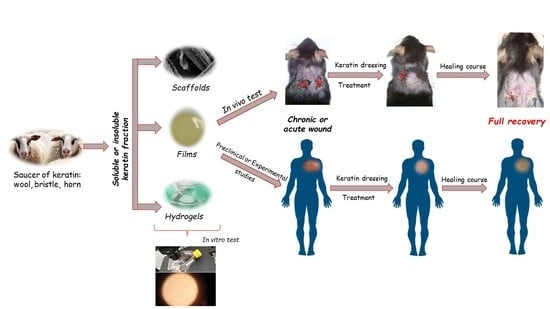
Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review
Abstract
:1. Introduction
2. Features of the Ideal Wound Dressing
3. Structure and Function of the Skin
3.1. Epidermis
3.2. Basement Membrane Zone (BMZ)
3.3. Dermis and Subcutaneous Tissues
4. Role of Keratin in Wound Healing
5. Biomedical Properties of Keratin
Antibacterial Properties of Keratin Biomaterials
6. Effect of Keratin on Hyperpigmentation
7. Biomedical Propertied of Keratin Biomaterials
Diabetic Condition
8. Clinical Application of Keratin Biomaterial in Wound Healing
9. Concluding Remarks and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrlich, F.; Lachner, J.; Hermann, M.; Tschachler, E.; Eckhart, L. Convergent Evolution of Cysteine-Rich Keratins in Hard Skin Appendages of Terrestrial Vertebrates. Mol. Biol. Evol. 2020, 37, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, F.; Fischer, H.; Langbein, L.; Praetzel-Wunder, S.; Ebner, B.; Figlak, K.; Weissenbacher, A.; Sipos, W.; Tschachler, E.; Eckhart, L. Differential Evolution of the Epidermal Keratin Cytoskeleton in Terrestrial and Aquatic Mammals. Mol. Biol. Evol. 2019, 36, 328–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, A.; Cavaco-Paulo, A. The use of keratin in biomedical applications. Curr. Drug Targets 2013, 14, 612–619. [Google Scholar] [CrossRef]
- Shah, A.; Tyagi, S.; Bharagava, R.N.; Belhaj, D.; Kumar, A.; Saxena, G.; Saratale, G.D.; Mulla, S.I. Keratin Production and Its Applications: Current and Future Perspective. In Keratin as a Protein Biopolymer: Extraction from Waste Biomass and Applications; Sharma, S., Kumar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-02901-2. [Google Scholar]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.-D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, L.; Wei, Y.; Chen, T.; Ji, X.; Ye, K.; Yu, J.; Tang, B.; Sun, X.; Hu, J. Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model. J. Mater. Sci. Mater. Med. 2019, 30, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konop, M.; Czuwara, J.; Klodzinska, E.; Laskowska, A.K.; Zielenkiewicz, U.; Brzozowska, I.; Nabavi, S.M.; Rudnicka, L. Development of a novel keratin dressing which accelerates full-thickness skin wound healing in diabetic mice: In vitro and in vivo studies. J. Biomater. Appl. 2018, 33, 527–540. [Google Scholar] [CrossRef]
- Konop, M.; Sulejczak, D.; Czuwara, J.; Kosson, P.; Misicka, A.; Lipkowski, A.W.; Rudnicka, L. The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017, 25, 62–74. [Google Scholar] [CrossRef]
- Kurzepa, K.; Róycki, K.; Bochyñska, M.; Konop, M.; Urbanczyk-Lipkowska, Z.; Lipkowski, A.W. Molecular scaffolds for three-dimensional cell and tissue cultures. Polimery 2013, 58, 663–669. [Google Scholar] [CrossRef]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Nasipuri, P.; Herschend, J.; Brejnrod, A.D.; Madsen, J.S.; Espersen, R.; Svensson, B.; Burmølle, M.; Jacquiod, S.; Sørensen, S.J. Community-intrinsic properties enhance keratin degradation from bacterial consortia. PLoS ONE 2020, 15, e0228108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.D. The development of wound managment products. Wounds 1989, 1, 155–171. [Google Scholar]
- Swenty, C.F. Principles to Guide Your Dressing Choice. J. Nurse Pract. 2016, 12, e125–e127. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- RǍdulescu, M.; Holban, A.M.; MogoantǍ, L.; BǍlşeanu, T.A.; Mogoşanu, G.D.; Savu, D.; Popescu, R.C.; FufǍ, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, characterization, and evaluation of bionanocomposites based on natural polymers and antibiotics for wound healing applications. Molecules 2016, 21, 761. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.W.; Chen, Y.K.; Tang, K.C.; Yang, K.C.; Cheng, N.C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef]
- Roy, D.C.; Tomblyn, S.; Isaac, K.M.; Kowalczewski, C.J.; Burmeister, D.M.; Burnett, L.R.; Christy, R.J. Ciprofloxacin-loaded keratin hydrogels reduce infection and support healing in a porcine partial-thickness thermal burn. Wound Repair Regen. 2016, 24, 657–668. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Gao, Y.; Wang, F.; Wang, Y.; Cao, M.; Zhang, W.; Liang, Y.; Song, M.; Jiang, G. Toxicity of silver nanoparticles on wound healing: A case study of zebrafish fin regeneration model. Sci. Total Environ. 2020, 717, 137178. [Google Scholar] [CrossRef]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postep. Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Joffe, R.; Plaza, J.A.; Kajoian, A. Tip Chapter: Histology and Physiology of the Skin. In Minimally Invasive Aesthetic Procedures: A Guide for Dermatologists and Plastic Surgeons; Da Costa, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 179–192. ISBN 978-3-319-78265-2. [Google Scholar]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Epithelial Skin Biology. Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128029565. [Google Scholar]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- McMillan, J.R.; Akiyama, M.; Shimizu, H. Epidermal basement membrane zone components: Ultrastructural distribution and molecular interactions. J. Dermatol. Sci. 2003, 31, 169–177. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L.; Has, C. Disorders of the cutaneous basement membrane zone-The paradigm of epidermolysis bullosa. Matrix Biol. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Chan, L.S. Human skin basement membrane in health and in autoimmune diseases. Front. Biosci. 1997, 2, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai-Cheong, J.E.; Arita, K.; McGrath, J.A. Genetic diseases of junctions. J. Investig. Dermatol. 2007, 127, 2713–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J.; Has, C.; Vahidnezhad, H.; Youssefian, L.; Bruckner-Tuderman, L. Molecular pathology of the basement membrane zone in heritable blistering diseases: The paradigm of epidermolysis bullosa. Matrix Biol. 2017, 57, 76–85. [Google Scholar] [CrossRef]
- Daniel, B.S.; Murrell, D.F. Review of autoimmune blistering diseases: The Pemphigoid diseases. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1685–1694. [Google Scholar] [CrossRef]
- Didona, D.; Maglie, R.; Eming, R.; Hertl, M. Pemphigus: Current and future therapeutic strategies. Front. Immunol. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 366. [Google Scholar] [CrossRef] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Dehdashtian, A.; Stringer, T.P.; Warren, A.J.; Mu, E.W.; Amirlak, B.; Shahabi, L. Anatomy and Physiology of the Skin. In Melanoma: A Modern Multidisciplinary Approach; Riker, A.I., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 15–26. ISBN 978-3-319-78310-9. [Google Scholar]
- Singh, V.; Wang, S.; Ng, K.W. 2.25 Keratin as a Biomaterial. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 542–557. ISBN 978-0-08-100692-4. [Google Scholar]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Heid, H.W.; Moll, I.; Franke, W.W. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation 1988, 37, 137–157. [Google Scholar] [CrossRef]
- Heid, H.W.; Werner, E.; Franke, W.W. The complement of native α-keratin polypeptides of hair-forming cells: A subset of eight polypeptides that differ from epithelial cytokeratins. Differentiation 1986, 32, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Ogawa, H. Coexpression of keratins characteristic of skin and hair differentiation in nail cells. J. Investig. Dermatol. 1993, 100, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II keratin intermediate filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, R.G. Apoptosis and keratin intermediate filaments. Cell Death Differ. 2002, 9, 486–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, R.P.; Lessard, J.C.; Coulombe, P.A. Keratin intermediate filament proteins—Novel regulators of inflammation and immunity in skin. J. Cell Sci. 2012, 125, 5257–5258. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.; Colucci-Guyon, E.; Takahashi, K.; Gu, C.; Babinet, C.; Coulombe, P.A. Introducing a null mutation in the mouse K6α and K6β genes reveals their essential structural role in the oral mucosa. J. Cell Biol. 2000, 150, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.; Coulombe, P.A. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 2003, 276, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Rotty, J.D.; Coulombe, P.A. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J. Cell Biol. 2012, 197, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Wong, P.; Coulombe, P.A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 2006, 441, 362–365. [Google Scholar] [CrossRef]
- Yang, L.; Jin, L.; Ke, Y.; Fan, X.; Zhang, T.; Zhang, C.; Bian, H.; Wang, G. E3 ligase Trim21 ubiquitylates and stabilizes Keratin 17 to induce STAT3 nuclear transport in psoriasis. J. Investig. Dermatol. 2018, 138, 2568–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Keller, L.; Pantel, K. Epithelial keratins: Biology and implications as diagnostic markers for liquid biopsies. Mol. Asp. Med. 2020, 72, 100817. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C 2016, 66, 178–184. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y. Development of keratin based hydrogels for biomedical applications. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 702, p. 012031. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Reichl, S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 2009, 30, 6854–6866. [Google Scholar] [CrossRef]
- Zafar, K.; Jamal, S.; Ghafoor, R. Bio-active cements-Mineral Trioxide Aggregate based calcium silicate materials: A narrative review. J. Pak. Med. Assoc. 2020, 70, 497–504. [Google Scholar] [CrossRef]
- Bochynska-Czyz, M.; Redkiewicz, P.; Kozlowska, H.; Matalinska, J.; Konop, M.; Kosson, P. Can keratin scaffolds be used for creating three-dimensional cell cultures? Open Med. 2020, 15, 249–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, K.; Maniwa, M.; Mori, T. Cultivation of fibroblast cells on keratin-coated substrata. J. Biomater. Sci. Polym. Ed. 1998, 9, 259–270. [Google Scholar] [CrossRef]
- Park, M.; Shin, H.K.; Kim, B.-S.; Kim, M.J.; Kim, I.-S.; Park, B.-Y.; Kim, H.-Y. Effect of discarded keratin-based biocomposite hydrogels on the wound healing process in vivo. Mater. Sci. Eng. C 2015, 55, 88–94. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices-regulations: In vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Peplow, P.V.; Dias, G.J. A study of the relationship between mass and physical strength of keratin bars in vivo. J. Mater. Sci. Mater. Med. 2004, 15, 1217–1220. [Google Scholar] [CrossRef]
- Patrucco, A.; Visai, L.; Fassina, L.; Magenes, G.; Tonin, C. Keratin-based matrices from wool fibers and human hair. In Materials for Biomedical Engineering: Biopolymer Fibers; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128168721. [Google Scholar]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng. 2017, 45, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, R.; Rahimi, M.K.; Abbasipour, M.; Brendjchi, A.H. Antibacterial nanofibrous scaffolds with lowered cytotoxicity using keratin extracted from quail feathers. J. Bioact. Compat. Polym. 2016, 31, 60–71. [Google Scholar] [CrossRef]
- Amajuoyi, J.N.; Ilomuanya, M.O.; Asantewaa-Osei, Y.; Azubuike, C.P.; Adeosun, S.O.; Igwilo, C.I. Development of electrospun keratin/coenzyme Q10/poly vinyl alcohol nanofibrous scaffold containing mupirocin as potential dressing for infected wounds. Future J. Pharm. Sci. 2020, 6, 25. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun Silver Nanoparticles-Embedded Feather Keratin/Poly(vinyl alcohol)/Poly(ethylene oxide) Antibacterial Composite Nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, M.; Legadevi, R.; Afrin Banu, N.; Gayathri, V.; Palanisammy, A. A study on anti bacterial activity of keratin nanoparticles from chicken feather waste against Staphylococcus aureus (Bovine Mastitis Bacteria) and its anti oxidant activity. Eur. J. Biotechnol. Biosci. 2015, 3, 1–5. [Google Scholar]
- Gu, L.-H.; Coulombe, P.A. Keratin function in skin epithelia: A broadening palette with surprising shades. Curr. Opin. Cell Biol. 2007, 19, 13–23. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Kerns, M.L.; Fuchs, E. Epidermolysis bullosa simplex: A paradigm for disorders of tissue fragility. J. Clin. Investig. 2009, 119, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uttam, J.; Hutton, E.; Coulombe, P.A.; Anton-Lamprecht, I.; Yu, Q.C.; Gedde-Dahl, T.J.; Fine, J.D.; Fuchs, E. The genetic basis of epidermolysis bullosa simplex with mottled pigmentation. Proc. Natl. Acad. Sci. USA 1996, 93, 9079–9084. [Google Scholar] [CrossRef] [Green Version]
- Betz, R.C.; Planko, L.; Eigelshoven, S.; Hanneken, S.; Pasternack, S.M.; Bussow, H.; Van Den Bogaert, K.; Wenzel, J.; Braun-Falco, M.; Rutten, A.; et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am. J. Hum. Genet. 2006, 78, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugassy, J.; Itin, P.; Ishida-Yamamoto, A.; Holland, K.; Huson, S.; Geiger, D.; Hennies, H.C.; Indelman, M.; Bercovich, D.; Uitto, J.; et al. Naegeli-Franceschetti-Jadassohn syndrome and dermatopathia pigmentosa reticularis: Two allelic ectodermal dysplasias caused by dominant mutations in KRT14. Am. J. Hum. Genet. 2006, 79, 724–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitch, K.R.; McGowan, K.A.; van Raamsdonk, C.D.; Fuchs, H.; Lee, D.; Puech, A.; Hérault, Y.; Threadgill, D.W.; Hrabé de Angelis, M.; Barsh, G.S. Genetics of dark skin in mice. Genes Dev. 2003, 17, 214–228. [Google Scholar] [CrossRef] [Green Version]
- McGowan, K.A.; Aradhya, S.; Fuchs, H.; de Angelis, M.H.; Barsh, G.S. A mouse keratin 1 mutation causes dark skin and epidermolytic hyperkeratosis. J. Investig. Dermatol. 2006, 126, 1013–1016. [Google Scholar] [CrossRef] [Green Version]
- McGowan, K.A.; Fuchs, H.; Hrabé de Angelis, M.; Barsh, G.S. Identification of a Keratin 4 mutation in a chemically induced mouse mutant that models white sponge nevus. J. Investig. Dermatol. 2007, 127, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Omary, M.B.; Coulombe, P.A.; McLean, W.H.I. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004, 351, 2087–2100. [Google Scholar] [CrossRef]
- Loan, F.; Cassidy, S.; Marsh, C.; Simcock, J. Keratin-based products for effective wound care management in superficial and partial thickness burns injuries. Burns 2016, 42, 541–547. [Google Scholar] [CrossRef]
- de Chalain, T.M.; Tang, C.; Thomson, H.G. Burn area color changes after superficial burns in childhood: Can they be predicted? J. Burn Care Rehabil. 1998, 19, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bhawan, J.; Whren, K.; Panova, I.; Yaar, M. Keratin 16 expression in epidermal melanocytes of normal human skin. Am. J. Dermatopathol. 2005, 27, 476–481. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin—Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Konop, M.; Laskowska, A.K.; Rybka, M.; Kłodzińska, E.; Sulejczak, D.; Schwartz, R.A.; Czuwara, J. Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules 2021, 26, 2554. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, B.J.; Lee, Y.; Park, N.J.; Park, K.M.; Hwang, Y.S.; Park, K.D. Human hair keratin-based hydrogels as dynamic matrices for facilitating wound healing. J. Ind. Eng. Chem. 2019, 73, 142–151. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Kan, J.; Deng, J.; Wang, B.; Hao, S. Synthesis and fabrication of a keratin-conjugated insulin hydrogel for the enhancement of wound healing. Colloids Surf. B Biointerfaces 2019, 175, 436–444. [Google Scholar] [CrossRef]
- Poranki, D.; Whitener, W.; Howse, S.; Mesen, T.; Howse, E.; Burnell, J.; Greengauz-Roberts, O.; Molnar, J.; Van Dyke, M. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J. Biomater. Appl. 2014, 29, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, W.; Deng, J.; Kan, J.; Guo, T.; Wang, B.; Hao, S. Recombinant Human Hair Keratin Nanoparticles Accelerate Dermal Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 18681–18690. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, D.; Wang, B.; Hao, S.; Song, J.; Peng, Z. Hair keratin promotes wound healing in rats with combined radiation-wound injury. J. Mater. Sci. Mater. Med. 2020, 31, 28. [Google Scholar] [CrossRef]
- Shanmugasundaram, O.L.; Syed Zameer Ahmed, K.; Sujatha, K.; Ponnmurugan, P.; Srivastava, A.; Ramesh, R.; Sukumar, R.; Elanithi, K. Fabrication and characterization of chicken feather keratin/polysaccharides blended polymer coated nonwoven dressing materials for wound healing applications. Mater. Sci. Eng. C 2018, 92, 26–33. [Google Scholar] [CrossRef]
- Vakilian, S.; Jamshidi-adegani, F.; Al-Shidhani, S.; Anwar, M.U.; Al-Harrasi, R.; Al-Wahaibi, N.; Qureshi, A.; Alyaqoobi, S.; Al-Amri, I.; Al-Harrasi, A.; et al. A Keratin-based biomaterial as a promising dresser for skin wound healing. Wound Med. 2019, 25, 100155. [Google Scholar] [CrossRef]
- Veerasubramanian, P.K.; Thangavel, P.; Kannan, R.; Chakraborty, S.; Ramachandran, B.; Suguna, L.; Muthuvijayan, V. Corrigendum to “An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing” [Colloids Surf. B Biointerfaces 165 (2018) 92–102] (S0927776518300973) (10.1016/j.colsurfb.2018.02.022). Colloids Surf. B Biointerfaces 2018, 171, 319. [Google Scholar] [CrossRef] [PubMed]
- Ponrasu, T.; Veerasubramanian, P.K.; Kannan, R.; Gopika, S.; Suguna, L.; Muthuvijayan, V. Morin incorporated polysaccharide-protein (psyllium-keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv. 2018, 8, 2305–2314. [Google Scholar] [CrossRef] [Green Version]
- Vahidnezhad, H.; Youssefian, L.; Saeidian, A.H.; Mozafari, N.; Barzegar, M.; Sotoudeh, S.; Daneshpazhooh, M.; Isaian, A.; Zeinali, S.; Uitto, J. KRT5 and KRT14 Mutations in Epidermolysis Bullosa Simplex with Phenotypic Heterogeneity, and Evidence of Semidominant Inheritance in a Multiplex Family. J. Investig. Dermatol. 2016, 136, 1897–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khani, P.; Ghazi, F.; Zekri, A.; Nasri, F.; Behrangi, E.; Aghdam, A.M.; Mirzaei, H. Keratins and epidermolysis bullosa simplex. J. Cell. Physiol. 2018, 234, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Than, M.P.; Smith, R.A.; Cassidy, S.; Kelly, R.; Marsh, C.; Maderal, A.; Kirsner, R.S. Use of a keratin-based hydrogel in the management of recessive dystrophic epidermolysis bullosa. J. Dermatol. Treat. 2013, 24, 290–291. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Cassidy, S.; Marsh, C.; Vivas, A.; Kelly, R.J. Use of a Keratin-Based Wound Dressing in the Management of Wounds in a Patient with Recessive Dystrophic Epidermolysis Bullosa. Adv. Skin Wound Care 2012, 25, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Than, M.P.; Smith, R.A.; Hammond, C.; Kelly, R.; Marsh, C.; Maderal, A.D.; Kirsner, R.S. Keratin-based Wound Care Products for Treatment of Resistant Vascular Wounds. J. Clin. Aesthet. Dermatol. 2012, 5, 31–35. [Google Scholar]
- Davidson, A.; Jina, N.H.; Marsh, C.; Than, M.; Simcock, J.W. Do functional keratin dressings accelerate epithelialization in human partial thickness wounds? A randomized controlled trial on skin graft donor sites. Eplasty 2013, 13, e45. [Google Scholar] [PubMed]
- Denyer, J.; Marsh, C.; Kirsner, R.S. Keratin gel in the management of Epidermolysis bullosa. J. Wound Care 2015, 24, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Batzer, A.T.; Marsh, C.; Kirsner, R.S. The use of keratin-based wound products on refractory wounds. Int. Wound J. 2016, 13, 110–115. [Google Scholar] [CrossRef]
- Paulsen, E.; Bygum, A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: A case report. Acta Derm. Venereol. 2019, 99, 234–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Author | Source of Keratin | Type of Keratin Wound Dressing | Healing Rate (p-Value) | Wound Status | |
|---|---|---|---|---|---|
| Control Wound | Dressed Wound | ||||
| Experimental Studies | |||||
| Lin et al. [22] | Human hair | Keratin scaffold seeded with hASCs | p < 0.05. | Injected with 100 μL of PBS + semiocclusive adhesive dressing | Keratin scaffold seeded with hASCs + semiocclusive adhesive dressing |
| Kim et al. [94] | Human hair | Keratin-based hydrogel | p < 0.05. | PBS | Keratin-based hydrogels (100 μL) |
| Li et al. [95] | Human hair | Keratin hydrogel conjugated insulin | p < 0.05. | Untreated | Treated with keratin, insulin, and the Ins-K hydrogels |
| Poranki et al. [96] | Human hair | Keratin-hydrogel | Mice chemical burns model: at days 4 through 16. p < 0.05. Swine thermal burn model: at days 3, 6, and 12. p < 0.05. | Saline (occlusive dressing) and chitosan hydrogel | Keratin-hydrogel |
| Gao et al. [97] | Human hair | Recombinant human hair keratin proteins (RKNP37 and RKNP81) and keratin nanoparticles (KNP) | p < 0.05 on day 7, 14. | Tegaderm film | 0.500 mg of RKNP37, RKNP81, or KNPs and fixed with Tegaderm film |
| Chen et. al. [98] | Human hair | Keratin hydrogel | p < 0.01 (Keratin and irradiated wound versus exposed and irradiated wound), p < 0.05 (Keratin and irradiated wound versus non-keratin non-irradiated wound). | Wounds exposed, one exposed and irradiated | Keratin hydrogel |
| Konop et al. [8] | Mice fur | Keratin scaffolds (FKDP) | p < 0.05. | No dressing | Keratin scaffolds |
| Shanmugasundaram et al. [99] | Chicken feather | Chicken feather keratin (CFK-NW), keratin-sodium alginate (CFK-SA-NW), and keratin-chitosan (CFK-CS-NW) | No available statistical analysis. | Nonwoven fabric | Chicken feather keratin (CFK-NW), keratin-sodium alginate (CFK-SA-NW), and keratin-chitosan (CFK-CS-NW) |
| Vakilian et al. [100] | Sneak shed skin (Puff and Cat Snakes) | Puff snake shed skin (P) Cat snake shed skin (C) | p < 0.05. | No dressing (negative control) Solcoseryl ointment (positive control) | Puff snake shed skin (P) Cat snake shed skin (C) |
| Veerasubramanian et al. [101] | Human hair | Konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts | p < 0.05. | Group I—control, rats dressed in non-medicated cotton gauze | Group II–rats dressed with KGM + KER scaffolds; and Group III–rats dressed with KGM + KER + OAT scaffolds |
| Ponrasu et al. [102] | Human hair | Keratin hydrogel (KER) supplemented with Psyllium seed husk (PSH) or Morin (MOR) | p < 0.05. | Group I—rats dressed in cotton gauze | Group II–rats dressed with PSH + KER scaffolds; group III–rats dressed with PSH + KER + 0.50% MOR scaffolds; group IV–rats dressed with PSH + KER + 1% MOR scaffolds |
| Konop et al. [7] | Mice fur | Keratin scaffolds (FKDP) | p < 0.05. | No dressing | Keratin scaffolds (FKDP) |
| Konop et al. [10] | Mice fur | Keratin scaffolds (FKDP) | p < 0.05. | No dressing | Keratin scaffolds + AgNP (FKDP-AgNP) |
| Konop et al. [93] | Mice fur | Keratin scaffolds (FKDP) | p < 0.05. | No dressing | Keratin scaffolds + 0.1% Casomorphin |
| Author | Source of Keratin | Type of Keratin Wound Dressing | Healing Rate (p-Value) | Wound Status | |
|---|---|---|---|---|---|
| Control Wound | Dressed Wound | ||||
| Than et al. [105] | Sheep’s wool | Keragel®—keratin-based hydrogel | No data | Lack of control site | Keragel® |
| Kirsner et al. [106] | Sheep’s wool | KeragelT®—keratin-enriched gel | No data (healing reduced from 14 to 7 days) | Saline cleansing, soft silicone-based, nonadherent primary dressing, also absorbent foam dressing for the feet, a tubular gauze bandage wrap for hands | KeragelT® |
| Than et al. [107] | Robust matrix dressing derived from freeze-dried keratin protein | No data (venous ulcer completely healed after 30 weeks) | Lack of control site | Keraderm (Blacksburg, Virginia) | |
| Davidson et al. [108] | Sheep’s wool | Keramatrix—absorbable matrix rich in keratin protein | No data | Alginate dressing | Keramatrix |
| Dayner et al. [109] | Sheep’s wool | KeragelT®—keratin-enriched gel | No data | Lack of control site | KeragelT® |
| Batzer et al. [110] | Sheep’s wool | Keramatrix, Keragel® | No data | Lack of control site | Keragel®, Keramatrix |
| Paulsen and Bygum [111] | Sheep’s wool | Keragel® | No data | Lack of control site | Keragel® |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. https://doi.org/10.3390/pharmaceutics13122029
Konop M, Rybka M, Drapała A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics. 2021; 13(12):2029. https://doi.org/10.3390/pharmaceutics13122029
Chicago/Turabian StyleKonop, Marek, Mateusz Rybka, and Adrian Drapała. 2021. "Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review" Pharmaceutics 13, no. 12: 2029. https://doi.org/10.3390/pharmaceutics13122029
APA StyleKonop, M., Rybka, M., & Drapała, A. (2021). Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics, 13(12), 2029. https://doi.org/10.3390/pharmaceutics13122029