Nanoformulations of α-Mangostin for Cancer Drug Delivery System
Abstract
:1. Introduction
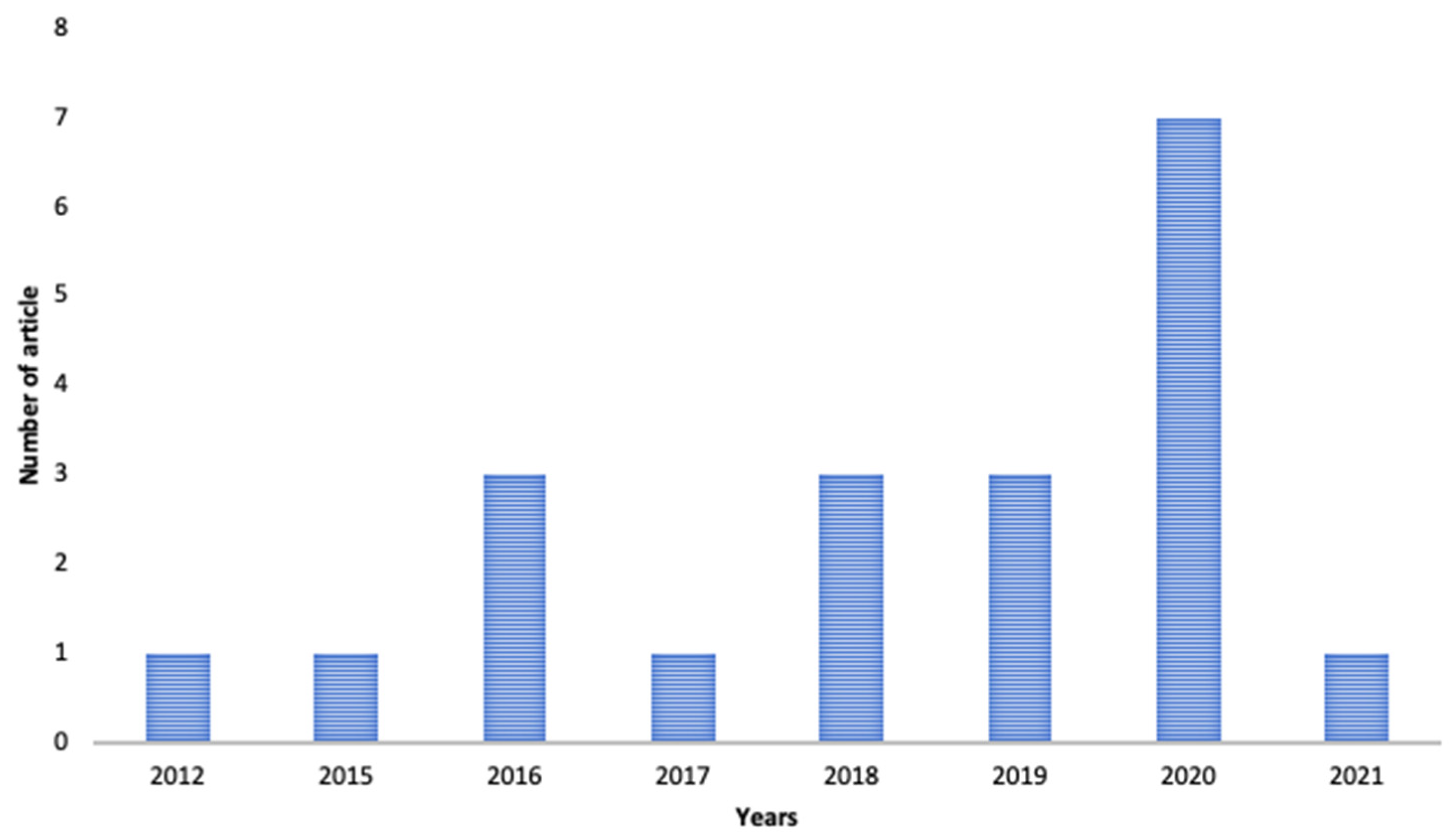
2. Methodology
3. α-Mangostin
4. α-Mangostin Nanoformulation
4.1. Nanofibers
4.2. Solid Lipid Nanoparticle
4.3. Nanostructured Lipid Carriers
4.4. Polymeric Nanoparticle
4.4.1. PLGA Nanoparticle
4.4.2. PEG-PLA Nanoparticles
4.4.3. Chitosan-Alginate Nanoparticles
4.4.4. Chitosan-Kappa Carrageenan Nanoparticles
4.5. Cyclodextrin Nanoparticles
4.6. Nanomicelles
4.7. Liposomal Nanoparticles
4.8. Gold Nanoparticles
5. The Perspective of the Authors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surfaces B Biointerfaces 2019, 181, 705–713. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2018, 77, 1548–1552. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M. Chemotherapy: Updates and New Perspectives. Oncologist 2011, 16, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Muralidhar Reddy, P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine 2017, 7, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- Nirmala, M.J.; Samundeeswari, A.; Sankar, P.D. Natural plant resources in anti-cancer therapy—A review. Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing delivery of plant based anti-cancer drugs. Front. Pharmacol. 2018, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mary Lazer, L.; Sadhasivam, B.; Palaniyandi, K.; Muthuswamy, T.; Ramachandran, I.; Balakrishnan, A.; Pathak, S.; Narayan, S.; Ramalingam, S. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 2018, 107, 1988–1998. [Google Scholar] [CrossRef]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-loaded hyaluronan-coated PLGA nanoparticles: Physico-chemical properties and in vitro anticancer features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Bahadori, M.B.; Vandghanooni, S.; Eskandani, M.; Nakhlband, A.; Eskandani, M. Preparation, characterization and anti-proliferative effects of sclareol-loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J. Drug Deliv. Sci. Technol. 2018, 45, 272–280. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef] [Green Version]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.H.; Zhang, K.J.; Gu, Q.L.; Bi, X.L.; Wang, J.X. Pharmacology of mangostins and their derivatives: A comprehensive review. Chin. J. Nat. Med. 2017, 15, 81–93. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from Mangosteen Extracts as Natural Chemopreventive Agents: Potential Anticancer Drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Watanapokasin, R.; Jarinthanan, F.; Nakamura, Y.; Sawasjirakij, N.; Jaratrungtawee, A.; Suksamrarn, S. Effects of α-mangostin on apoptosis induction of human colon cancer. World J. Gastroenterol. 2011, 17, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Kang, K.; Jho, E.H.; Chin, Y.W.; Kim, J.; Nho, C.W. α- and γ-Mangostin inhibit the proliferation of colon cancer cells via β-catenin gene regulation in Wnt/cGMP signalling. Food Chem. 2011, 129, 1559–1566. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, G.; Shen, Y. The naturally occurring xanthone α-mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J. Biol. Sci. 2018, 25, 1090–1095. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, W.; Shrivastava, A.; Srivastava, R.K.; Shankar, S. Inhibition of pancreatic cancer stem cell characteristics by α-Mangostin: Molecular mechanisms involving Sonic hedgehog and Nanog. J. Cell. Mol. Med. 2019, 23, 2719–2730. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer 2014, 13, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Sanderson, B.J.S.; Zhang, W. Significant anti-invasive activities of α-mangostin from the mangosteen pericarp on two human skin cancer cell lines. Anticancer Res. 2012, 32, 3805–3816. [Google Scholar] [PubMed]
- Chen, J.J.; Long, Z.J.; Xu, D.F.; Xiao, R.Z.; Liu, L.L.; Xu, Z.F.; Qiu, S.X.; Lin, D.J.; Liu, Q. Inhibition of autophagy augments the anticancer activity of α-mangostin in chronic myeloid leukemia cells. Leuk. Lymphoma 2014, 55, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Sodalee, K.; Sapsuphan, P.; Wongsirikul, R.; Puttipipatkhachorn, S. Preparation and evaluation of alpha-mangostin solid self-emulsifying drug delivery system. Asian J. Pharm. Sci. 2016, 11, 225–226. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice. Sci. Rep. 2016, 6, 32743. [Google Scholar] [CrossRef]
- Li, L.; Brunner, I.; Han, A.R.; Hamburger, M.; Kinghorn, A.D.; Frye, R.; Butterweck, V. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol. Nutr. Food Res. 2011, 55, 67–74. [Google Scholar] [CrossRef]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug 2013, 65, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Nanoparticle therapeutics: Technologies and methods for overcoming cancer. Eur. J. Pharm. Biopharm. 2015, 97, 140–151. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; nJoni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle drug delivery systems for α-mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Muchtaridi, M.; Wijaya, C.A. Anticancer potential of α-mangostin. Asian J. Pharm. Clin. Res. 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Ahmad, M.; Yamin, B.M.; Mat Lazim, A. A study on dispersion and characterisation of α-mangostin loaded pH sensitive microgel systems. Chem. Cent. J. 2013, 7, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Wang, X.; Lu, X.; Wang, H.; Brodelius, P.E. α-Mangostin extraction from the native mangosteen (Garcinia mangostana L) and the binding mechanisms of α-mangostin to HSAorTRF. PLoS ONE 2016, 11, e0161566. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.A.; Ismail, Z.; Abu-Salah, K.M.; Majid, A.M.S.A. Solid Dispersions of α-Mangostin Improve Its Aqueous Solubility Through Self-Assembly of Nanomicelles. J. Pharm. Sci. 2012, 101, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Bumrung, J.; Chanchao, C.; Intasanta, V.; Palaga, T.; Wanichwecharungruang, S. Water-dispersible unadulterated α -mangostin particles for biomedical applications. R. Soc. Open Sci. 2020, 7, 200543. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, S.Y.; Kim, Y.J.; Kim, Y.M.; Chin, Y.W. Absorption, tissue distribution, tissue metabolism and safety of α-mangostin in mangosteen extract using mouse models. Food Chem. Toxicol. 2014, 66, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ma, J.; Lei, J.; Duan, W.; Sheng, L.; Chen, X.; Hu, A.; Wang, Z.; Wu, Z.; Wu, E.; et al. α-mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. Biomed. Res. Int. 2014, 2014, 546353. [Google Scholar] [CrossRef]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. A-Mangostin, a Xanthone From Mangosteen Fruit, Promotes Cell Cycle Arrest in Prostate Cancer and Decreases Xenograft Tumor Growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.N.; Jang, H.Y.; Kim, H.J.; Shin, S.A.; Choo, G.S.; Park, Y.S.; Kim, S.K.; Jung, J.Y. Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int. J. Mol. Med. 2016, 37, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Ying, T.H.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Chou, R.H.; Hsieh, Y.H. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef]
- Sakagami, Y.; Thevanesam, V.; Programme, N.P.; Lanka, S.; Lanka, S.; Sakagami, Y. Natural Product Research: Formerly Natural Product Letters Antibacterial activity of xanthones from Garcinia mangostana (L.) and their structure—Activity relationship studies. Nat. Prod. Res. 2013, 27, 938–941. [Google Scholar]
- Sivaranjani, M.; Leskinen, K.; Aravindraja, C.; Saavalainen, P.; Pandian, S.K.; Skurnik, M.; Ravi, A.V. Deciphering the antibacterial mode of action of alpha-mangostin on Staphylococcus epidermidis RP62A through an integrated transcriptomic and proteomic approach. Front. Microbiol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Hepatoprotective effect of α-mangostin against lipopolysaccharide/D-galactosamine-induced acute liver failure in mice. Biomed. Pharmacother. 2018, 106, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Buelna-Chontal, M.; Correa, F.; Hernández-Reséndiz, S.; Zazueta, C.; Pedraza-Chaverri, J. Protective effect of α-mangostin on cardiac reperfusion damage by attenuation of oxidative stress. J. Med. Food 2011, 14, 1370–1374. [Google Scholar] [CrossRef]
- Upegui, Y.; Robledo, S.M.; Gil Romero, J.F.; Quiñones, W.; Archbold, R.; Torres, F.; Escobar, G.; Nariño, B.; Echeverri, F. In vivo Antimalarial Activity of α-Mangostin and the New Xanthone δ-Mangostin. Phyther. Res. 2015, 29, 1195–1201. [Google Scholar] [CrossRef]
- Taher, M.; Mohamed Amiroudine, M.Z.A.; Tengku Zakaria, T.M.F.S.; Susanti, D.; Ichwan, S.J.A.; Kaderi, M.A.; Ahmed, Q.U.; Zakaria, Z.A. α -mangostin improves glucose uptake and inhibits adipocytes differentiation in 3T3-L1 cells via PPAR γ, GLUT4, and leptin expressions. Evid-Based Complement. Altern. Med. 2015, 2015, 740238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xia, Z.; Xu, J.R.; Wang, Y.X.; Hou, L.N.; Qiu, Y.; Chen, H.Z. α-Mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 2012, 62, 871–881. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281650, Alpha-Mangostin. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin (accessed on 10 November 2021).
- Ravindran, R. Nano Technology in Cancer Diagnosis and Treatment: An Overview. Oral Maxillofac. Pathol. J. 2011, 2, 101–106. [Google Scholar]
- Karra, N.; Benita, S. The Ligand Nanoparticle Conjugation Approach for Targeted Cancer Therapy. Curr. Drug Metab. 2011, 13, 22–41. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-formulations of drugs: Recent developments, impact and challenges. Biochimie 2016, 128, 99–112. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. In Computational Toxicology: Methods and Protocols; Nicolotti, O., Ed.; Springer: New York, NY, USA, 2018; pp. 347–365. ISBN 978-1-4939-7899-1. [Google Scholar]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, L.M.; Dawidczyk, C.M.; Searson, P.C. Quantitative Evaluation of the Enhanced Permeability and Retention (EPR) Effect. Cancer Nanotechnol. 2017, 1530, 41–195. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Khobragade, D.S.; Trambak Patil, A.; Shamrao Khobragade, D.; Annaji Chafle, S.; Prasadrao Ujjainkar, A.; Niranjanrao Umathe, S.; Laxminarayan Lakhotia, C. Development and evaluation of a hot-melt coating technique for enteric coating. Brazilian J. Pharm. Sci. 2012, 48, 69–77. [Google Scholar]
- Taokaew, S.; Chiaoprakobkij, N.; Siripong, P.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Multifunctional cellulosic nanofiber film with enhanced antimicrobial and anticancer properties by incorporation of ethanolic extract of Garcinia mangostana peel. Mater. Sci. Eng. C 2021, 120, 111783. [Google Scholar] [CrossRef]
- Bonafè, F.; Pazzini, C.; Marchionni, S.; Guarnieri, C.; Muscari, C. Complete disaggregation of MCF-7-derived breast tumour spheroids with very low concentrations of α-mangostin loaded in CD44 thioaptamer-tagged nanoparticles. Int. J. Med. Sci. 2019, 16, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surfaces B Biointerfaces 2017, 149, 301–311. [Google Scholar] [CrossRef]
- Chandra Boinpelly, V.; Verma, R.K.; Srivastav, S.; Srivastava, R.K.; Shankar, S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J. Cell. Mol. Med. 2020, 24, 11343–11354. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.T.H.; Lee, J.H.; Takahashi, R.; Nguyen, P.T.M.; Nguyen, V.A.T.; Pham, H.T.T.; Fujii, S.; Sakurai, K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: Potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52, 457–466. [Google Scholar] [CrossRef]
- Doan, V.T.H.; Takano, S.; Doan, N.A.T.; Nguyen, P.T.M.; Nguyen, V.A.T.; Pham, H.T.T.; Nakazawa, K.; Fujii, S.; Sakurai, K. Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin. Polym. J. 2020, 53, 481–492. [Google Scholar] [CrossRef]
- Nguyen Thi, M.P.; Tran Dai, L.; Ta Thu, M.; Nguyen Trung, H. Cytotoxicity of α-mangostin encapsulated polymeric nanoparticles against lung cancer cells. Acad. J. Biol. 2018, 40, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of alpha mangostin-loaded chitosan/alginate controlled-release nanoparticles using genipin as crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Wathoni, N.; Meylina, L.; Rusdin, A.; Abdelwahab Mohammed, A.F.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; Lesmana, R.; et al. The potential cytotoxic activity enhancement of α-mangostin in chitosan-kappa carrageenan-loaded nanoparticle against mcf-7 cell line. Polymers 2021, 13, 1681. [Google Scholar] [CrossRef]
- Yang, S.; Gao, X.; He, Y.; Hu, Y.; Xu, B.; Cheng, Z.; Xiang, M.; Xie, Y. Applying an innovative biodegradable self-assembly nanomicelles to deliver α-mangostin for improving anti-melanoma activity. Cell Death Dis. 2019, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Liu, J.; Faried, A.; Richard, S.A.; Gao, X. Novel chemically synthesized, alpha-mangostin-loaded nano-particles, enhanced cell death through multiple pathways against malignant glioma. J. Biomed. Nanotechnol. 2018, 14, 1866–1882. [Google Scholar] [CrossRef]
- Benjakul, R.; Kongkaneramit, L.; Sarisuta, N.; Moongkarndi, P.; Müller-Goymann, C.C. Cytotoxic effect and mechanism inducing cell death of α-mangostin liposomes in various human carcinoma and normal cells. Anticancer. Drugs 2015, 26, 824–834. [Google Scholar] [CrossRef]
- Trang Phan, T.K.; Tran, T.Q.; Nguyen Pham, D.T.; Nguyen, D.T. Characterization, Release Pattern, and Cytotoxicity of Liposomes Loaded With α-Mangostin Isolated from Pericarp of Mangosteen (Garcinia mangostana L.). Nat. Prod. Commun. 2020, 15, 1934578X20974559. [Google Scholar] [CrossRef]
- Qiu, S.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Pouget, C.; Micallef, L.; Sothea-Ouk, T.; Leger, D.Y.; Liagre, B.; Chaleix, V.; et al. Delivery of tanshinone IIA and α-mangostin from gold/PEI/cyclodextrin nanoparticle platform designed for prostate cancer chemotherapy. Bioorg. Med. Chem. Lett. 2016, 26, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Adam Khan, P.; Sasikanth, K.; Nama, S.; Suresh, P.; Brahmaiah, B. Nanofibers—A New Trend in Nano Drug Delivery Systems. Int. J. Pharm. Res. Anal. 2013, 3, 47–55. [Google Scholar]
- Poláková, L.; Širc, J.; Hobzová, R.; Cocârță, A.I.; Heřmánková, E. Electrospun nanofibers for local anticancer therapy: Review of in vivo activity. Int. J. Pharm. 2019, 558, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Serio, F.; Kale, N.R.; D’Amone, E.; Gigli, G.; Del Mercato, L.L. Electrospun nanofibers in cancer research: From engineering of: From vitro 3D cancer models to therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef]
- Sasikala, A.R.K.; Unnithan, A.R.; Park, C.H.; Kim, C.S. Nanofiber-Based Anticancer Drug Delivery Platform; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128149447. [Google Scholar]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; Supriyanto, E.; Razak, S.I.A. An insight on electrospun-nanofibers-inspired modern drug delivery system in the treatment of deadly cancers. RSC Adv. 2015, 5, 57984–58004. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Prabhu, P. Nanofibers for Medical Diagnosis and Therapy; Springer: Berlin, Germany, 2019; ISBN 9783319536552. [Google Scholar]
- Jastrzebska, K.; Kucharczyk, K.; Florczak, A.; Dondajewska, E.; Mackiewicz, A.; Dams-Kozlowska, H. Silk as an innovative biomaterial for cancer therapy. Reports Pract. Oncol. Radiother. 2015, 20, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Kumar, V. Solid Lipid Nanoparticle of Alpha-Mangostin Exerts Diethylnitrosamine-Induced Hepatocellular Carcinoma via Alteration of PI3K/Akt Pathway. Gut Liver. 2019, 13, 209. [Google Scholar]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured Lipid Carriers: A Novel Platform for Chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26. [Google Scholar] [CrossRef]
- Zieli’nska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkates, S.N.; Durazzo, A.; Lucarini, M.; Ede, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Madkour, L.H. Polymer nanoparticle drug-nucleic acid combinations. In Nucleic Acids as Gene Anticancer Drug Delivery Therapy; Academic Press: London, UK, 2019; pp. 241–255. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Peltonen, L.; Singhal, M.; Hirvonen, J. Principles of Nanosized Drug Delivery Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780081029855. [Google Scholar]
- Li, B.; Li, Q.; Mo, J.; Dai, H. Drug-loaded polymeric nanoparticles for cancer stem cell targeting. Front. Pharmacol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Suriya Prabha, A.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Senthil Kumaran, S. Recent Advances in the Study of Toxicity of Polymer-Based Nanomaterials. Nanotoxicity 2020, 2020, 143–165. [Google Scholar]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Katuwavila, N.P.; Perera, A.D.L.C.; Samarakoon, S.R.; Soysa, P.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Chitosan-Alginate Nanoparticle System Efficiently Delivers Doxorubicin to MCF-7 Cells. J. Nanomater. 2016, 2016, 3178904. [Google Scholar] [CrossRef] [Green Version]
- Samprasit, W.; Opanasopit, P. Chitosan-Based Nanoparticles for Controlled-Release Delivery of α–Mangostin. Int. J. Pharma Med. Biol. Sci. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Anila, M.M.; Franklin, S. Synthesis characterization and biological evaluation of alginate nanoparticle for the targeted delivery of curcumin Synthesis characterization and biological evaluation of alginate nanoparticle for the targeted delivery of curcumin. Mater. Sci. Eng. C 2019, 78, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, D. Cyclodextrins and Their Inclusion Complexes. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; Bilensoy, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–18. [Google Scholar] [CrossRef]
- Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Comprehensive Supramolecular Chemistry II: Cyclodextrin. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 9, pp. 269–316. ISBN 9780128031988. [Google Scholar]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirmahani, N.; Mahmoodi, N.O.; Mohammadi Galangash, M.; Ghavidast, A. Advances in nanomicelles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Mohamed, S.; Parayath, N.N.; Taurin, S.; Greish, K. Polymeric nano-micelles: Versatile platform for targeted delivery in cancer. Ther. Deliv. 2014, 5, 1101–1121. [Google Scholar] [CrossRef]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Trinh, H.M.; Joseph, M.; Cholkar, K.; Mitra, R.; Mitra, A.K. Nanomicelles in Diagnosis and Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 45–58. ISBN 9780323429979. [Google Scholar]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Pawar, H.R.; Bhosale, S.S.; Derle, N.D. Use of liposomes in cancer therapy: A review. Int. J. Pharm. Sci. Res. 2012, 3, 3585–3590. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 329, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.R.; Thomas, R.T. Gold Nanoparticles in Cancer Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780323353038. [Google Scholar]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Austin, L.A.; MacKey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [Green Version]

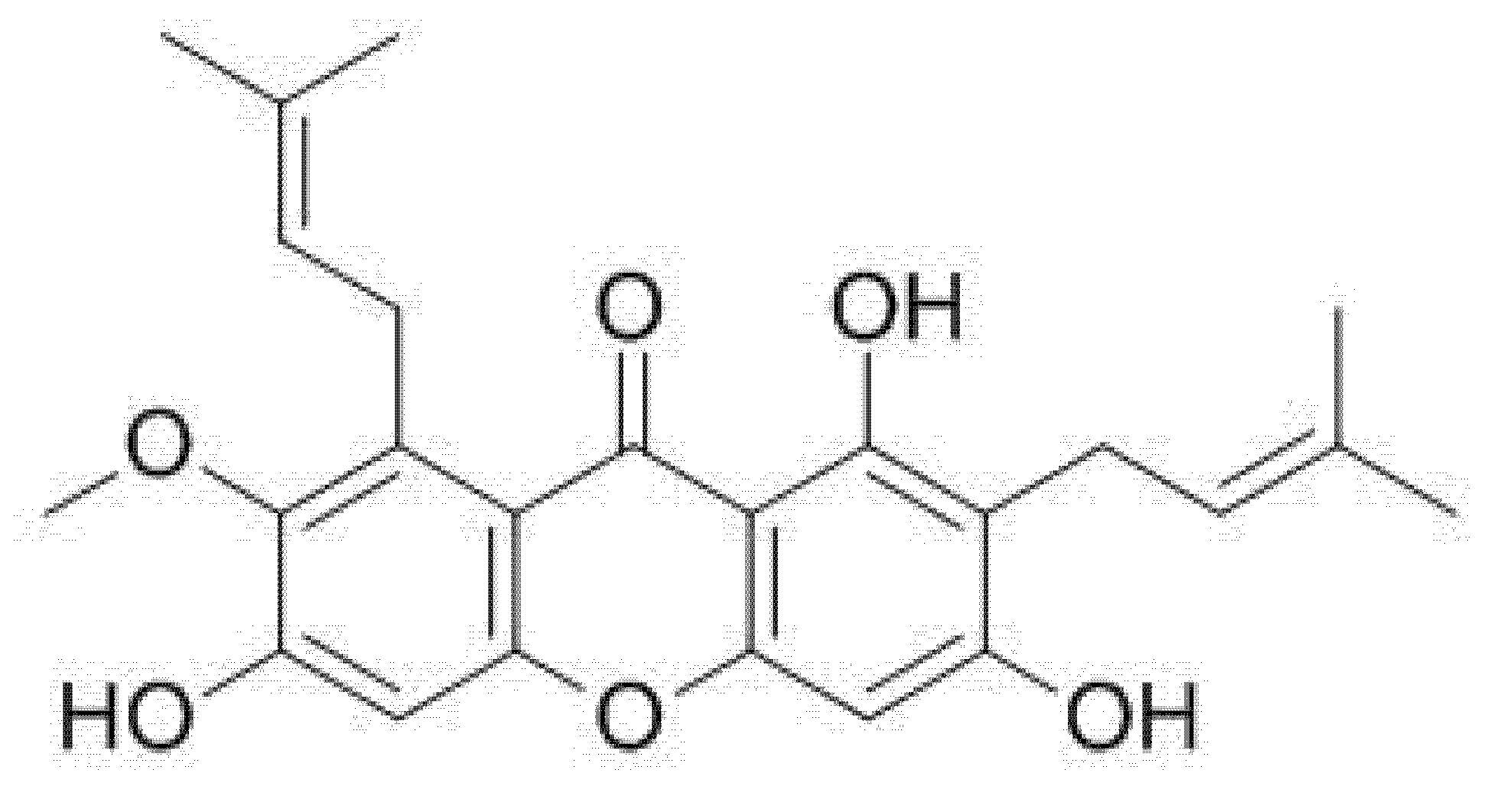


| Property | Description |
|---|---|
| Molecular formula | C24H26O6 |
| IUPAC name | 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9H-xanthen-9-one |
| Molecular weight | 410.5 |
| Color/Form | Faint yellow to yellow powder |
| Melting point | 180–181 °C |
| Solubility | Soluble in methanol, in water (2.03 × 10−4 mg/L at 25 °C) |
| LogP | log Kow = 7.71 |
| Stability | Stable under normal temperatures and pressures |
| Dissociation constants | pKa1 = 3.68 (primary carbonyl); pKa2 = 7.69 (secondary carbonyl); pKa3 = 9.06 (tertiary carbonyl) |
| Carrier | Cell Line | Outcome | Ref. |
|---|---|---|---|
| Silk fibroin N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and polyethylenimine (crosslinker) | Caco-2 MCF-7 |
| [3] |
| Acetobacter xylinum | B16F10; MCF-7; hGF; HaCaT |
| [70] |
| PLGA, soybean lecithin, DSPE–PEG2000–COOH Thioaptamer (ligand) | MCF-7 |
| [71] |
| Miglyol 812, cetyl paomitate, montanov 82, and oleoyl chitosan (coating agent) | Caco-2 Hela |
| [72] |
| PLGA | Pancreatic cancer cell line (AsPC-1, PANC-1, and Mia-Paca-2) Cancer stem cells (CSCs) |
| [35] |
| PLGA | HCT116 and HT29 Normal epithelial cells (CRL-1831) |
| [73] |
| α, β dan γ cyclodextrin (CD) Epichlorohydrid (ECH) as linker | CT26WT |
| [74,75] |
| β-cyclodextrin | A549 |
| [76] |
| Chitosan and alginate Genipin as crosslinker | HT-29 |
| [77] |
| Poly-(ethylene glycol)–poly(l-lactide) (PEG–PLA) CREKA peptide (ligand) | PANC-1 NIH3T3, PANC-1-Luc2 |
| [78] |
| Chitosan and Kappa Carrageenan | MCF-7 |
| [79] |
| Polyvinylpyrrolidone (PVP) | HCT 116 |
| [43] |
| Monomethoxy poly (ethylene glycol)-polycaprolactones (MPEG-PCL) | A375 and B16 non-tumor cell lines (LO2, Vero, and HEK293T cells) |
| [80] |
| Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) | U87 |
| [81] |
| Phosphatidylcholine and cholesterol | Calu-3; HT-29; MCF-7; Caco-2; HaCaT; HDF |
| [82] |
| Dioleoylphosphatidylcholine and cholesterol | Hep-G2 |
| [83] |
| Gold citrate | PC-3 DU145 |
| [84] |
| Formulation | Type of Tumor | Reduction of Tumor Volume | Ref. |
|---|---|---|---|
| PLGA | Pancreatic | More than 60% of tumor reduction with 20 mg/kg dosage | [35] |
| Cyclodextrin nanoparticle | Colon | Approximately 56% of tumor reduction with 10 mg/kg dosage | [75] |
| PEG-PLA nanomicelles coated with CREKA peptide | Pancreatic | More than 70% of tumor reduction with 20 mg/kg dosage | [78] |
| MPEG-PCL nanomicelles | Melanoma | Almost 50% of tumor growth reduction with 50 mg/kg dosage | [80] |
| MPEG-PLA nanomicelles | Glioma | Approximately 65% tumor reduction with 50 mg/kg dosage | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics 2021, 13, 1993. https://doi.org/10.3390/pharmaceutics13121993
Meylina L, Muchtaridi M, Joni IM, Mohammed AFA, Wathoni N. Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics. 2021; 13(12):1993. https://doi.org/10.3390/pharmaceutics13121993
Chicago/Turabian StyleMeylina, Lisna, Muchtaridi Muchtaridi, I Made Joni, Ahmed Fouad Abdelwahab Mohammed, and Nasrul Wathoni. 2021. "Nanoformulations of α-Mangostin for Cancer Drug Delivery System" Pharmaceutics 13, no. 12: 1993. https://doi.org/10.3390/pharmaceutics13121993
APA StyleMeylina, L., Muchtaridi, M., Joni, I. M., Mohammed, A. F. A., & Wathoni, N. (2021). Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics, 13(12), 1993. https://doi.org/10.3390/pharmaceutics13121993








