Internalization and Transport of PEGylated Lipid-Based Mixed Micelles across Caco-2 Cells Mediated by Scavenger Receptor B1
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Mixed Micelles of Different Compositions
2.3. Cell Culture
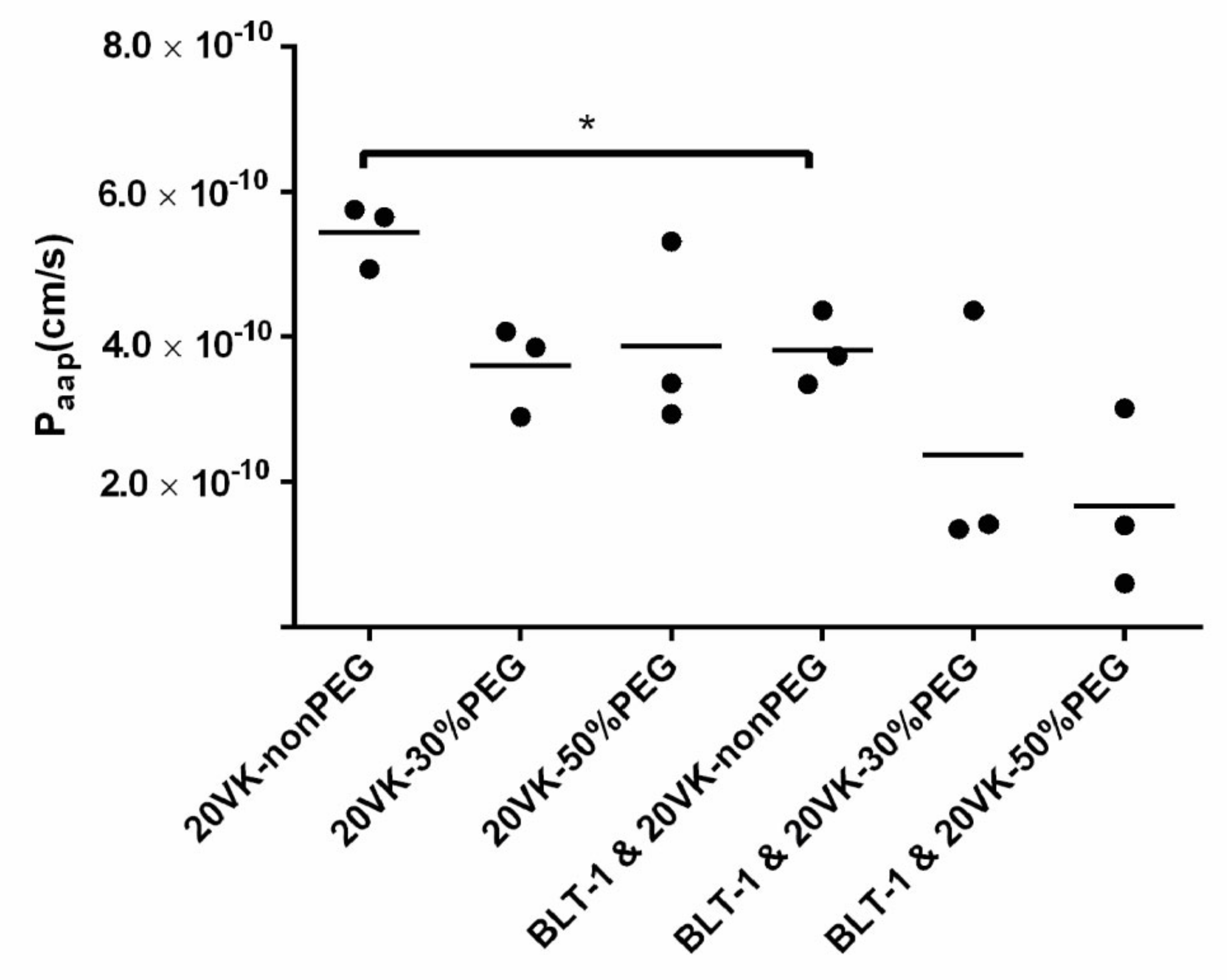
2.4. Transport of Vitamin-K-Loaded Mixed Micelles with Different PEG Densities through Differentiated Caco-2 Monolayers
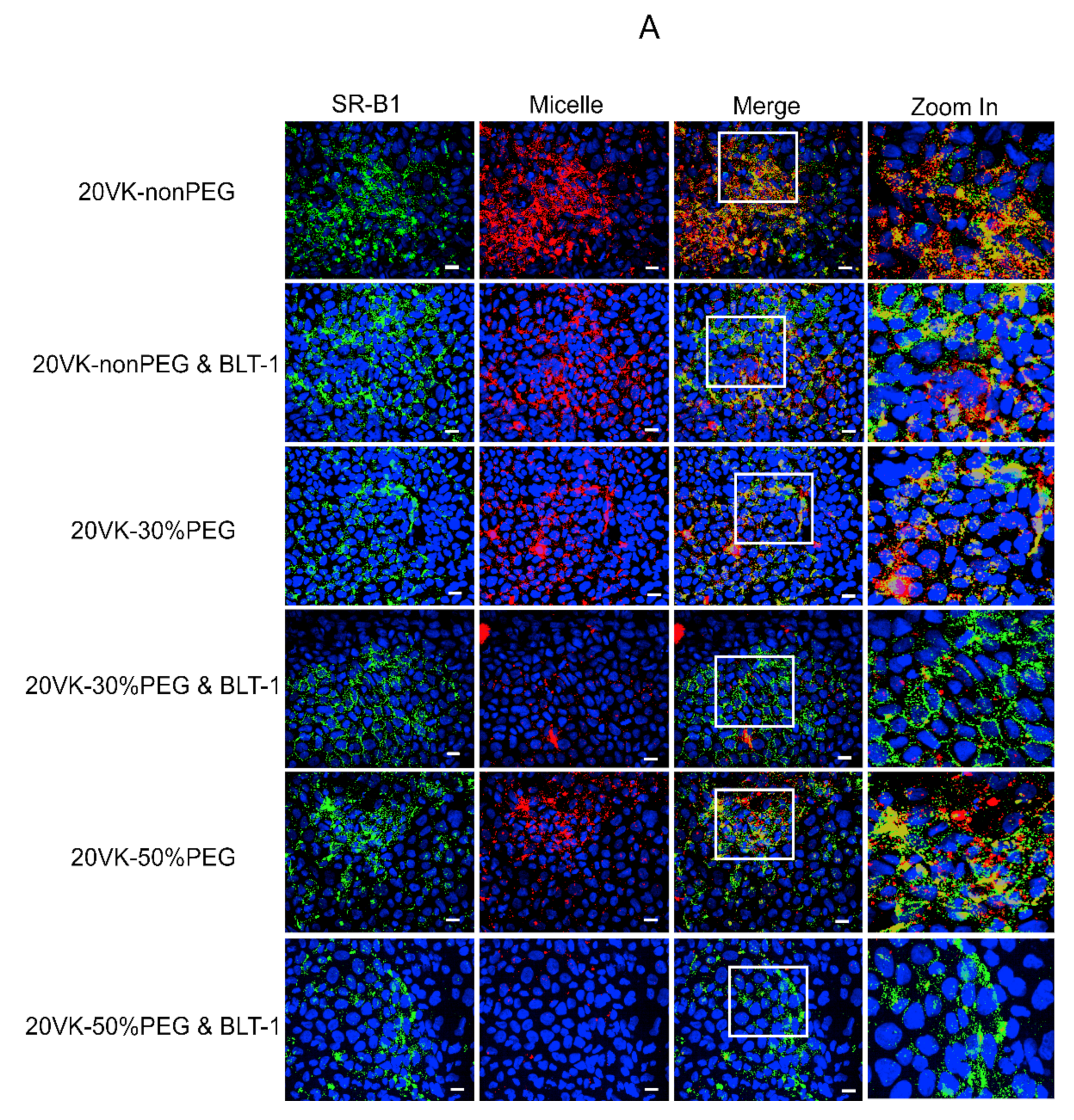
2.5. Uptake of Rhodamine-Labeled Mixed Micelles and Colocalization with SR-B1 as Studied by Confocal Microscopic Analysis
2.6. Binding and Uptake of Mixed Micelles by Hela Cells Overexpressing SR-B1 Studies by Confocal Microscopic Analysis
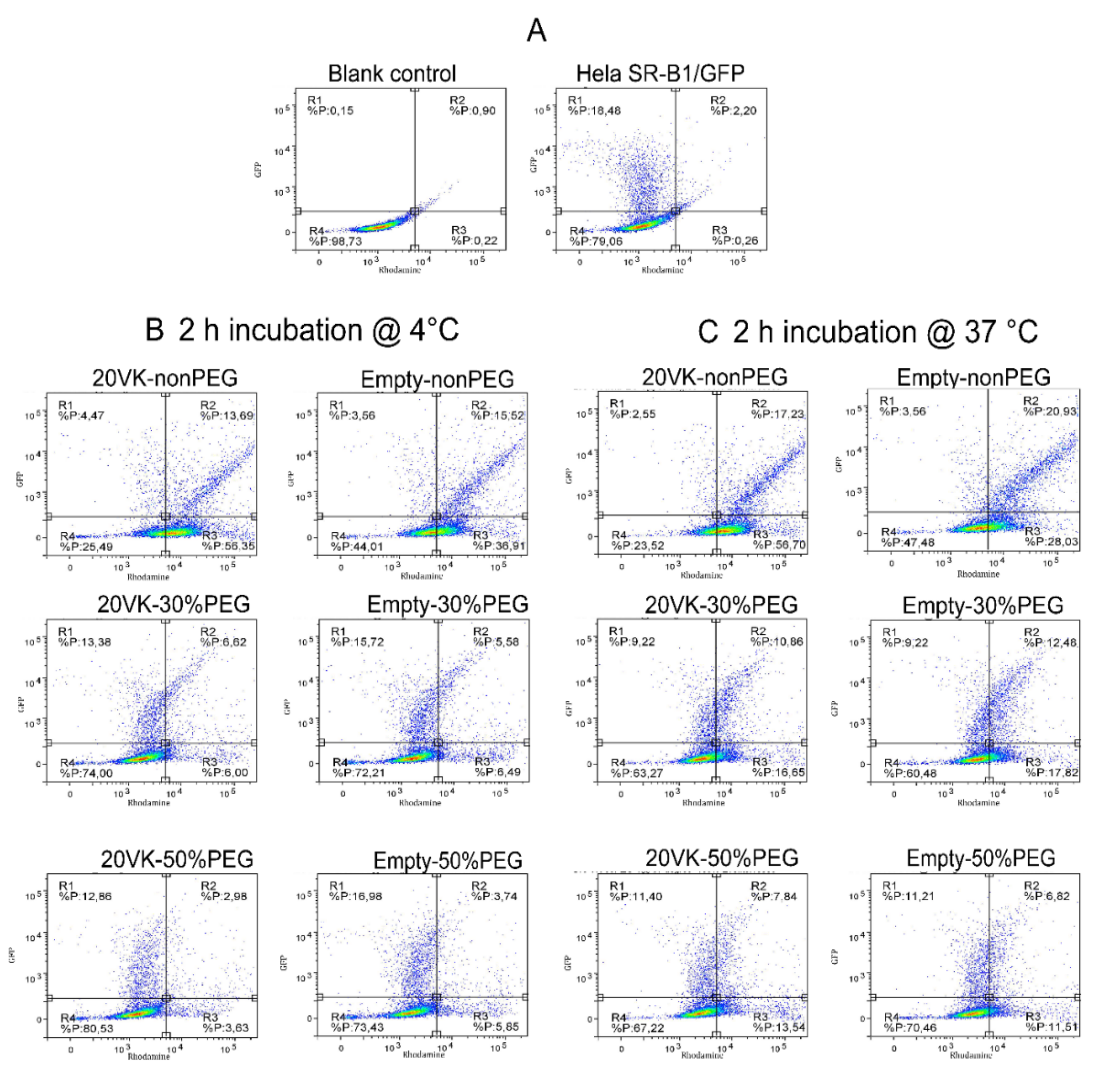
2.7. Binding and Uptake of Mixed Micelles by Hela Cells Overexpressing SR-B1 Measured by Fluorescence-Activated Cell Sorting (FACS)
2.8. Surface Plasmon Resonance (SPR) to Study the Interaction between SR-B1 and Mixed Micelles of Different Compositions
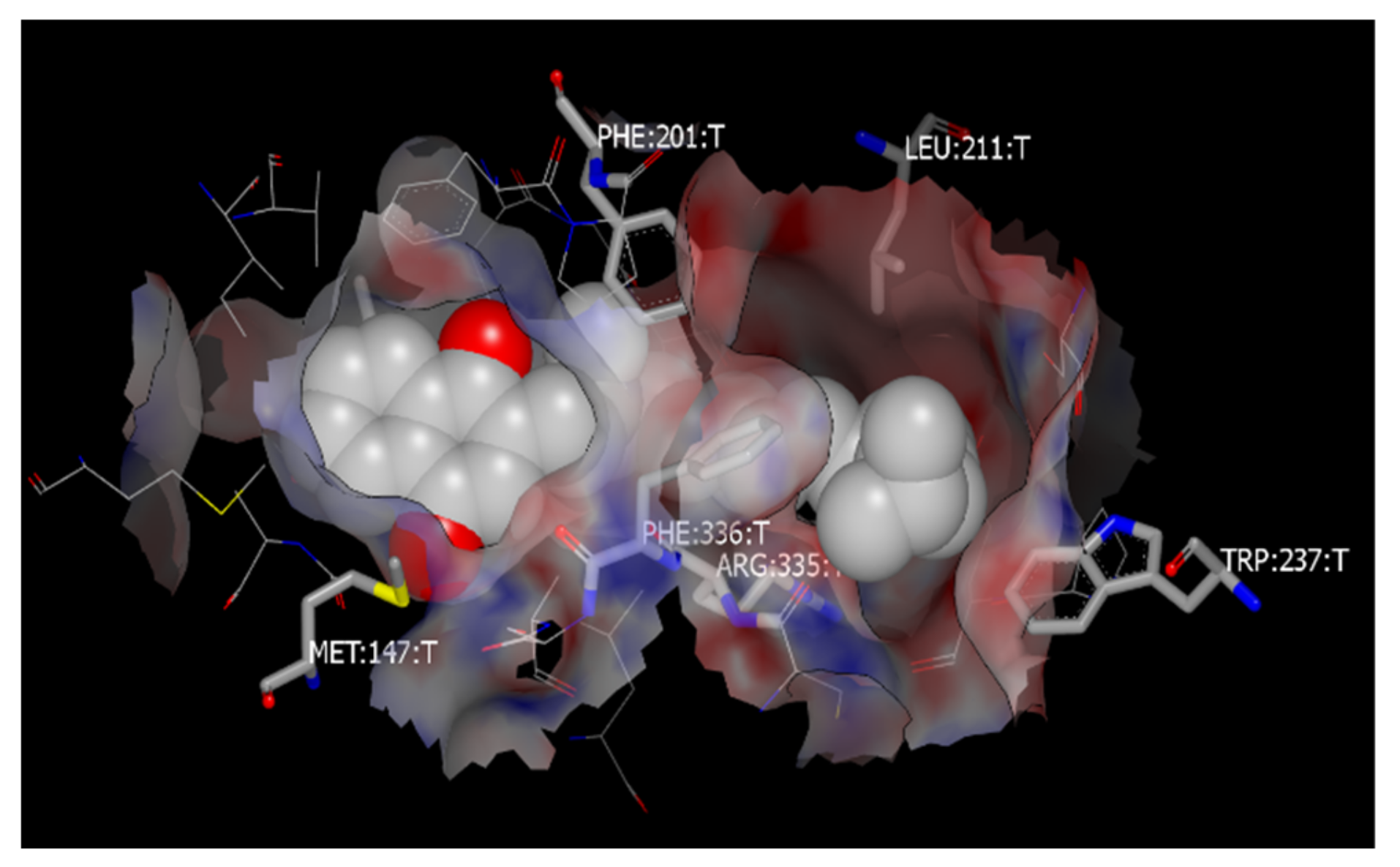
2.9. Molecular Docking to Study the Binding Sites of SR-B1 with Vitamin K and PEG
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Vitamin-K-Loaded Mixed Micelles
3.2. Transport of Vitamin-K-Loaded Mixed Micelles through Differentiated Caco-2 Monolayers
3.3. Colocalization Studies of SR-B1 and Vitamin-K-Loaded Mixed Micelles
3.4. Binding and Uptake of Mixed Micelles by Hela Cells Overexpressing SR-B1/GFP Revealed by Confocal Microscopy
3.5. FACS Analysis of Binding and Uptake of Mixed Micelles by Hela Cells Overexpressing SR-B1
3.6. SPR Analysis to Study the Affinity between SR-B1 and Mixed Micelles
3.7. Molecular Docking to Study the Binding Sites of SR-B1 with Vitamin K and PEG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamazoe, E.; Fang, J.Y.; Tahara, K. Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm. 2021, 593, 120148. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Sun, F.; Jaspers, T.C.; van Hasselt, P.M.; Hennink, W.E.R.; van Nostrum, C.F. A mixed micelle formulation for oral delivery of vitamin K. Pharm. Res. 2016, 33, 2168–2179. [Google Scholar] [CrossRef]
- Von Kries, R.; Hachmeister, A.; Göbel, U. Oral mixed micellar vitamin K for prevention of late vitamin K deficiency bleeding. Arch. Dis Child. Fetal Neonatal Ed. 2003, 88, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Lowensteyn, Y.N.; Jansen, N.J.G.; van Heerde, M.; Klein, R.H.; Kneyber, M.C.J.; Kuiper, J.W.; Riedijk, M.A.; Verlaat, C.W.M.; Visser, I.H.E.; van Waardenburg, D.A.; et al. Increasing the dose of oral vitamin K prophylaxis and its effect on bleeding risk. Eur. J. Pediatr. 2019, 178, 1033–1042. [Google Scholar] [CrossRef]
- Hernell, O.; Staggers, J.E.; Carey, M.C. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry 1990, 29, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Adrian, M.; Beztsinna, N.; van den Dikkenberg, J.B.; Maas-Bakker, R.F.; van Hasselt, P.M.; van Steenbergen, M.J.; Su, X.; Kapitein, L.C.; Hennink, W.E.; et al. Influence of PEGylation of Vitamin-K-Loaded Mixed Micelles on the Uptake by and Transport through Caco-2 Cells. Mol. Pharm. 2018, 15, 3786–3795. [Google Scholar] [CrossRef]
- Rooimans, T.; Minderhoud, T.C.; Leal, N.; Vromans, H.; van Nostrum, C.F.; van Hasselt, P.M. Novel Orally Formulated Mixed Micelles Optimize Vitamin K Absorption Under Bile Deficient Conditions. Gastroenterology 2021, 161, 1056–1059. [Google Scholar] [CrossRef]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Fogelman, A.M. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract.Cardiovasc. Med. 2006, 3, 540–547. [Google Scholar] [CrossRef]
- Goncalves, A.; Gontero, B.; Nowicki, M.; Margier, M.; Masset, G.; Amiot, M.J.; Reboul, E. Micellar lipid composition affects micelle interaction with class B scavenger receptor extracellular loops. J. Lipid Res. 2015, 56, 1123–1133. [Google Scholar] [CrossRef]
- Hoekstra, M.; van Berkel, T.J.; van Eck, M. Scavenger receptor BI: A multi-purpose player in cholesterol and steroid metabolism. World J. Gastroenterol. 2010, 16, 5916–5924. [Google Scholar]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Williams, D.L.; Wong, J.S.; Hamilton, R.L. SR-BI is required for microvillar channel formation and the localization of HDL particles to the surface of adrenocortical cells in vivo. J. Lipid Res. 2002, 43, 544–549. [Google Scholar] [CrossRef]
- Raith, M.; Kauffman, S.J.; Asoudeh, M.; Buczek, J.A.; Kang, N.G.; Mays, J.W.; Dalhaimer, P. Elongated PEO-based nanoparticles bind the high-density lipoprotein (HDL) receptor scavenger receptor class B I (SR-BI). J. Control. Release 2021, 337, 448–457. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Sakurai, M.; Takechi-Haraya, Y.; Nanjo, K.; Goda, Y. Involvement of scavenger receptor class B type 1 and low-density lipoprotein receptor in the internalization of liposomes into HepG2 cells. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2253–2258. [Google Scholar] [CrossRef]
- Koide, N.; Fujita, K.; Kuroda, S.; Hinuma, S. Binding of liposomes composed of phosphatidylcholine to scavenger receptor class B type 1 and its modulation by phosphatidic acid in HEK293T cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119043. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Bhargav, A.G.; Perez, J.G.; Wadajkar, A.S.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Surface plasmon resonance as a high throughput method to evaluate specific and non-specific binding of nanotherapeutics. J. Control. Release 2015, 219, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Leloup, N.; Chataigner, L.M.P.; Janssen, B.J.C. Structural insights into SorCS2-Nerve Growth Factor complex formation. Nat. Commun. 2018, 9, 2979. [Google Scholar] [CrossRef]
- Hawkins, P. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
- Neculai, D.; Schwake, M.; Ravichandran, M.; Zunke, F.; Collins, R.F.; Peters, J.; Neculai, M.; Plumb, J.; Loppnau, P.; Pizarro, J.C.; et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013, 504, 172–176. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef] [PubMed]
- McGann, M.R. Gaussian Docking Functions. Biopolymers 2003, 68, 76–90. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, G.B.; Sheridan, R.P.; Bayly, C.I.; Culberson, J.C.; Kreatsoulas, C.; Lindsley, S.; Maiorov, V.; Truchon, J.F.; Cornell, W.D. Comparison of Topological, Shape, and Docking Methods in Virtual Screening. J. Chem. Inf. Model. 2007, 47, 1504–1519. [Google Scholar] [CrossRef] [PubMed]
- McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- Bhattacharjee, S. In relation to the following article “DLS and zeta potential—What they are and what they are not”? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Vukovic, L.; Khatib, F.A.; Drake, S.P.; Madriaga, A.; Brandenburg, K.S.; Kral, P.; Onyuksel, H. Structure and dynamics of highly PEG-ylated sterically stabilized micelles in aqueous media. J. Am. Chem. Soc. 2011, 133, 13481–13488. [Google Scholar] [CrossRef]
- Stamp, D.H. Three hypotheses linking bile to carcinogenesis in the gastrointestinal tract: Certain bile salts have properties that may be used to complement chemotherapy. Med. Hypotheses 2002, 59, 398–405. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Rammohan, R.; Lukyanov, A.N.; Whiteman, K.R.; Torchilin, V.P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002, 240, 95–102. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Pearce, S.C.; Coia, H.G.; Karl, J.P.; Pantoja-Feliciano, I.G.; Zachos, N.C.; Racicot, K. Intestinal in vitro and ex vivo Models to Study Host-Microbiome Interactions and Acute Stressors. Front. Physiol. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A unique multifunctional receptor for cholesterol influx and efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lau, T.Y.; Carr, S.A.; Krieger, M. Exoplasmic cysteine Cys384 of the HDL receptor SR-BI is critical for its sensitivity to a small-molecule inhibitor and normal lipid transport activity. Proc. Natl. Acad. Sci. USA 2011, 108, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.N.; Patil, S.A.; Sistla, S.; Jadhav, J.P. Comparative biophysical characterization: A screening tool for acetylcholinesterase inhibitors. PLoS ONE 2019, 14, e0215291. [Google Scholar] [CrossRef]
- Vishnyakova, T.G.; Bocharov, A.V.; Baranova, I.N.; Chen, Z.; Remaley, A.T.; Csako, G.; Eggerman, T.L.; Patterson, A.P. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 2003, 278, 22771–22780. [Google Scholar] [CrossRef]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Abdiche, Y.N.; Miles, A.; Eckman, J.; Foletti, D.; van Blarcom, T.J.; Yeung, Y.A.; Pons, J.; Rajpal, A. High-throughput epitope binning assays on label-free array-based biosensors can yield exquisite epitope discrimination that facilitates the selection of monoclonal antibodies with functional activity. PLoS ONE 2014, 9, e92451. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Li, P.Y.; Deng, J.; Arhontoulis, D.C.; Li, C.Y.; Bowne, W.B.; Cheng, H. Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation. ACS Nano. 2018, 12, 10130–10141. [Google Scholar] [CrossRef]
- Pannuzzo, M.; Esposito, S.; Wu, L.P.; Key, J.; Aryal, S.; Celia, C.; di Marzio, L.; Moghimi, S.M.; Decuzzi, P. Overcoming Nanoparticle-Mediated Complement Activation by Surface PEG Pairing. Nano Lett. 2020, 20, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Gomez-Garcia, M.J.; Rinker, K.D.; Cramb, D.T. Surface-grafted polyethylene glycol conformation impacts the transport of PEG-functionalized liposomes through a tumour extracellular matrix model. RSC Advances 2018, 8, 7697–7708. [Google Scholar] [CrossRef]
- Li, Y.; Kroger, M.; Liu, W.K. Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef]
- Mansbach, C.M.; Cohen, R.S.; Leff, P.B. Isolation and properties of the mixed lipid micelles present in intestinal content during fat digestion in man. J. Clin. Investig. 1975, 56, 781–791. [Google Scholar] [CrossRef]
- Field, F.J.; Watt, K.; Mathur, S.N. TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J. Lipid Res. 2010, 51, 1407–1415. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Yang, J.; Shi, Y.; Ma, G.; Zhang, Z.; Zhang, X. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Calvo, D.; Vega, M.A. Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. J. Biol. Chem. 1993, 268, 18929–18935. [Google Scholar] [CrossRef]
- McGann, M. FRED and HYBRID docking performance on standardized datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Berglund, L.; Rasmussen, M.; Petersen, T. Assignment of disulfide bridges in bovine CD36. Eur. J. Biochem. 1998, 257, 488–494. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Shen, W.J.; Nomoto, A.; Azhar, S. Differential roles of cysteine residues in cellular trafficking, dimerization, and function of the HDL receptor, SR-BI. Biochemistry 2011, 50, 10860–10875. [Google Scholar] [CrossRef][Green Version]
- Mooberry, L.K.; SabnisAndras, N.A.; Lacko, G. Targeting the SR-B1 receptor as a gateway for cancer therapy and imaging. Front. Pharmacol. 2016, 7, 466. [Google Scholar] [CrossRef]
- Yu, M.; Lau, T.; Carr, S.; Krieger, M. Contributions of a disulfide bond and a reduced cysteine side chain to the intrinsic activity of the HDL receptor SR-BI. Biochemistry 2012, 51, 10044–10055. [Google Scholar] [CrossRef]
- Faloon, P.W.; Dockendorff, C.; Youngsaye, W.; Yu, M.; Nag, P.P.; Lewis, T.A.; Bennion, M.; Paterson, C.; Lam, G.; Dandapani, S.; et al. A Small Molecule Inhibitor of Scavenger Receptor BI-mediated Lipid Uptake—Probe 1; National Center for Biotechnology Information: Bethseda, MD, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK133420/ (accessed on 19 October 2021).
- Heybrock, S.; Kanerva, K.; Meng, Y.; Ing, C.; Liang, A.; Xiong, Z.J.; Weng, X.; Ah Kim, Y.; Collins, R.; Trimble, W.; et al. Lysosomal integral membrane protein-2 (LIMP-2/SCARB2) is involved in lysosomal cholesterol export. Nat. Commun. 2019, 10, 3521. [Google Scholar] [CrossRef]
- Reaven, E.; Leers-Sucheta, S.; Nomoto, A.; Azhar, S. Expression of scavenger receptor class B type 1 (SR-BI) promotes microvillar channel formation and selective cholesteryl ester transport in a heterologous reconstituted system. Proc. Natl. Acad. Sci. USA 2001, 98, 1613–1618. [Google Scholar] [CrossRef]
- Reaven, E.; Spicher, M.; Azhar, S. Microvillar channels: A unique plasma membrane compartment for concentrating lipoproteins on the surface of rat adrenal cortical cells. J. Lipid Res. 1989, 30, 1551–1560. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Y. Biological fate of ingested lipid-based nanoparticles: Current understanding and future directions. Nanoscale 2019, 11, 11048–11063. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Suzuki, K.; Cho, H.; Youn, Y.S.; Bae, Y.H. Oral Nanoparticles Exhibit Specific High-Efficiency Intestinal Uptake and Lymphatic Transport. ACS Nano 2018, 12, 8893–8900. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Bae, Y.H. Bile acid transporter-mediated oral drug delivery. J. Control. Release 2020, 327, 100–116. [Google Scholar] [CrossRef] [PubMed]




| Formulations * | EPC/DSPE-PEG (Total 25 mM) | Glyco- Cholic Acid (mM) | Vitamin K (mM) | Encapsulation Efficiency (%) | Z-Average Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 20VK-nonPEG | 100/0 | 30 | 5.55 | 95 ± 2 | 7.5 ± 0.2 | −20.6 ± 2.2 |
| 10VK-nonPEG | 100/0 | 30 | 2.78 | 91 ± 3 | 7.3 ± 0.1 | −18.9 ± 0.6 |
| 5VK-nonPEG | 100/0 | 30 | 1.39 | 96 ± 2 | 7.3 ± 0.1 | −17.7 ± 0.5 |
| Empty-non-PEG | 100/0 | 30 | 0 | - | 7.4 ± 0.1 | −20.4 ± 1.3 |
| 20VK-15%PEG | 85/15 | 30 | 5.55 | 91 ± 2 | 9.1 ± 0.1 | −14.7 ± 1.1 |
| Empty-15%PEG | 85/15 | 30 | 0 | - | 8.4 ± 0.1 | −12.9 ± 0.7 |
| 20VK-30%PEG | 70/30 | 30 | 5.55 | 93 ± 2 | 9.7 ± 0.6 | −9.2 ± 0.7 |
| Empty-30%PEG | 70/30 | 30 | 0 | - | 8.7 ± 0.1 | −8.5 ± 0.8 |
| 20VK-50%PEG | 50/50 | 30 | 5.55 | 91 ± 4 | 10.7 ± 0.4 | −5.1 ± 0.2 |
| Empty-50%PEG | 50/50 | 30 | 0 | - | 10.5 ± 0.1 | −7.3 ± 0.6 |
| DSPE-PEG | 0/100 | 0 | 0 | - | 14.1 ± 0.1 | −5.9 ± 0.7 |
| Formulations | EPC (%) | DSPE-PEG (%) | Glycocholic Acid (%) | Vitamin K (%) | KD (μM) | Bmax (Ru) |
|---|---|---|---|---|---|---|
| 20VK-nonPEG | 55.2 | 0 | 37.9 | 6.6 | 9.2 ± 0.1 | 408 ± 25 |
| 10VK-nonPEG | 57.1 | 0 | 39.3 | 3.3 | - | - |
| 5VK-nonPEG | 58.2 | 0 | 40 | 1.8 | - | - |
| Empty-nonPEG | 59.3 | 0 | 40.7 | 0 | 159 ± 19 | 168 ± 13 |
| 20VK-15%PEG | 38.6 | 24.5 | 31.2 | 5.4 | - | - |
| Empty-15%PEG | 40.9 | 26 | 33.1 | 0 | - | - |
| 20VK-30%PEG | 27 | 41.7 | 26.5 | 4.7 | 59 ± 9 | 408 ± 35 |
| Empty-30%PEG | 28.4 | 43.8 | 27.8 | 0 | 37 ± 7 | 188 ± 27 |
| 20VK-50%PEG | 16.1 | 57.8 | 22.1 | 3.6 | 133 ± 34 | 319 ± 14 |
| Empty-50%PEG | 16.7 | 60.3 | 23 | 0 | 128 ± 25 | 226 ± 19 |
| DSPE-PEG | 0 | 100 | 0 | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Ramírez-Escudero, M.; Sun, F.; van den Dikkenberg, J.B.; van Steenbergen, M.J.; Pieters, R.J.; Janssen, B.J.C.; van Hasselt, P.M.; Hennink, W.E.; van Nostrum, C.F. Internalization and Transport of PEGylated Lipid-Based Mixed Micelles across Caco-2 Cells Mediated by Scavenger Receptor B1. Pharmaceutics 2021, 13, 2022. https://doi.org/10.3390/pharmaceutics13122022
Su X, Ramírez-Escudero M, Sun F, van den Dikkenberg JB, van Steenbergen MJ, Pieters RJ, Janssen BJC, van Hasselt PM, Hennink WE, van Nostrum CF. Internalization and Transport of PEGylated Lipid-Based Mixed Micelles across Caco-2 Cells Mediated by Scavenger Receptor B1. Pharmaceutics. 2021; 13(12):2022. https://doi.org/10.3390/pharmaceutics13122022
Chicago/Turabian StyleSu, Xiangjie, Mercedes Ramírez-Escudero, Feilong Sun, Joep B. van den Dikkenberg, Mies J. van Steenbergen, Roland J. Pieters, Bert J. C. Janssen, Peter M. van Hasselt, Wim E. Hennink, and Cornelus F. van Nostrum. 2021. "Internalization and Transport of PEGylated Lipid-Based Mixed Micelles across Caco-2 Cells Mediated by Scavenger Receptor B1" Pharmaceutics 13, no. 12: 2022. https://doi.org/10.3390/pharmaceutics13122022
APA StyleSu, X., Ramírez-Escudero, M., Sun, F., van den Dikkenberg, J. B., van Steenbergen, M. J., Pieters, R. J., Janssen, B. J. C., van Hasselt, P. M., Hennink, W. E., & van Nostrum, C. F. (2021). Internalization and Transport of PEGylated Lipid-Based Mixed Micelles across Caco-2 Cells Mediated by Scavenger Receptor B1. Pharmaceutics, 13(12), 2022. https://doi.org/10.3390/pharmaceutics13122022







