Ultrasound and Microbubbles Enhance Uptake of Doxorubicin in Murine Kidneys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Doxorubicin and Maximum Tolerated Dose
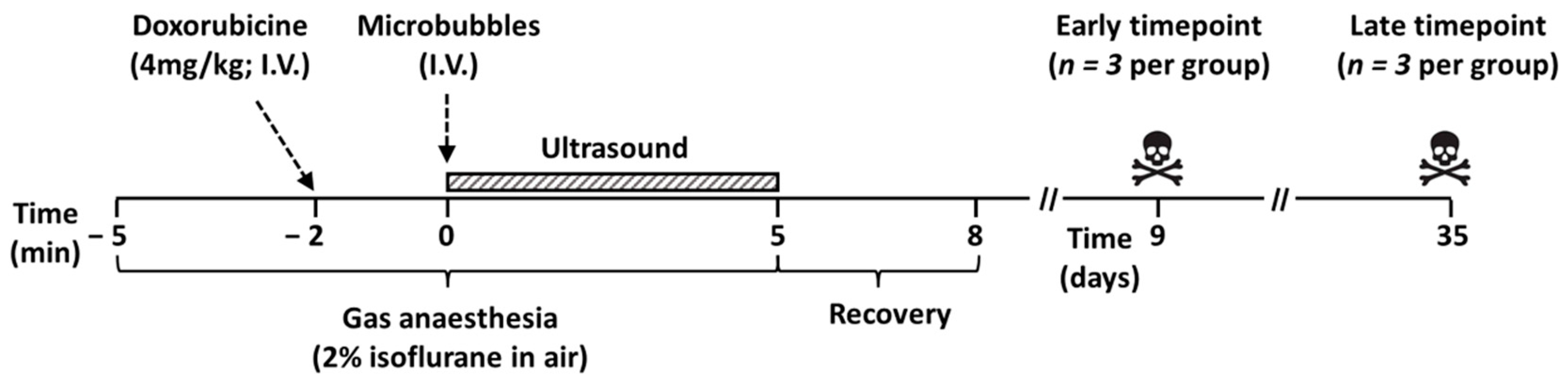
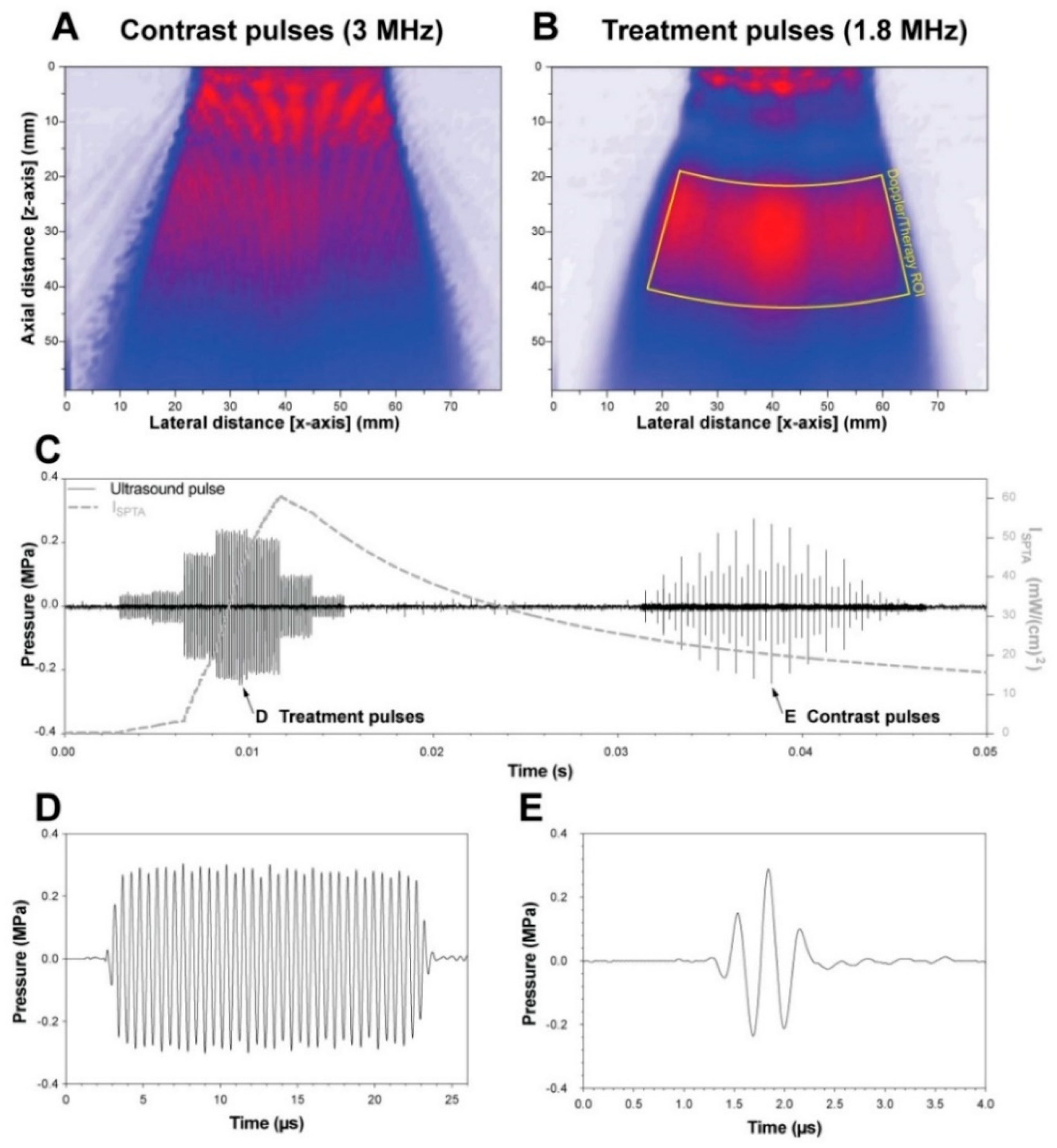
2.3. Ultrasound and Microbubble Treatment
2.4. Toxicity Evaluation
2.5. Drug Delivery and Uptake: Doxorubicin and Doxorubicinol Measurements
2.6. Histology
2.7. Kidney Function
2.8. Statistical Analyses
3. Results and Discussion
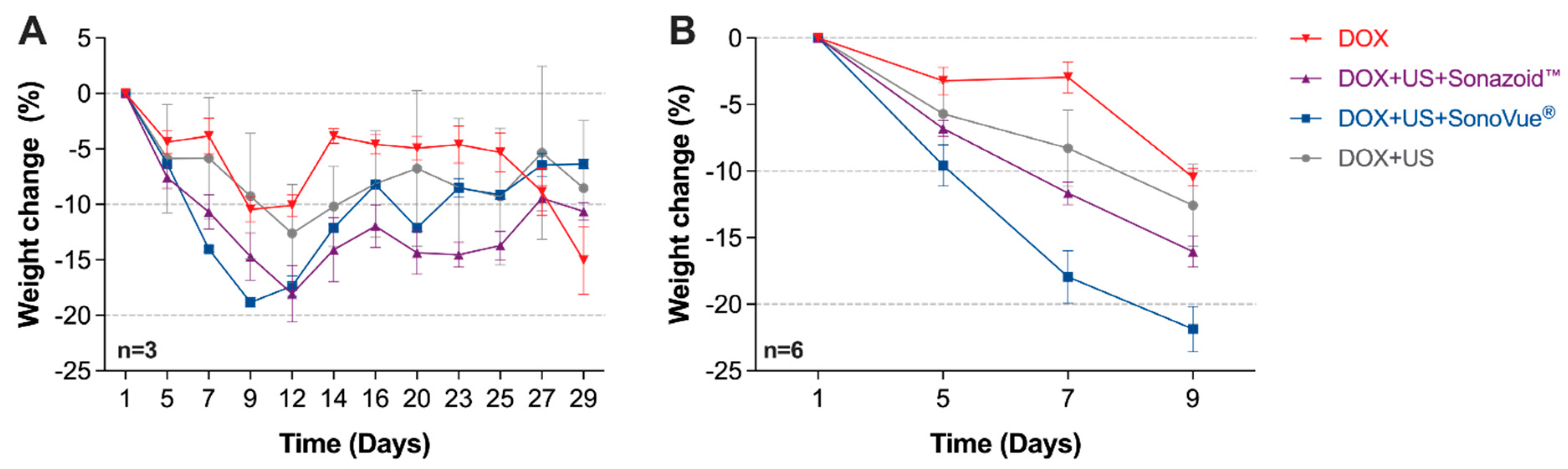
3.1. Body Weight
3.2. Doxorubicin Uptake and Its Metabolite
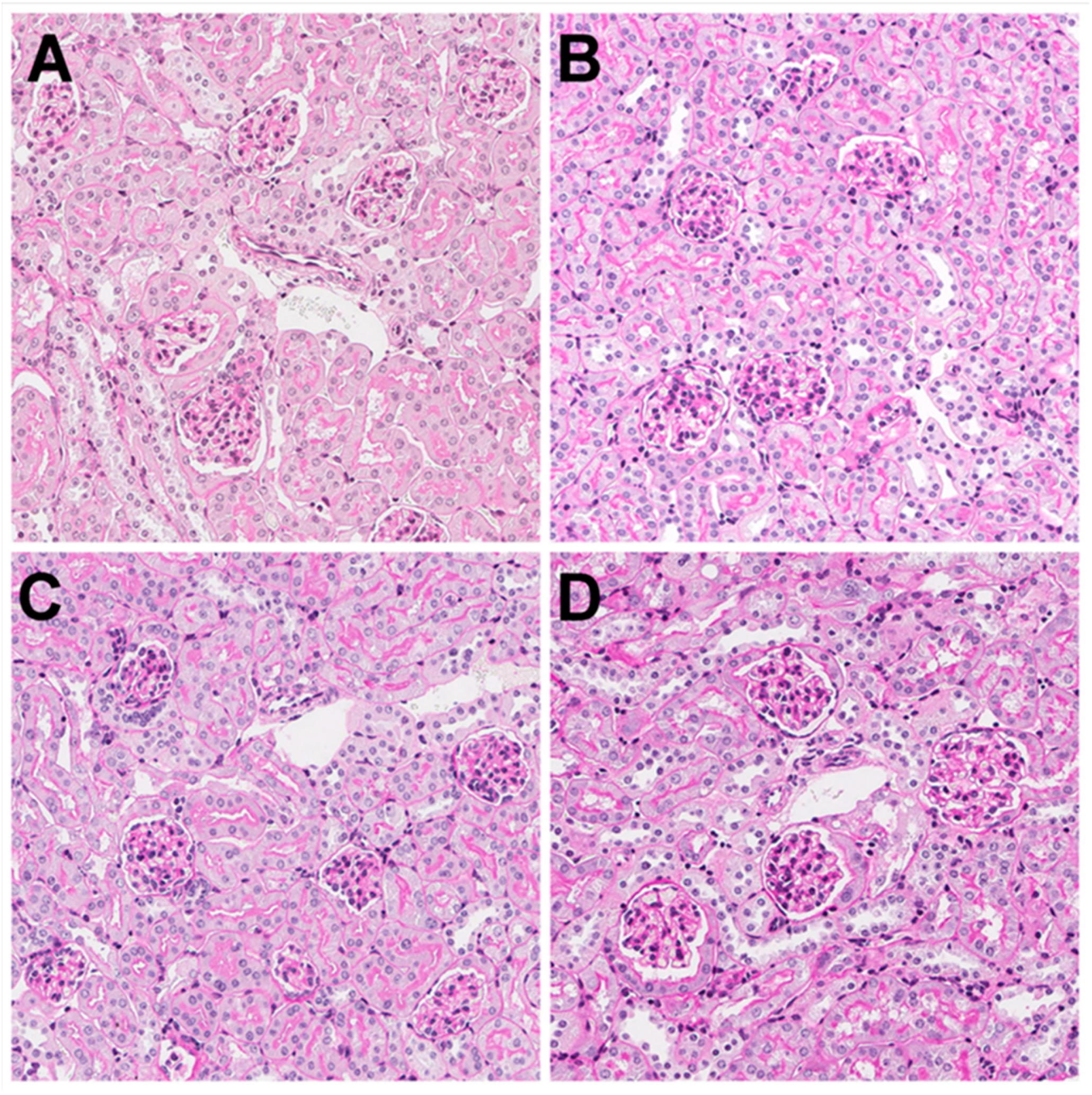
3.3. Kidney Histology
3.4. Kidney Function
3.5. Study Limitations and Future Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.L.; Quddus, J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med. Biol. 2000, 26, 661–667. [Google Scholar] [CrossRef]
- Derieppe, M.; Rojek, K.; Escoffre, J.M.; de Senneville, B.D.; Moonen, C.; Bos, C. Recruitment of endocytosis in sonopermeabilization-mediated drug delivery: A real-time study. Phys. Biol. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lammertink, B.H.; Bos, C.; van der Wurff-Jacobs, K.M.; Storm, G.; Moonen, C.T.; Deckers, R. Increase of intracellular cisplatin levels and radiosensitization by ultrasound in combination with microbubbles. J. Control. Release 2016, 238, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; Geers, B.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Mol. Ther. 2010, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Baues, M.; Ojha, T.; Mockel, D.; Veettil, S.K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J. Control. Release 2016, 231, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Van Wamel, A.; Sontum, P.C.; Healey, A.; Kvale, S.; Bush, N.; Bamber, J.; de Lange Davies, C. Acoustic cluster therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane(R) for treatment of human prostate adenocarcinoma in mice. J. Control. Release 2016, 236, 15–21. [Google Scholar] [CrossRef]
- Phenix, C.P.; Togtema, M.; Pichardo, S.; Zehbe, I.; Curiel, L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J. Pharm. Pharm. Sci. 2014, 17, 136–153. [Google Scholar] [CrossRef]
- Shapiro, G.; Wong, A.W.; Bez, M.; Yang, F.; Tam, S.; Even, L.; Sheyn, D.; Ben-David, S.; Tawackoli, W.; Pelled, G.; et al. Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation. J. Control. Release 2016, 223, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Postema, M.; Kotopoulis, S.; Delalande, A.; Gilja, O.H. Sonoporation: Why microbubbles create pores. Ultraschall Med. Georg. Thieme Verl. 2012, 33, 97–98. [Google Scholar]
- Beekers, I.; Vegter, M.; Lattwein, K.R.; Mastik, F.; Beurskens, R.; van der Steen, A.F.W.; de Jong, N.; Verweij, D.M.; Kooiman, K. Opening of endothelial cell-cell contacts due to sonoporation. J. Control. Release 2020, 322, 426–438. [Google Scholar] [CrossRef]
- Qin, P.; Han, T.; Yu, A.C.H.; Xu, L. Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery. J. Control. Release 2018, 272, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Delalande, A.; Kotopoulis, S.; Rovers, T.; Pichon, C.; Postema, M. Sonoporation at a low mechanical index. Bubble Sci. Eng. Technol. 2011, 3, 3–12. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjanes, T.; Hoem, D.; Schjott, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Hull, T.D.; Agarwal, A.; Hoyt, K. New ultrasound techniques promise further advances in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.S.; Lee, D.C.H. Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology 2010, 16, 30–38. [Google Scholar]
- Hole, L.D.; Larsen, T.H.; Fossan, K.O.; Lime, F.; Schjott, J. A short-time model to study relevant indices of cardiotoxicity of doxorubicin in the rat. Toxicol. Mech. Methods 2013, 23, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotopoulis, S.; Delalande, A.; Popa, M.; Mamaeva, V.; Dimcevski, G.; Gilja, O.H.; Postema, M.; Gjertsen, B.T.; McCormack, E. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol. Imaging Biol. 2014, 16, 53–62. [Google Scholar] [CrossRef]
- Song, K.H.; Trudeau, T.; Kar, A.; Borden, M.A.; Gutierrez-Hartmann, A. Ultrasound-mediated delivery of siESE complexed with microbubbles attenuates HER2+/- cell line proliferation and tumor growth in rodent models of breast cancer. Nanotheranostics 2019, 3, 212–222. [Google Scholar] [CrossRef]
- Patel, K.J.; Tredan, O.; Tannock, I.F. Distribution of the anticancer drugs doxorubicin, mitoxantrone and topotecan in tumors and normal tissues. Cancer Chemother. Pharmacol. 2013, 72, 127–138. [Google Scholar] [CrossRef]
- Pippin, J.W.; Brinkkoetter, P.T.; Cormack-Aboud, F.C.; Durvasula, R.V.; Hauser, P.V.; Kowalewska, J.; Krofft, R.D.; Logar, C.M.; Marshall, C.B.; Ohse, T.; et al. Inducible rodent models of acquired podocyte diseases. Am. J. Physiol. Renal. Physiol. 2009, 296, F213–F229. [Google Scholar] [CrossRef]
- Duck, F.A. Physical Properties of Tissue: A Comprehensive Reference Book: Acoustic Properties of Tissue at Ultrasonic Frequencies; Academic Press Limited: London, UK, 1990. [Google Scholar]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. Characteristics of SonoVuetrade mark. Echocardiography 1999, 16, 743–746. [Google Scholar] [CrossRef]
- Tu, J.; Guan, J.; Qiu, Y.; Matula, T.J. Estimating the shell parameters of SonoVue microbubbles using light scattering. J. Acoust. Soc. Am. 2009, 126, 2954–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontum, P.C. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med. Biol. 2008, 34, 824–833. [Google Scholar] [CrossRef]
- Paul, S.; Katiyar, A.; Sarkar, K.; Chatterjee, D.; Shi, W.T.; Forsberg, F. Material characterization of the encapsulation of an ultrasound contrast microbubble and its subharmonic response: Strain-softening interfacial elasticity model. J. Acoust. Soc. Am. 2010, 127, 3846–3857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, C.K.; Adamo, B.; Karginova, O.; Deal, A.M.; Rawal, S.; Darr, D.; Schorzman, A.; Santos, C.; Bash, R.; Tafri, T.; et al. Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer. PLoS ONE 2013, 8, e61359. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jeon, H.; Shim, S.; Im, M.; Kim, J.; Kim, J.H.; Lee, B.C. Preclinical study to improve microbubble-mediated drug delivery in cancer using an ultrasonic probe with an interchangeable acoustic lens. Sci. Rep. 2021, 11, 12654. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.G.; Mima, T.; Ohnishi, S.T.; Mori, K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000, 66, 148–151. [Google Scholar] [CrossRef]
- Olson, L.E.; Bedja, D.; Alvey, S.J.; Cardounel, A.J.; Gabrielson, K.L.; Reeves, R.H. Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res. 2003, 63, 6602–6606. [Google Scholar]
- Chapman, K.; Sewell, F.; Allais, L.; Delongeas, J.L.; Donald, E.; Festag, M.; Kervyn, S.; Ockert, D.; Nogues, V.; Palemr, H.; et al. A global pharmaceutical company initiative: An evidence-based approach to define the upper limit of body weight loss in short term toxicity studies. Regul. Toxicol. Pharmacol. 2013, 67, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Schloss, J.A. How to get genomes at one ten-thousandth the cost. Nat. Biotechnol. 2008, 26, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Traverso, G. Low-frequency ultrasound for drug delivery in the gastrointestinal tract. Expert Opin. Drug Deliv. 2016, 13, 1045–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zeng, D.; Wang, Z.; Fang, L.; Li, F.; Wang, Z. Ultrasound-enhanced delivery of doxorubicin/all-trans retinoic acid-loaded nanodiamonds into tumors. Nanomedicine 2018, 13, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kaddur, K.; Lebegue, L.; Tranquart, F.; Midoux, P.; Pichon, C.; Bouakaz, A. Transient transmembrane release of green fluorescent proteins with sonoporation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, N.; Kimmel, E.; Tsarfaty, I.K. The effect of low-intensity ultrasound and met signaling on cellular motility and morphology. Appl. Acoust. 2019, 143, 1–6. [Google Scholar] [CrossRef]
- Rubin, D.M.; Anderton, N.; Smalberger, C.; Polliack, J.; Nathan, M.; Postema, M. On the behaviour of living cells under the influence of ultrasound. Fluids 2018, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Snipstad, S.; Sulheim, E.; de Lange Davies, C.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to improve drug delivery to tumors: From fundamental understanding to clinical translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lin, L.; Cheng, M.; Jin, L.; Du, L.; Han, T.; Xu, L.; Yu, A.C.H.; Qin, P. Effect of acoustic parameters on the cavitation behavior of SonoVue microbubbles induced by pulsed ultrasound. Ultrason. Sonochem. 2017, 35, 176–184. [Google Scholar] [CrossRef]
- Chen, W.S.; Matula, T.J.; Brayman, A.A.; Crum, L.A. A comparison of the fragmentation thresholds and inertial cavitation doses of different ultrasound contrast agents. J. Acoust. Soc. Am. 2003, 113, 643–651. [Google Scholar] [CrossRef]
- Schultz, C.W.; Ruiz de Garibay, G.; Langer, A.; Liu, J.B.; Dhir, T.; Leitch, C.; Wessner, C.; Mayoral, M.; Zhang, B.; Popa, M.; et al. Selecting the optimal parameters for sonoporation of pancreatic cancer in a pre-clinical model. Cancer Biol. Ther. 2021, 22, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Nejad, S.M.; Hosseini, H.; Akiyama, H.; Tachibana, K. Reparable cell sonoporation in suspension: Theranostic potential of microbubble. Theranostics 2016, 6, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Aron, M.; Vince, O.; Gray, M.; Mannaris, C.; Stride, E. Investigating the role of lipid transfer in microbubble-mediated drug delivery. Langmuir 2019, 35, 13205–13215. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.B. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother. Pharmacol. 1981, 5, 267–270. [Google Scholar] [CrossRef]
- Cusack, B.J.; Young, S.P.; Driskell, J.; Olson, R.D. Doxorubicin and doxorubicinol pharmacokinetics and tissue concentrations following bolus injection and continuous infusion of doxorubicin in the rabbit. Cancer Chemother. Pharmacol. 1993, 32, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; van Tellingen, O.; Tijssen, F.; Schinkel, A.H.; Beijnen, J.H. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br. J. Cancer 1999, 79, 108–113. [Google Scholar] [CrossRef]



| Posttreatment Analysis | ||
|---|---|---|
| Treatment Regimen | Day 9 (Early Time Point) n = 3 | Day 35 (Late Time Point) n = 3 |
| DOX (n = 6) |
|
|
| US + DOX (n = 6) | ||
| US + DOX + SonazoidTM (n = 6) | ||
| US + DOX + SonoVue® (n = 6) | ||
| Microbubble | Manufacturer | Stock Concentration (×108 ppmL) | Mean Diameter (µm) | Resonance Frequency (MHz) | Shell Elasticity (Nm−1) |
|---|---|---|---|---|---|
| SonoVue® | Bracco Imaging S.p.A | 2.5 [23] | 2.5 [23] | 3.0 [23] | 0.22 [24] |
| Sonazoid™ | GE Healthcare | 12.0 [25] | 2.1 [25] | 4.3 [25] | 0.53 [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eikrem, O.; Kotopoulis, S.; Popa, M.; Mayoral Safont, M.; Fossan, K.O.; Leh, S.; Landolt, L.; Babickova, J.; Gudbrandsen, O.A.; Gilja, O.H.; et al. Ultrasound and Microbubbles Enhance Uptake of Doxorubicin in Murine Kidneys. Pharmaceutics 2021, 13, 2038. https://doi.org/10.3390/pharmaceutics13122038
Eikrem O, Kotopoulis S, Popa M, Mayoral Safont M, Fossan KO, Leh S, Landolt L, Babickova J, Gudbrandsen OA, Gilja OH, et al. Ultrasound and Microbubbles Enhance Uptake of Doxorubicin in Murine Kidneys. Pharmaceutics. 2021; 13(12):2038. https://doi.org/10.3390/pharmaceutics13122038
Chicago/Turabian StyleEikrem, Oystein, Spiros Kotopoulis, Mihaela Popa, Mireia Mayoral Safont, Kjell Ove Fossan, Sabine Leh, Lea Landolt, Janka Babickova, Oddrun Anita Gudbrandsen, Odd Helge Gilja, and et al. 2021. "Ultrasound and Microbubbles Enhance Uptake of Doxorubicin in Murine Kidneys" Pharmaceutics 13, no. 12: 2038. https://doi.org/10.3390/pharmaceutics13122038






