Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HPLC Method of Genistein
2.2.2. Phase Solubility Study
2.2.3. Physical Mixture
2.2.4. Binary and Ternary Inclusion Complex
2.2.5. Saturation Solubility Study
2.2.6. Dissolution Study
2.2.7. Drug Content
2.2.8. Fourier Transform Infra-Red (FTIR)
2.2.9. Differential Scanning Calorimetry (DSC)
2.2.10. X-ray Diffraction Analysis
2.2.11. Nuclear Magnetic Resonance (NMR)
2.2.12. Scanning Electron Microscope (SEM)
2.2.13. Antioxidant Activity
2.2.14. Cytotoxicity Study
2.2.15. Antimicrobial Activity
2.2.16. Statistical Analysis
3. Result and Discussion
3.1. Phase Solubility Study
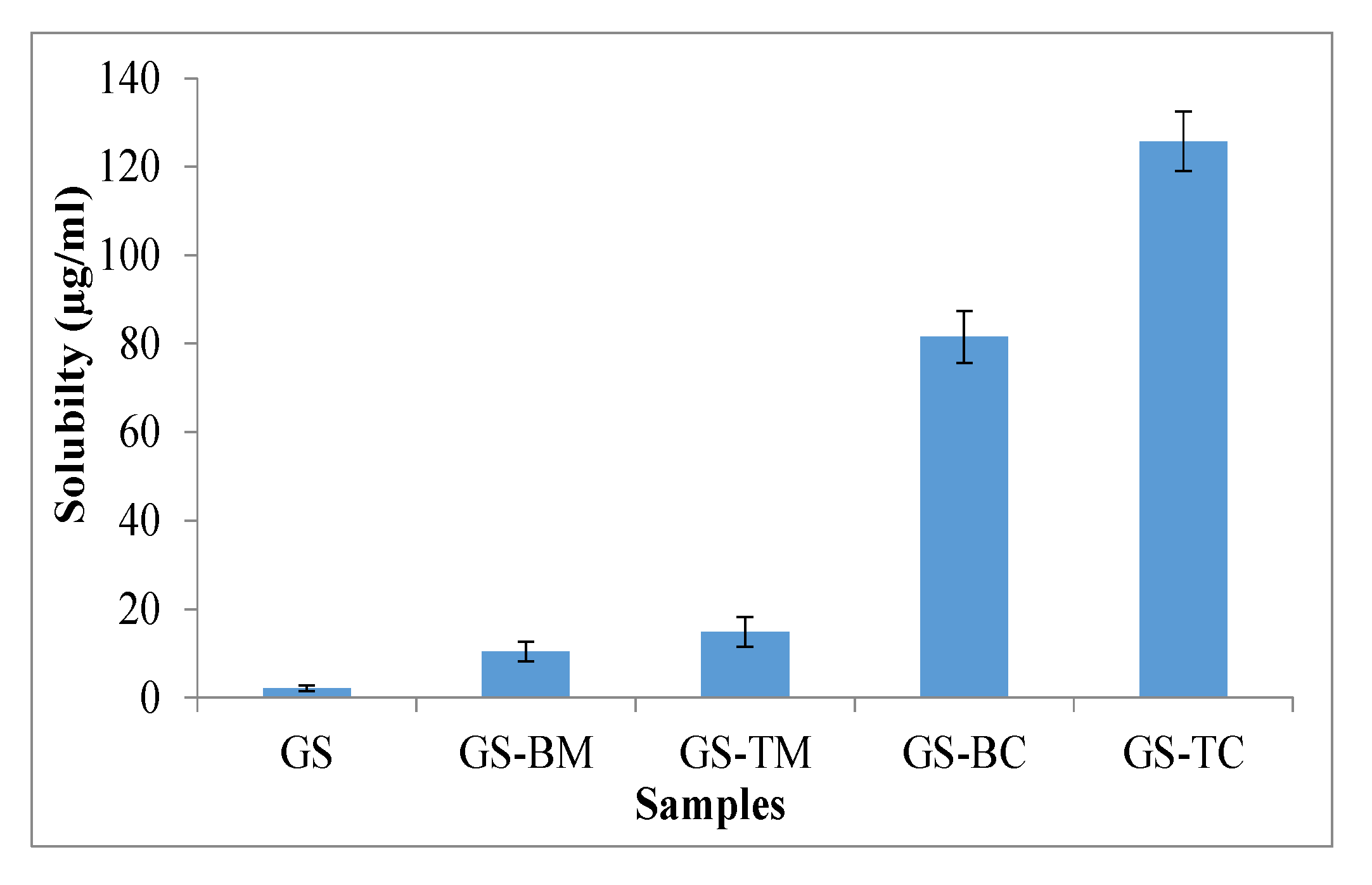
3.2. Saturation Solubility Study
3.3. Dissolution Study
3.4. Drug Content
3.5. Fourier Transform Infra-Red
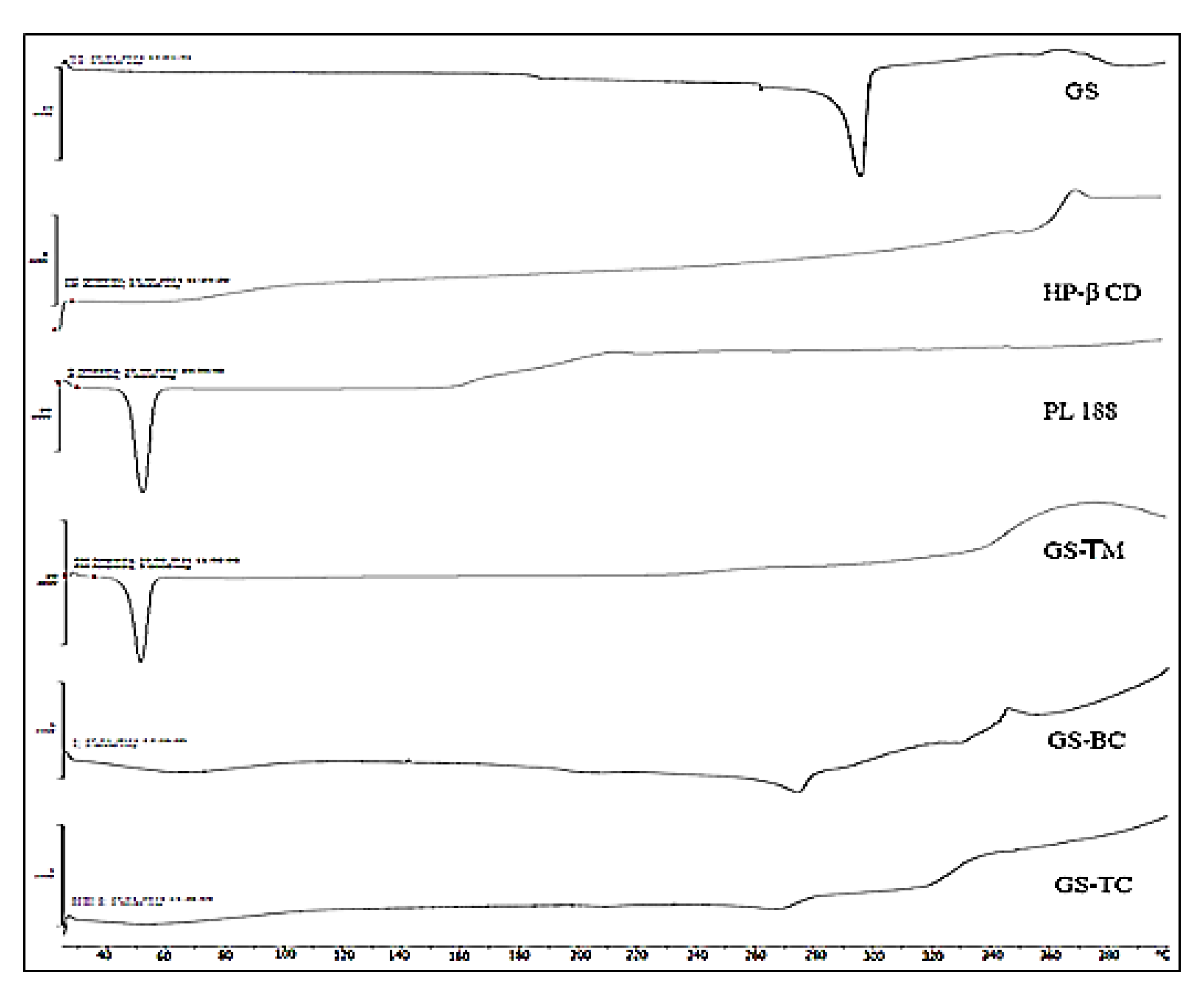
3.6. Differential Scanning Calorimetry
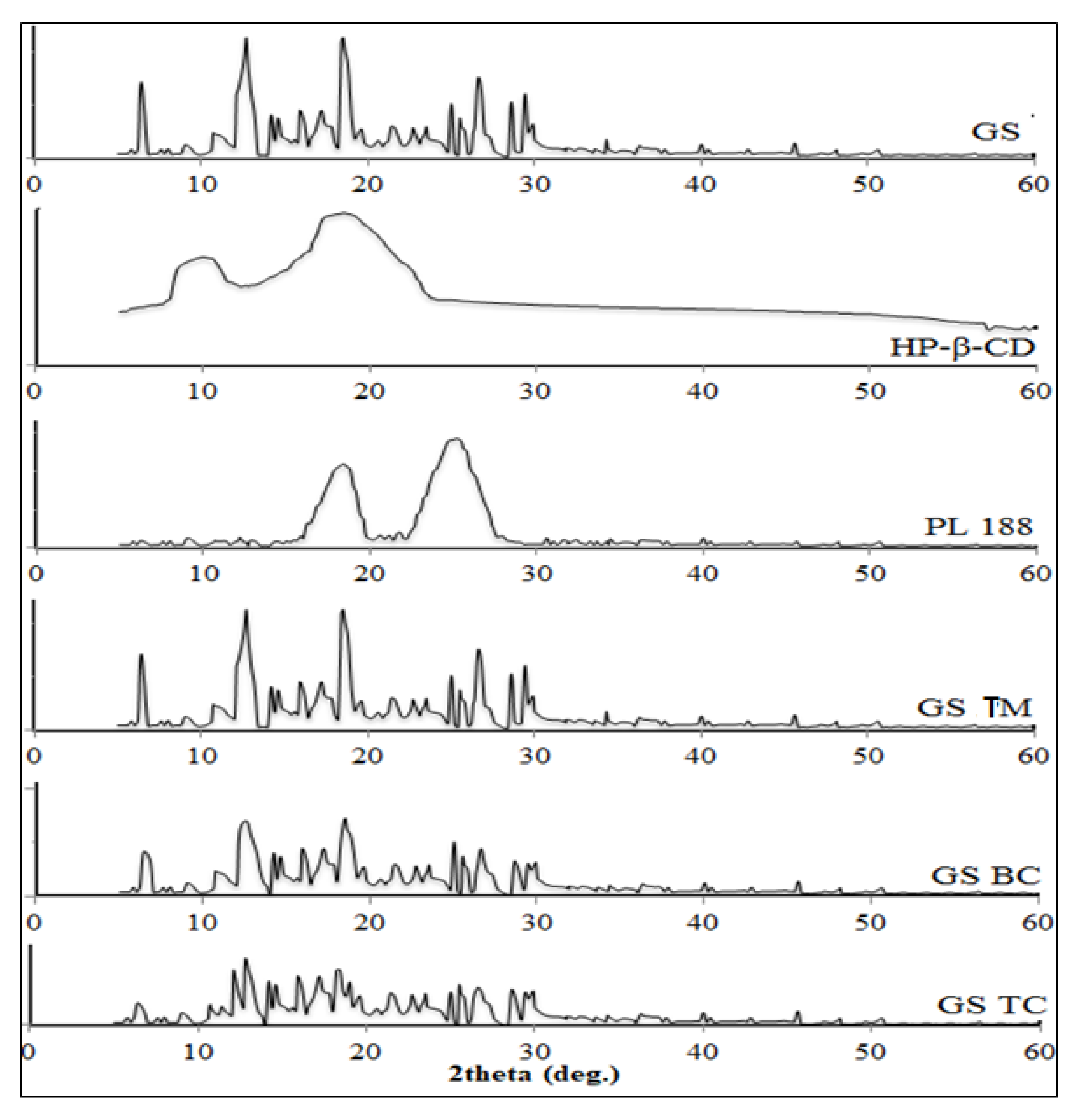
3.7. X-ray Diffraction Analysis
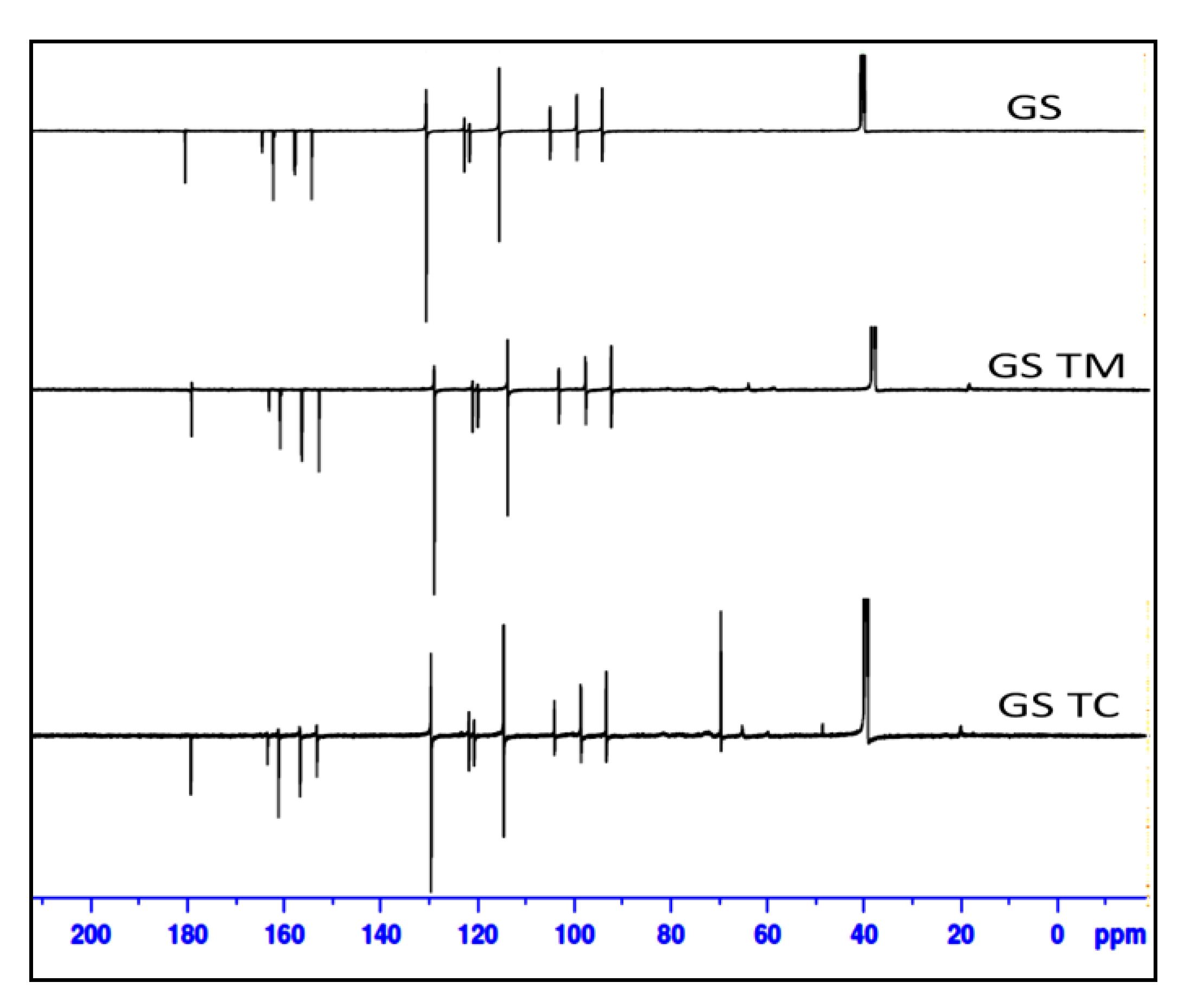
3.8. Nuclear Magnetic Resonance
3.9. Scanning Electron Microscope (SEM)
3.10. Antioxidant Scavenging Activity
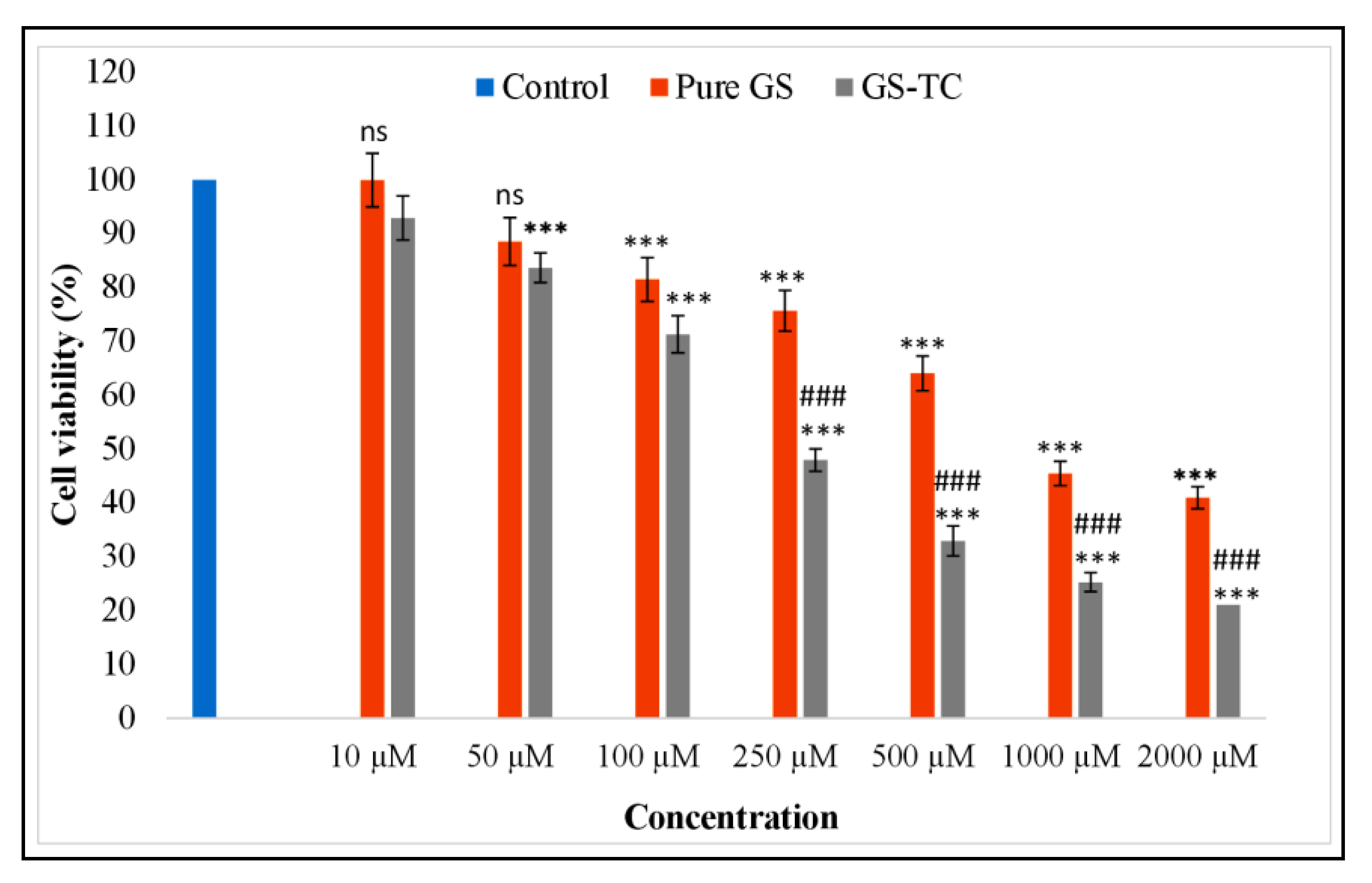
3.11. Cytotoxicity Study
3.12. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amirsaadat, S.; Jafari-Gharabaghlou, D.; Alijani, S.; Mousazadeh, H.; Dadashpour, M.; Zarghami, N. Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102107. [Google Scholar] [CrossRef]
- Sarhadi, S.; Sadeghi, S.; Nikmanesh, F.; Soltanahmadi, Y.P.; Shahabi, A.; Aval, S.F.; Zarghami, N. A systems biology approach provides deeper insights into differentially expressed genes in taxane-anthracycline chemoresistant and nonresistant breast cancers. Asian Pac. J. Cancer Prev. 2017, 18, 2629. [Google Scholar] [PubMed]
- Sharma, D.; Soni, M.; Kumar, S.; Gupta, G.D. Solubility enhancement—eminent role in poorly soluble drugs. Res. J. Pharm. Technol. 2009, 2, 220–224. [Google Scholar]
- Kim, N.A.; Oh, H.K.; Lee, J.C.; Choi, Y.H.; Jeong, S.H. Comparison of solubility enhancement by solid dispersion and micronized butein and its correlation with in vivo study. J. Pharm. Investig. 2021, 51, 53–60. [Google Scholar] [CrossRef]
- Giri, B.R.; Lee, J.; Lim, D.Y.; Kim, D.W. Docetaxel/dimethyl-β-cyclodextrin inclusion complexes: Preparation, in vitro evaluation and physicochemical characterization. Drug Dev. Ind. Pharm. 2021, 47, 319–328. [Google Scholar] [CrossRef]
- Jafar, M.; Khalid, M.S.; Aldossari, M.F.E.; Amir, M.; Alshaer, F.I.; Adrees, F.A.A.; Gilani, S.J.; Alshehri, S.; Hassan, M.Z.; Imam, S.S. Formulation of Curcumin-β-cyclodextrin-polyvinylpyrrolidone supramolecular inclusion complex: Experimental, molecular docking, and preclinical anti-inflammatory assessment. Drug Dev. Ind. Pharm. 2020, 46, 1524–1534. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Buchanan, C.M.; Klein, S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur. J. Pharm. Biopharm. 2013, 83, 378–387. [Google Scholar] [CrossRef]
- Cheng, Q.; Qin, W.; Yu, Y.; Li, G.; Wu, J.; Zhuo, L. Preparation and Characterization of PEG-PLA Genistein Micelles Preparation and Characterization of PEG-PLA Genistein Micelles using modified emulsion-evaporation method. J. Nanomater. 2020, 2020, 3278098. [Google Scholar] [CrossRef]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.H.; Ko, S. Curcumin and Genistein Coloaded Nanostructured Lipid Carriers: In Vitro Digestion and Antiprostate Cancer Activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef]
- Xiao, Y.; Ho, C.T.; Chen, Y.; Wang, Y.; Wei, Z.; Dong, M.; Huang, Q. Synthesis Characterization, and Evaluation of Genistein-Loaded Zein/Carboxymethyl Chitosan Nanoparticles with Improved Water Dispersibility, Enhanced Antioxidant Activity, and Controlled Release Property. Foods 2020, 9, 1604. [Google Scholar] [CrossRef]
- Wu, B.; Liang, Y.; Tan, Y.; Xie, C.; Shen, J.; Zhang, M.; Liu, X.; Yang, L.; Zhang, F.; Liu, L.; et al. Genistein-loaded nanoparticles of star-shaped diblock copolymer mannitol-core PLGA–TPGS for the treatment of liver cancer. Mater. Sci. Eng. C 2016, 59, 792–800. [Google Scholar] [CrossRef]
- Soleimanpour, M.; Tamaddon, A.M.; Kadivar, M.; Abolmaali, S.S.; Shekarchizadeh, H. Fabrication of nanostructured mesoporous starch encapsulating soy-derived phytoestrogen (genistein) by well-tuned solvent exchange method. Int. J. Biol. Macromol. 2020, 159, 1031–1047. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Su, Y.; Zhang, H.; Ding, L.; Yan, X.; Zhao, D.; Shao, N.; Ye, X.; Cheng, Y. Inclusion complexes of isoflavones with two commercially available dendrimers: Solubility, stability, structures, release behaviors, cytotoxicity, and anti-oxidant activities. Int. J. Pharm. 2011, 421, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Orlova, Y.; Kovalev, M.; Ungar, Y.; Shimoni, E. Structural and functional properties of amylose complexes with genistein. J. Agric. Food Chem. 2008, 56, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; McClements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Ashwaq, A.A.S.; Rasedee, A.; Abdul, A.B.; Taufiq-Yap, Y.H.; Al-Qubaisi, M.S.; Eid, E.E. Characterization drug release profile and cytotoxicity of Dentatin-Hydroxypropyl-β-Cyclodextrin complex. J. Incl. Phenom. Macrocycl. Chem. 2017, 87, 167–178. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, M.; Lu, J.; Zhu, X. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 17–24. [Google Scholar] [CrossRef]
- Shah, M.; Pore, Y.; Dhawale, S.; Burade, K.; Kuchekar, B. Physicochemical characterization of spray dried ternary micro-complexes of cefuroxime axetil with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 391–401. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A. Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes 2020, 8, 1450. [Google Scholar] [CrossRef]
- Soe, H.M.H.; Chamni, S.; Mahalapbutr, P.; Kongtaworn, N.; Rungrotmongkol, T.; Jansook, P. The investigation of binary and ternary sulfobutylether-β-cyclodextrin inclusion complexes with asiaticoside in solution and in solid state. Carbohydr. Res. 2020, 498, 108190. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. Aaps Pharmscitech. 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Ansari, M.N.; Ezzeldin, E.; Khalil, N.Y.; Ali, R. Improving the Solubilization and Bioavailability of Arbidol Hydrochloride by the. Preparation of Binary and Ternary β-Cyclodextrin Complexes with Poloxamer 188. Pharmaceuticals 2021, 14, 411. [Google Scholar] [CrossRef]
- Huang, Z.; Xia, M. HPLC Method for Simultaneous Determination of Six Isoflavones in Soybean Yoghurt. IOP Conf. Ser. Mater. Sci. Eng. 2019, 562, 012132. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Suliman, F.O.; Elbashir, A.A. Experimental and molecular modeling investigations of inclusion complexes of imazapyr with 2-hydroxypropyl(β/γ) cyclodextrin. J. Mol. Liq. 2018, 262, 504–513. [Google Scholar] [CrossRef]
- Imam, S.S.; AlShehri, S.M.; Alzahrani, T.A.; Altamimi, M.A.; Altamimi, M.A. Formulation and Evaluation of Supramolecular Food-Grade Piperine HP β CD and TPGS Complex: Dissolution, Physicochemical Characterization, Molecular Docking, In Vitro Antioxidant Activity, and Antimicrobial Assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a myricetin/hydroxypropyl-rmbeta-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydrate Polymers. Carbohydr. Polym. 2014, 22, 329–337. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Kanaan, R.; El-Elimat, T.; Aludatt, M.; Lahham, J.; El-Oqlah, A. In vitro propagation, genetic stability, and secondary metabolite analysis of wild lavender (Lavandula coronopifolia Poir.). Hortic. Environ. Biotechnol. 2017, 58, 393–405. [Google Scholar] [CrossRef]
- Akar, Z.; Kuçuk, M.; Dogan, H. A new colorimetric DPPH scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Chunlian, T.; Xin, L.; Yu, C.; Ruxia, W.; Tianmeng, L.; Cancan, C.; Mingchun, L. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Yao, Q.; Lin, M.T.; Lan, Q.H.; Huang, Z.W.; Zheng, Y.W.; Jiang, X.; Zhu, Y.D.; Kou, L.; Xu, H.L.; Zhao, Y.Z. In vitro and in vivo evaluation of didymin cyclodextrin inclusion complexes: Characterization and chemosensitization activity. Drug Deliv. 2020, 27, 54–65. [Google Scholar] [CrossRef] [Green Version]
- EL Maghraby, G.M.; Alomrani, A.H. Synergistic Enhancement of Itraconazole Dissolution by Ternary System Formation with Pluronic F68 and Hydroxy propyl methyl cellulose. Sci. Pharm. 2009, 77, 401–417. [Google Scholar] [CrossRef] [Green Version]
- Danciu, C.; Soica, C.; Oltean, M.; Avram, S.; Borcan, F.; Csanyi, E.; Ambrus, R.; Zupko, I.; Muntean, D.; Dehelean, C.A.; et al. Genistein in 1:1 Inclusion Complexes with Ramified Cyclodextrins: Theoretical, Physicochemical and Biological Evaluation. Int. J. Mol. Sci. 2014, 15, 1962–1982. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Osada, M.; Murata, I.; Kobata, K.; Kanamoto, I. Evaluation of Solubility Characteristics of a Hybrid Complex of Components of Soy. ACS Omega 2019, 4, 8632–8640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, M.R.; Rolim, L.A.; Soares, M.F.D.L.R.; Rolim-Neto, P.J.; de Albuquerque, M.M.; Soares-Sobrinho, J.L. Inclusion complex of methyl-beta-cyclodextrin and olanzapine as potential drug delivery system for schizophrenia. Carbohydr. Polym. 2012, 89, 1095–1100. [Google Scholar] [CrossRef]
- Nogueiras-Nieto, L.; Sobarzo-Sánchez, E.; Gómez-Amoza, J.L.; Otero, F.J. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: Implications in drug solubilization and delivery. Eur. J. Pharm. Biopharm. 2012, 80, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Nunes, S.C.; Simoes, S.; Pais, A.A.; Veiga, F. Molecular interaction governing solubility and release profiles in supramolecular systems containing fenbufen, pluronics and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 395–407. [Google Scholar] [CrossRef]
- Pandit, N.T.; Patravale, V.B. Design and Optimization of a Novel Method for Extraction of Genistein. Indian J. Pharm. Sci. 2011, 73, 184–191. [Google Scholar] [PubMed] [Green Version]
- Delrivo, A.; Zoppi, A.; Longhi, M.R. Interaction of sulfadiazine with cyclodextrins in aqueous solution and solid state. Carbohydr. Polym. 2012, 87, 1980–1988. [Google Scholar] [CrossRef]
- Bera, H.; Chekuri, S.; Sarkar, S.; Kumar, S.; Muvva, N.B.; Mothe, S.; Nadimpalli, J. Novel pimozide-β-cyclodextrin-polyvinylpyrrolidone inclusion complexes for Tourette syndrome treatment. J. Mol. Liq. 2016, 215, 135–143. [Google Scholar] [CrossRef]
- Jabbari, M.; Jabbari, A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 392–399. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]





| Binary System | Ternary System | ||
|---|---|---|---|
| Binary physical mixture (GS BM) | Binary inclusion complex (GS BC) | Ternary physical mixture (GS TM) | Ternary inclusion complex (GS TC) |
| GS: HP β CD | GS: HP β CD | GS: HP β CD: PL188 | GS: HP β CD: PL188 |
| 1:1 | 1:1 | 1:1:0.5 * | 1:1:0.5 * |
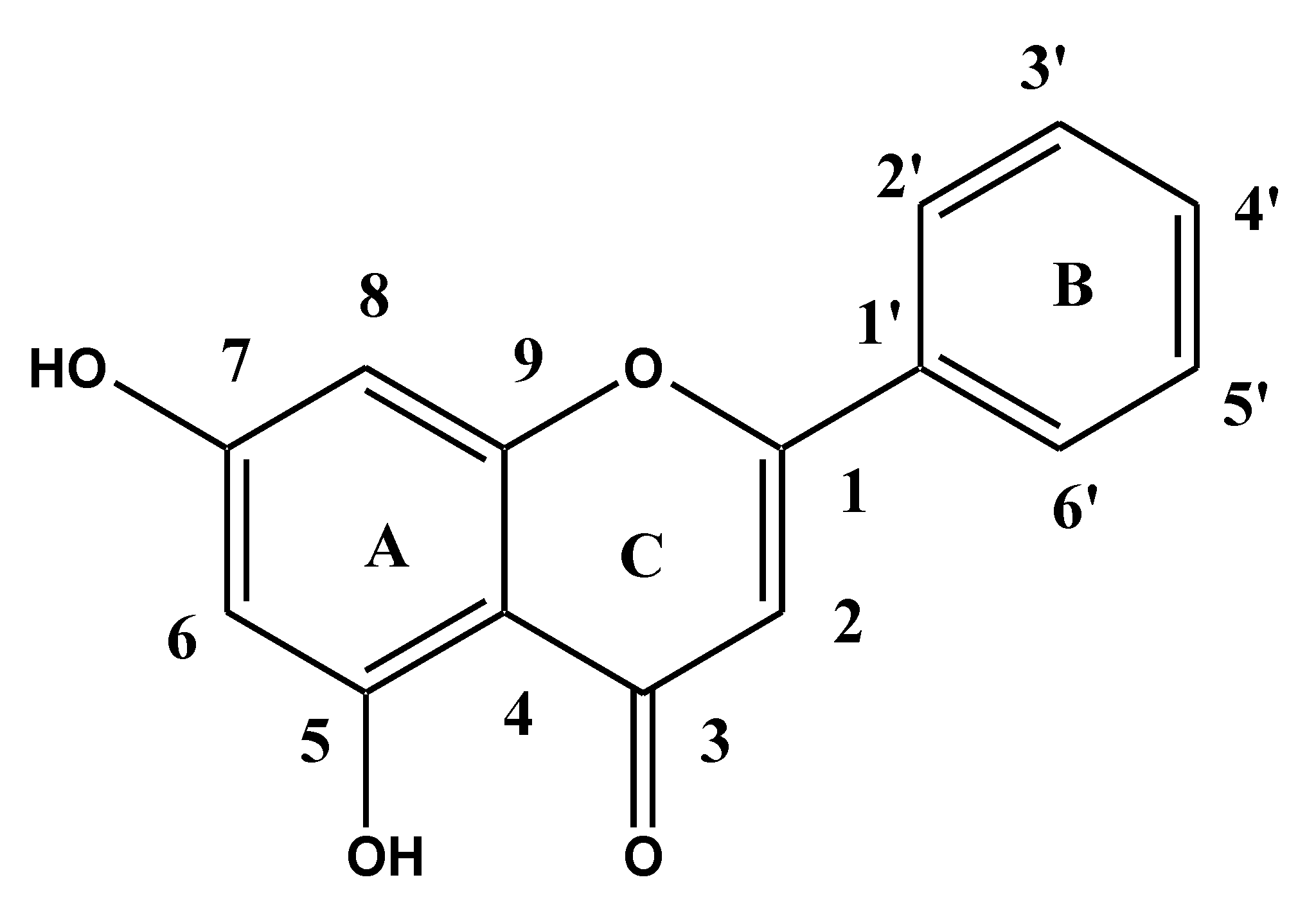
| Genistein (GS) | Poloxamer 188 (PL 188) | Hydroxy Propyl Beta Cyclodextrin (HP-β CD) | Genistein Ternary Mixture (GS-TM) | Genistein Ternary Complex (GS-TC) |
|---|---|---|---|---|
| 13C NMR Spectral Analysis | ||||
| 13C NMR (176 MHz, DMSO-d6) δ ppm: 130.64 (C-1), 115.54 (C-2), 99.46 (C-4), 94.22 (C-6) and 94.15 (C-8) of chromenyl moiety, 122.84 (C-2′), 122.78 (C-6′) benzene ring. | 13C NMR (176 MHz, DMSO-d6) δ ppm: 64 (C of CH2-OH),70.6 (C-O-C),72.6 (CH), 17.1 (CH3), 77.8 (CH2-CH-) | 13C NMR (176 MHz, DMSO-d6) δ ppm: 103.14(Glucose) | 13C NMR (176 MHz, DMSO-d6) δ ppm: 122.81(C-2′) of the benzene ring, 99.44 (C-4), 94.21 (C-6) and 94.14 (C-8) of chromenyl moiety, 65.80 (C of CH2-OH), 73.09 (CH), 20.20 (CH3) | 13C NMR (176 MHz, DMSO-d6) δ ppm: 130.64 (C-1), 121.68 (C-6′) and 124.19(C-2′) of the benzene ring, 115.53 (C-2), 99.44 (C-4), 94.21 (C-6), 94.14 (C-8) of chromenyl moiety, 65.80 (C of CH2-OH), 70.25 (C-O-C), 72.74 (CH), 104.94 (Glucose), 20.20 (CH3). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alkholifi, F.K.; Alharbi, K.S.; Mostafa, E.M.; Alanazi, A.S.; Gilani, S.J.; Musa, A.; et al. Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics 2021, 13, 1997. https://doi.org/10.3390/pharmaceutics13121997
Zafar A, Alruwaili NK, Imam SS, Alsaidan OA, Alkholifi FK, Alharbi KS, Mostafa EM, Alanazi AS, Gilani SJ, Musa A, et al. Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics. 2021; 13(12):1997. https://doi.org/10.3390/pharmaceutics13121997
Chicago/Turabian StyleZafar, Ameeduzzafar, Nabil K. Alruwaili, Syed Sarim Imam, Omar Awad Alsaidan, Faisal K. Alkholifi, Khalid Saad Alharbi, Ehab M. Mostafa, Abdullah S. Alanazi, Sadaf Jamal Gilani, Arafa Musa, and et al. 2021. "Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment" Pharmaceutics 13, no. 12: 1997. https://doi.org/10.3390/pharmaceutics13121997
APA StyleZafar, A., Alruwaili, N. K., Imam, S. S., Alsaidan, O. A., Alkholifi, F. K., Alharbi, K. S., Mostafa, E. M., Alanazi, A. S., Gilani, S. J., Musa, A., Alshehri, S., Rawaf, A., & Alquraini, A. (2021). Formulation of Genistein-HP β Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics, 13(12), 1997. https://doi.org/10.3390/pharmaceutics13121997









