions. In The Structure, Dynamics and Equilibrium Properties of Colloidal Systems; Springer: Dordrecht, The Netherlands, 1990; pp. 233–242. [Google Scholar]
- Madhav, S.; Gupta, D. A review on microemulsion based system. Int. J. Pharm. Sci. Res. 2011, 2, 1888–1899. [Google Scholar]
- El-Aasser, M.S.; Sudol, E.D. Miniemulsions: Overview of research and applications. JCT Res. 2004, 1, 21–32. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and curcumin co-loaded liposphere gel: Synergistic combination towards management of psoriasis. J. Control. Release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Walunj, M.; Doppalapudi, S.; Bulbake, U.; Khan, W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J. Liposome Res. 2020, 30, 68–79. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Katare, O.P.; Vyas, S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Lei, W.; Yu, C.; Lin, H.; Zhou, X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J. Pharm. Sci. 2013, 8, 336–345. [Google Scholar] [CrossRef] [Green Version]
- EI Gizaway, S.; Fadel, M.; Mourad, B.; Elnaby, F.E.A. Betamethasone dipropionate gel for treatment of localized plaque psoriasis. Int. J. Pharm. Pharm. Sci. 2017, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Radha, G.; Rani, T.S.; Sarvani, B. A review on proniosomal drug delivery system for targeted drug action. J. Basic Clin. Pharm. 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Irchhaiya, R. Niosomes: A potential tool for novel drug delivery. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- Lakshmi, P.K.; Devi, G.S.; Bhaskaran, S.; Sacchidanand, S. Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 157–161. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Rawat, B.S.; Singh, D.; Rawat, M.S.M. Pharmacosomes: The lipid-based new drug delivery system. Expert Opin. Drug Deliv. 2009, 6, 599–612. [Google Scholar] [CrossRef]
- Meshram, S.S.; Itankar, P.R.; Patil, A.T.; Meshram, M.S.S. To Study Pharmacognostic, Physicochemical and Phytochemical Study of Stem Bark of Bauhinia purpurea Linn. J. Pharmacogn. Phytochem. 2013, 2, 19–22. [Google Scholar]
- Jain, N.; Gupta, B.P.; Thakur, N.; Jain, R.; Banweer, J.; Jain, D.K.; Jain, S. Phytosome: A Novel Drug Delivery System for Herbal Medicine. Int. J. Pharm. Sci. Drug Res. 2010, 2, 224–228. [Google Scholar]
- Cevc, G.; Schätzlein, A.; Blume, G. Transdermal drug carriers: Basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J. Control. Release 1995, 36, 3–16. [Google Scholar] [CrossRef]
- Lin, H.W.; Xie, Q.C.; Huang, X.; Ban, J.F.; Wang, B.; Wei, X.; Chen, Y.Z.; Lu, Z.F. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Barreiros, L.; Segundo, M.A.; Torres, T.; Selores, M.; Costa Lima, S.A.; Reis, S. Topical co-delivery of methotrexate and etanercept using lipid nanoparticles: A targeted approach for psoriasis management. Colloids Surf. B Biointerfaces 2017, 159, 23–29. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem. Phys. Lipids 2015, 186, 9–16. [Google Scholar] [CrossRef]
- Abdel-Salam, F.S.; Elkheshen, S.A.; Mahmoud, A.A.; Ammar, H.O. Diflucortolone valerate loaded solid lipid nanoparticles as a semisolid topical delivery system. Bull. Fac. Pharm. Cairo Univ. 2016, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Singh, D.; Murthy, S.N.; Singh, M.R. Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids 2015, 101, 56–63. [Google Scholar] [CrossRef]
- Pinto, M.F.; Moura, C.C.; Nunes, C.; Segundo, M.A.; Costa Lima, S.A.; Reis, S. A new topical formulation for psoriasis: Development of methotrexate-loaded nanostructured lipid carriers. Int. J. Pharm. 2014, 477, 519–526. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gorain, B.; Choudhury, H.; Srivastava, A.; Kesharwani, P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon 2019, 5, e01343. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Badıllı, U.; Şen, T.; Tarımcı, N. Microparticulate based topical delivery system of clobetasol propionate. AAPS PharmSciTech 2011, 12, 949–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGAlga nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Dua, K.; Khude, P. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, Y.; Petkar, K.C.; Sawant, K.K. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010, 401, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Umeyor, E.C.; Kenechukwu, F.; Ogbonna, J.D.; Chime, S.; Attama, A. Investigation of solidified reverse micellar systems as novel carriers for oral delivery of gentamicin. J. Pharm. Res. 2012, 5, 4914–4920. [Google Scholar]
- Gaba, B.; Fazil, M.; Ali, A.; Baboota, S.; Sahni, J.K.; Ali, J. Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Deliv. 2015, 22, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Liu, M.; Fréchet, J.M.J. Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 1999, 2, 393–401. [Google Scholar] [CrossRef]
- Cloninger, M.J. Biological applications of dendrimers. Curr. Opin. Chem. Biol. 2002, 6, 742–748. [Google Scholar] [CrossRef]
- Narang, N. Aquasomes: Self-assembled systems for the delivery of bioactive molecules. Asian J. Pharm. 2014, 6, 95. [Google Scholar] [CrossRef]
- Müller, R. Junghanns Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Yadav, H.K. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Novel Approach: Herbal Remedies and Natural Products. Google Scholar. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Novel+approach%3A+Herbal+remedies+and+natural+products+in+pharmaceutical+science+as+nano+drug+delivery+systems.+&btnG= (accessed on 26 February 2021).
- Shirwaikar, A.; Shirwaikar, A.; Prabhu, S.; Kumar, G. Herbal excipients in novel drug delivery systems. Indian J. Pharm. Sci. 2008, 70, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Sungthongjeen, S.; Pitaksuteepong, T.; Somsiri, A.; Sriamornsak, P. Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Dev. Ind. Pharm. 1999, 25, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Promising Role of Nanopharmaceuticals in Drug Delivery. Google Scholar. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Promising+role+of+nanopharmaceuticals+in+drug+delivery&btnG= (accessed on 26 February 2021).
- Li, Y.; Dong, L.; Jia, A.; Chang, X.; Xue, H. Preparation and characterization of solid lipid nanoparticles loaded traditional chinese medicine. Int. J. Biol. Macromol. 2006, 38, 296–299. [Google Scholar] [CrossRef]
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.K.; Du Toit, L.C.; Choonara, Y.E. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef]
- Mainardi, T.; Kapoor, S.; Bielory, L. Complementary and alternative medicine: Herbs, phytochemicals and vitamins and their immunologic effects. J. Allergy Clin. Immunol. 2009, 123, 283–294. [Google Scholar] [CrossRef]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 1–9. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Ofelia Vila Buso, M.A.; Ruiz Sauri, A.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef]
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sonia, K.; Anupama, D. Microemulsion Based Transdermal Drug Delivery of Tea Tree Oil. Int. J. Drug Dev. Res. 2010, 3, 191–198. [Google Scholar]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Lanza, M.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical delivery of curcumin by choline-calix[4]arene-based nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef] [PubMed]





| Psoriasis Type (Affected Area) | Characteristic Features | Ref. |
|---|---|---|
| Guttate psoriasis (Head and limbs) | The lesions are monomorphic. Children and adolescents are predominantly affected. The upper respiratory tract infection is occurring followed by streptococcal infection. | [15,29,30] |
| Plaques psoriasis/Psoriasis vulgaris (all body but typically on elbows, knees, scalp areas) | The characteristic lesions are dry, sharp and oval in shape which seems as erythematous macules and form plaque. | [15] |
| Pustular psoriasis (Palms and soles) | The patient suffers from red, inflamed, skin. In localized pustulosis, two distinct types of psoriasis are acrodermatitis continua of hallopeau and palmoplantar pustulosis in which pustules are formed which spread all over feet. In generalized psoriasis, pustular lesions occur in pregnancy state or due to some drugs. | [31] |
| Erythrodermic psoriasis (All body) | This is unstable psoriasis which is occurred by various reasons like cardiac failure, hyperthermia or deficiency of vitamins | [32,33] |
| Nail psoriasis (Fingernail, toe-nails) | At proximal portion of nail, small pits are forms which are characterized by orange-yellow area below nail plate which is called as oil spots. | [3] |
| Scalp psoriasis (Hairline) | Selection of appropriate treatment is difficult to need of application at scalp area. | [34] |
| Parameter | Phytosome | Liposome | Solid Lipid Nanoparticles | Polymeric Nanoparticle |
|---|---|---|---|---|
| Bond | Present | Absent | Absent | Absent |
| Complexity in manufacturing | Less complex | More Complex | More Complex | More Complex |
| Drug leakage | Less | More | More | More |
| Lipid drug interaction | Yes | No | No | No |
| Stability | More stable | Less Stable | Less Stable | More Stable |
| Entrapment | High | Low | Low | Low |
| Nature of excipients | Lipid | Lipid | Lipid | Synthetic or natural polymer |
| Biocompatibility | High | High | High | Low |
| Safety profile of solvents | ICH Class III solvents | ICH Class II and III solvents | ICH Class II and III solvents | ICH Class II and III solvents |
| Bioavailability | High | Moderate | Moderate | Moderate |
| Absorption | High | Moderate | Moderate | Moderate |
| Nanocarrier System | Advantages |
|---|---|
| Vesicular drug delivery system | |
| Transferosome | Large molecular weight medications are delivered to skin in a non-invasive manner. |
| Ethosome | Highly permeable and compliance to patient as well as safer for skin |
| Liposome | Amphiphilic nature, biocompatibility, and ease of surface alteration |
| Phytosomes | Great oral and transdermal bioavailability and therapeutic effect |
| Niosome | Enhances skin penetration of drugs; improved bioavailability of insufficiently absorbed drugs |
| Particulate drug delivery system | |
| Solid lipid nanoparticle | Increased efficacy, biocompatible, biodegradable |
| Dendrimers | Easy to prepare and alterations; better skin penetration |
| Polymeric nanoparticle | Used for entrapment of various class of drugs; biocompatible, biodegradable |
| Nanostructured lipid carriers | Reduces drug leakage during storage, increases drug payload; biocompatible |
| Micelles drug delivery systems | |
| Polymeric micelles | Thermodynamic stability, self-assembling, and skin targeting potential. |
| Carrier/Matrix for Nanocarrier | Properties | Ref. |
|---|---|---|
| Gelucires | Gelucires are polyethylene glycol glycerides made up of mono-, di-, and triglycerides, as well as mono- and diesters of polyethylene glycol. | [50] |
| Transcutol | Protic solvent, faint odor, colorless limpid liquid, hygroscopic having viscosity 4.1 mPa.s at 25 °C; have exceptional solubilizing capacity due to an alcohol and ether function; Used for skin penetration enhancement | [51,52] |
| Phosphatidylcholine | Choline is head-group of phosphatidylcholine and is attached to glycerol of fatty acids via ester-bound to backbone | [53] |
| Poly-lactic acid-co-glycolic acid | It is made up of glycolic acid (hydroxy acetic acid) and lactic acid (α-hydroxy propanoic acid) and due to its biocompatible and biodegradable nature, this polymer has been extensively utilized in drug delivery system with superiour loading efficiency | [54] |
| Cholesterol | 27-carbon molecule which has amphiphilic nature and contains hydroxyl group linked with phospholipids by hydrogen bonds with the help of flexible carbohydrate linked with bulky steroid ring. During preparation of liposomes, cholesterol avoids aggregation of liposomes and enhances physical stability of membrane of liposomal vesicles and have tendency to generate stable vesicles with high drug loading capacity. | [55,56] |
| Chitosan | It is produced via N-deacetylation of chitin. It is biocompatible, and biodegradable polymer of natural origin, therefore considered as harmless substance for use as carrier in production of drug delivery system with high drug loading and entrapment efficiency of several drug molecules | [57] |
| Egg lecithin | Provides better stabilizing and encapsulation efficiency; better drug loading | [58] |
| Drug (Delivery System) | Excipients | Preparation Technique | Clinical Significance and Outcomes | Ref. |
|---|---|---|---|---|
| Hydrocortisone (Multiple emulsion) | Glycerol sorbitan fatty acid ester, Liquid paraffin | Oil-water-oil emulsification | Prolonged topical release of hydrocortisone in epidermis and dermis and the absorbed percentage of hydrocortisone was 1.5-fold greater from the simple emulsion compared to multiple emulsion | [43] |
| Cyclosporine (Micro-emulsion) | Oleic acid, Tween 80, Water, Vitamin E-TPGS, Transcutol, Propylene glycol | Emulsification | Quick skin uptake and superior skin concentrations achieved after 2 h of contact | [44] |
| Salicylic acid (Micro-emulsion) | Polyethylene glycol, Tween 20, Isopropyl myristate | O/W emulsification | ME with 10% SA does not show any change in storage stability after 6 months except decrease in viscosity after 1 month | [45] |
| 8-Methoxsalen (Micro-emulsion) | Octanediol, Isopropyl myristate, Tween 80, Span 80, Water | O/W emulsification | The skin accumulation of 8-Methoxsalen was enhanced 1.5–4.5-fold | [46] |
| Methoxsalen (Micro-emulsion) | Egg phosphatidyl-choline, Chitosan, Ethanol, Acetic acid, Soya oil | Emulsification | Methoxsalen loaded chitosan-coated ME show controlled release of drug i.e., 18.75% lesser release than the ME with high drug retention into skin | [59] |
| Methotrexate (Nano- emulsion) | Tween 80, Water, Chaulmoogra oil | Self-emulsification | Showed negligible skin irritation and increased penetration into the skin | [60] |
| Clobitasol Propionate & Calcipotriol (Nano- emulsion) | Cremophor RH 40, Capmul MCM C8 EP, Labrafil® M 1944 CS, Water | Oil in water emulsification | The nanoemulsion globules of size <100 nm also contributes to improved skin penetration, permeation and retention of drug in deep skin layers | [61] |
| Drug (Delivery System) | Excipients | Preparation Technique | Clinical Significance and Outcomes | Ref. |
|---|---|---|---|---|
| Tacrolimus and Curcumin (Liposphere) | Egg lecithin, Tricaprin tween 80, cremophor RH40, Isopropyl alcohol | Single emulsion solvent evaporation | Exhibited slow release of drugs and improvement in phenotypic/histopathological features of psoriatic skin | [71] |
| Cyclosporine (Cationic liposome) | N-(1-(2,3-dioleoyloxy) propyl)- cholesterol, chloroform, ethanol | Ethanol injection, thin film hydration, reverse phase evaporation | 1.67 times rise in the levels of IL-17 on application of IMQ as compared to normal group and showed shear thinning behavior and highly effective and facilitate in psoriasis treatment | [72] |
| Methotrexate (Ethosome) | Soya phosphatidyl-choline, chloroform, methanol, hydro-ethanolic solution | Mechanical dispersion Cast film | Provided improved transdermal flux and reduced lag time of 0.9 h across human cadaver skin | [73] |
| Dithranol (Liposome, Niosome) | Phosphatidyl choline, cholesterol, span 60, chloroform | Thin-film hydration | Drug leakage study carried out at 4–8, 25 ± 2 and 37 °C for a period of two months and results revealed that leakage increased at a higher temperature and enhanced permeation with vesicles as signified through flux of dithranol | [74] |
| Tacrolimus (Transferosome) | Lipoid E80, Tween 80, Span 80, Dehydrated alcohol | Thin film hydration | In vitro drug release was higher in TFs-gel after 24 h in comparision to commercial ointment and from TFs-gel cumulative drug release after 12 h in vitro was 37.6%. and efficient skin target for topical delivery of tacrolimus | [75] |
| Betamethasone dipropionate (Transferosome) | Soya phosphatidyl-choline, Sodium deoxycholate, Tween 80, chloroform | Film hydration technique | The vesiclecs have 90.19% EE, and have great stability at 25 °C and 4 °C for 6 months and showed significant clinical improvement along with a considerable boost in safety and tolerability | [76] |
| Drug (Delivery System) | Excipients | Preparation Technique | Clinical Significance and Outcomes | Ref. |
|---|---|---|---|---|
| Methotrexate (SLN) | Cetyl palmitate, Polysorbate 80 | Ultra-sonication | In-vitro results showed a sustained release for 8 h and enhanced skin deposition for effectual treatment of psoriasis | [87] |
| Fluocinolone acetonide (SLN) | Compritol 888 ATO, Soya lecithin, Poloxamer 188 | Modified emulsification ultrasonication | Stability results show that SLNs were stable at 4 °C for 3 months and is a promising modality for psoriasis treatment | [88] |
| Diflucortolone valerate (SLN) | Geleol, Precirol ATO5, Tristearin, Compritol 888ATO, Poloxamer 407 | High shear homogenization | TO produce SLNs semisolid preparation 10–20% w/w solid lipid is enough and lipid based surfactants incorporation increased entrapment efficiency and enhanced drug’s solubility | [89] |
| Cyclosporine and calcipotriol (SLN and NLC) | Compritol 888 ATO, Precirol, Behenic acid, Gellucire 44/14, Span 20, Cremophor RH-40, Tween 80 | Hot melt homogenization | Deeper and confined drug penetration in epidermal layers for superior psoriatic management | [90] |
| Fluocinolone acetonide (NLC) | Compritol, Miglyol 812, Polysorbate 80 | High speed homogenization | Stability findings (3 months) revealed 1.77% and 5.66% percent alteration in EE and particle size and respectively and is regarded higher potential system for psoriasis treatment | [91] |
| Methotrexate (NLC) | Witepsol, oleic acid, polysorbate 60, polysorbate 80 | High-shear homogenization | Provided higher drug fluxes of 0.88 μg/cm 2 /h in comparison to free drug (0.59 μg/cm 2 /h) flux | [92] |
| Dithranol (Dendrimer) | Poly (amido) amine, ethyl cellulose, carbopol 934, Polyvinyl alcohol | Quasi-emulsion solvent diffusion | The results revealed that EE of preparation was in between 71.33% to 49.21%, particle size 28 ± 1.12 mm to 130 ± 1.01 mm and %age yield 66.28% and the formulation produced prolonged efficacy without causing skin toxicities | [93] |
| Dithranol (Dendrimer) | Ethylenediamine | Divergent method | Primary irritation index of DIT–PPI was revealed to be 1 that means DIT-PPU causes less irritation and have high drug penetration in controlled manner | [94] |
| Dexa-methasone (Nano-crystal) | Polyvinyl alcohol, Sodium lauryl sulphate | Wet bead milling | Superior drug penetration and distribution within skin with reduced dose | [95] |
| Clobetasol propionate (Polymeric microsphere) | Poly (d,l-lactide co-glycolide), polyvinyl alcohol | Solvent evaporation | F8-coded preparation fabricated using PLGA 50:50 at 1:5 drug/polymer ratio and homogenised for 1 min at rpm 8000 was considered as the best preparation and having superior drug efficacy in topical applications | [96] |
| Apremilast (Polymeric nano-particle) | Poly (d,l-lactide co-glycolide), Polyvinyl alcohol | Single emulsion solvent evaporation | 2.25 folds increment in bio-availability of F3 nanoparticles than normal APM suspension and enhancement in half-life and mean residence time leads to long-term retention of nano-particles to provide once-daily regimen | [97] |
| Methotrexate (Gold nano-particle) | 3-mercapto-1-propansulfonate, Diethylaminoethanethiol hydrochloride, tetrachloroauric (III) acid, sodium borohydride | Bioconjugation and functionalization | Induced a diminution of keratinocytes hyper-proliferation, epidermal thickness as well as inflammatory infiltrate in imiquimod-induced psoriasis like mice model | [98] |
| Mometasone furoate (SLN) | Glycerol mono-stearate, Tefose-63, Tween-80 | Solvent injection method | 2.67 times more skin deposition as compared to marketed cream and 20 times more in comparsion to plain drug loaded gel and is promising for topical delivery of corticosteroid | [99] |
| Acitretin (NLC) | Precirol ATO5, oleic acid, tween 80, tetrahydrofuran | Solvent diffusion method | Increase in Acitretin deposition was found in cadaver skin from ActNLC gel (81.38 ± 1.23%) than to Act plain gel (47.28 ± 1.02%) and enhancement in therapeutic effect for psoriasis and decrease in local side effects | [100] |
| Herbal Constituent (Delivery System) | Excipients | Preparation Technique | Clinical Significance and Outcomes | Ref. |
|---|---|---|---|---|
| Celastrol isolated from Tripterygium regelii (Niosome) | Cholesterol, carbopol 934, span 20, span 60 | Thin film hydration | The developed nanoparticle has particle size of 147 nm and yield of up to 90% and increased water solubility and permeation of celastrol into skin which enhanced its anti-psoriasis activity in mice | [123] |
| Mangiferin isolated from leaves/bark of Mangifera indica (Nano-emulsion) | Lipoid® S75, hylouronic acid, Polysorbate 80 | Ultra-sonication | Nanoemulsions having mangiferin significantly reduce oedema ∼20-fold higher than empty nanoemulsions and reduce leucocyte infiltration and showed an anti-inflammatory activity | [124] |
| Acitretin and aloe-emodin Aloe-emodin isolated from plant of genus Aloe (Polymeric nanoparticle) | Chitin | Centrifugation | Revealed greater skin permeation and drug retention in deeper layer of skin with and improve compatibility | [125] |
| Tea tree oil isolated from leaves of Melaleuca alternifolia (Micro-emulsion) | Tween 80 | Emulsification | Showed superior drug solubilization and bioavailability for topical applications of anti-psoriatic active moieties and bio-actives | [126] |
| Curcumin (Nano-hydrogel) | Curcumin, choline-calix[4]arene amphiphile | Supra-molecular nano-hydrogel | Exhibited no significant toxicity and showed effective anti-psoriatic activity in an IMQ-induced psoriasis mouse via decreased pro-inflammation | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sharma, N.; Behl, T.; Sarkar, B.C.; Saha, H.R.; Garg, K.; Singh, S.K.; Arora, S.; Amran, M.S.; Abdellatif, A.A.H.; et al. Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management. Pharmaceutics 2021, 13, 1978. https://doi.org/10.3390/pharmaceutics13111978
Singh S, Sharma N, Behl T, Sarkar BC, Saha HR, Garg K, Singh SK, Arora S, Amran MS, Abdellatif AAH, et al. Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management. Pharmaceutics. 2021; 13(11):1978. https://doi.org/10.3390/pharmaceutics13111978
Chicago/Turabian StyleSingh, Sukhbir, Neelam Sharma, Tapan Behl, Bidhan Chandra Sarkar, Hasi Rani Saha, Kanika Garg, Supriya Kamari Singh, Sandeep Arora, Md. Shah Amran, Ahmed A. H. Abdellatif, and et al. 2021. "Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management" Pharmaceutics 13, no. 11: 1978. https://doi.org/10.3390/pharmaceutics13111978
APA StyleSingh, S., Sharma, N., Behl, T., Sarkar, B. C., Saha, H. R., Garg, K., Singh, S. K., Arora, S., Amran, M. S., Abdellatif, A. A. H., Bilgrami, A. L., Ashraf, G. M., & Rahman, M. S. (2021). Promising Strategies of Colloidal Drug Delivery-Based Approaches in Psoriasis Management. Pharmaceutics, 13(11), 1978. https://doi.org/10.3390/pharmaceutics13111978









