Spray-Dried Powder Formulation of Capreomycin Designed for Inhaled Tuberculosis Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray-Dried Powders
2.3. Drug Content
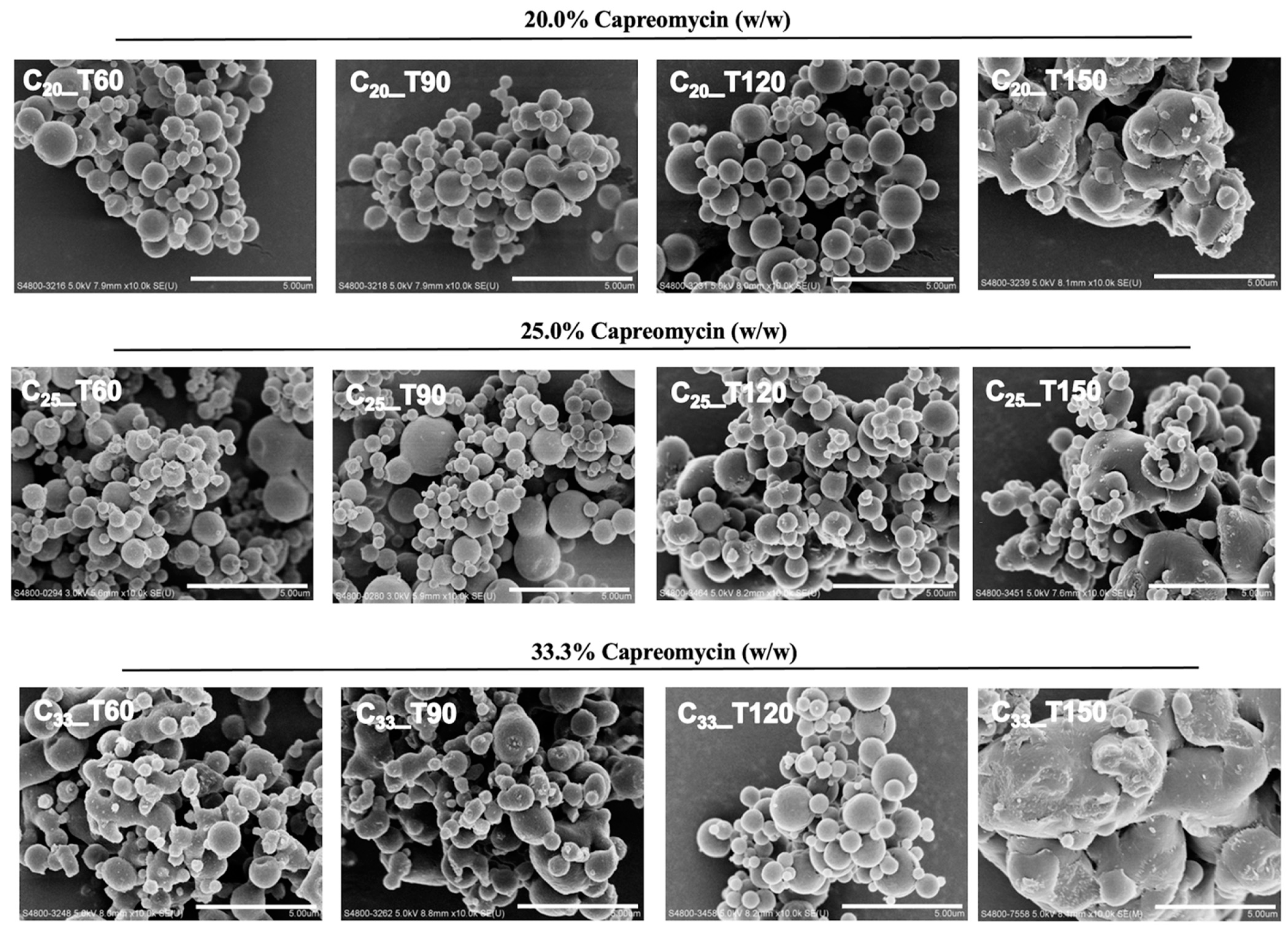
2.4. Morphology Study
2.5. Particle Size Distribution
2.6. In Vitro Aerosol Performance
2.7. Thermogravimetric Analysis (TGA)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Powder X-ray Diffraction (PXRD)
2.10. X-ray Photoelectron Spectroscopy (XPS)
2.11. Animal Study
2.12. Pharmacokinetic Study
2.13. Extraction of Capreomycin
2.14. Data Analysis
3. Results
3.1. Production Yield of Spray Drying
3.2. Drug Content and Residual Moisture
3.3. Particle Morphology
3.4. Particle Size Distribution
3.5. In Vitro Aerosol Performance
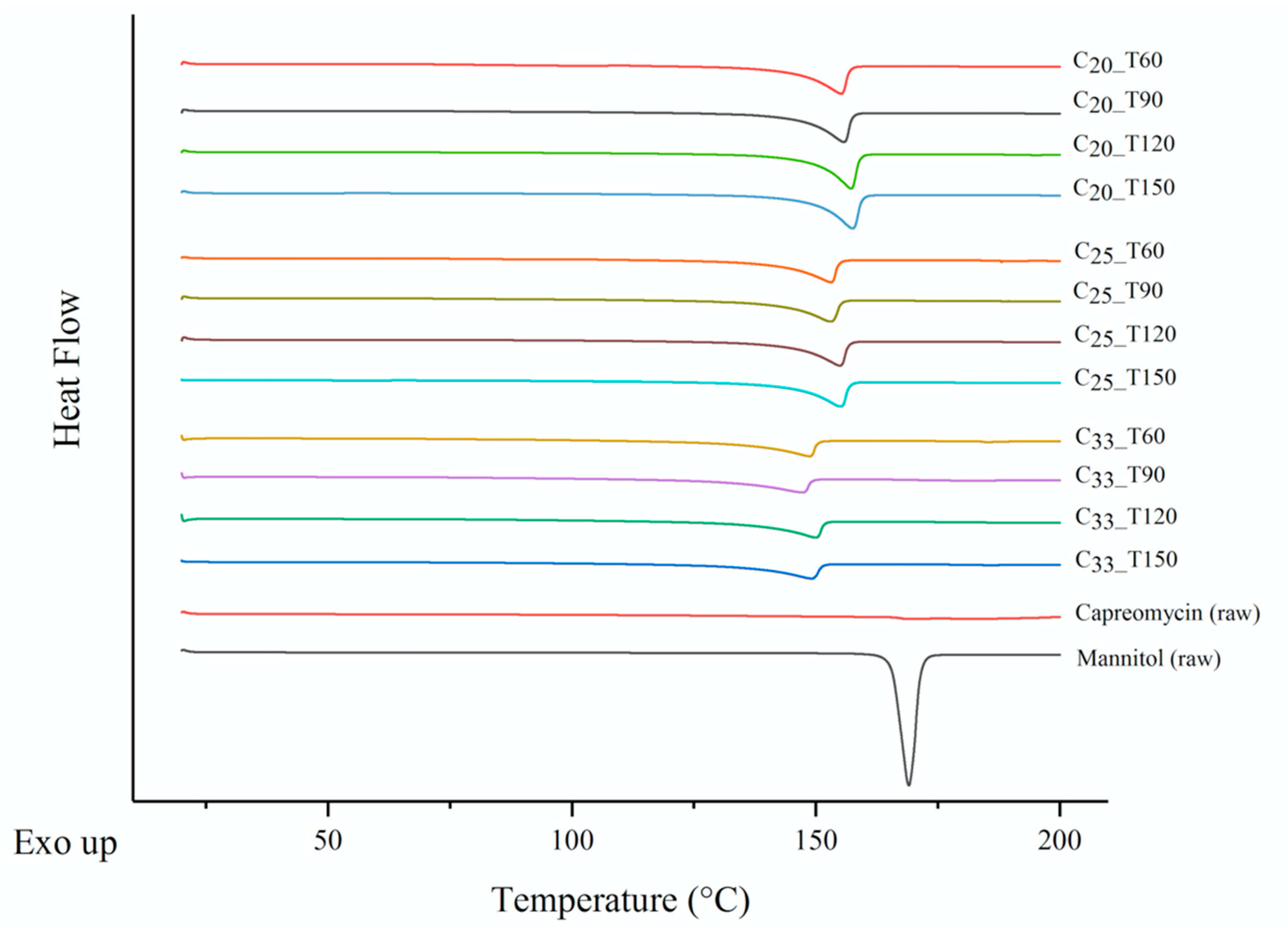
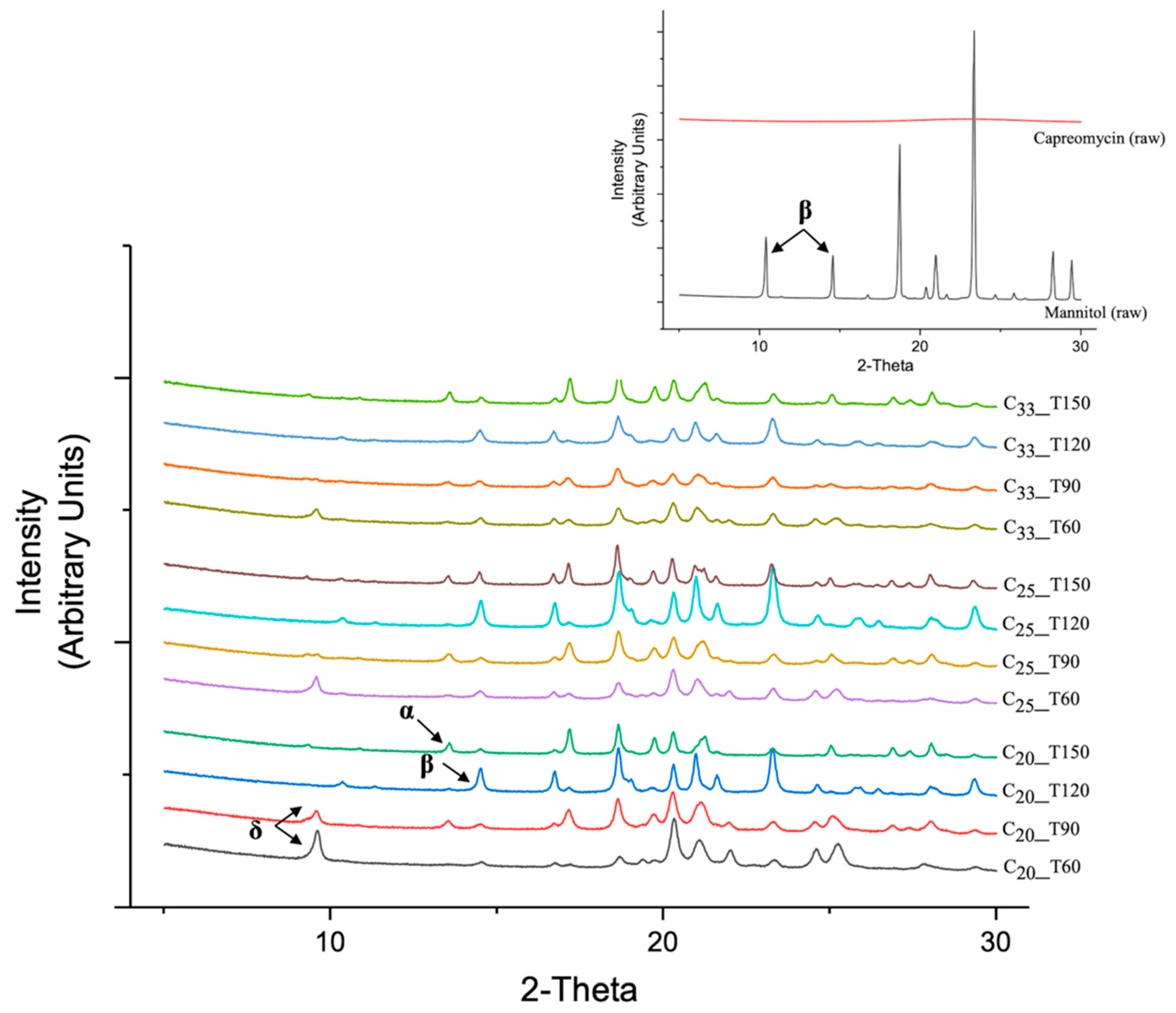
3.6. Thermoanalysis and Powder Crystallinity
3.7. Surface Composition
3.8. Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; p. 232. [Google Scholar]
- Garcia-Contreras, L.; Muttil, P.; Fallon, J.K.; Kabadi, M.; Gerety, R.; Hickey, A.J. Pharmacokinetics of Sequential Doses of Capreomycin Powder for Inhalation in Guinea Pigs. Antimicrob. Agents Chemother. 2012, 56, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Luan, B.; Liang, G.; Xing, L.; Huang, L.; Wang, C.; Xu, Y. Isolation and identification of four major impurities in capreomycin sulfate. J. Chromatogr. A 2018, 1571, 155–164. [Google Scholar] [CrossRef]
- Johansen, S.K.; Maus, C.E.; Plikaytis, B.B.; Douthwaite, S. Capreomycin binds across the ribosomal subunit interface using tlyA-encoded 2′-O-methylations in 16S and 23S rRNAs. Mol. Cell 2006, 23, 173–182. [Google Scholar] [CrossRef]
- Fu, L.M.; Shinnick, T.M. Genome-wide exploration of the drug action of capreomycin on Mycobacterium tuberculosis using Affymetrix oligonucleotide GeneChips. J. Infect. 2007, 54, 277–284. [Google Scholar] [CrossRef]
- Reisfeld, B.; Metzler, C.P.; Lyons, M.A.; Mayeno, A.N.; Brooks, E.J.; DeGroote, M.A. A Physiologically Based Pharmacokinetic Model for Capreomycin. Antimicrob. Agents Chemother. 2012, 56, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Manion, J.A.R.; Cape, S.P.; McAdams, D.H.; Rebits, L.G.; Evans, S.; Sievers, R.E. Inhalable Antibiotics Manufactured Through Use of Near-Critical or Supercritical Fluids. Aerosol Sci. Technol. 2012, 46, 403–410. [Google Scholar] [CrossRef]
- Pitner, R.A.; Durham, P.G.; Stewart, I.E.; Reed, S.G.; Cassell, G.H.; Hickey, A.J.; Carter, D. A Spray-Dried Combination of Capreomycin and CPZEN-45 for Inhaled Tuberculosis Therapy. J. Pharm. Sci. 2019, 108, 3302–3311. [Google Scholar] [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Blasi, P.; Vescovi, C.; Fardella, G.; Chiappini, I.; Perioli, L.; Ricci, M.; Rossi, C. Unilamellar vesicles as potential capreomycin sulfate carriers: Preparation and physicochemical characterization. AAPS PharmSciTech 2004, 4, 549–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoubben, A.; Blasi, P.; Giovagnoli, S.; Ricci, M.; Rossi, C. Simple and scalable method for peptide inhalable powder production. Eur. J. Pharm. Sci. 2010, 39, 53–58. [Google Scholar] [CrossRef]
- Garcia-Contreras, L.; Fiegel, J.; Telko, M.J.; Elbert, K.; Hawi, A.; ThomaS, A.; VerBerkmoes, J.; Germishuizen, W.A.; Fourie, P.B.; Hickey, A.J.; et al. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob. Agents Chemother. 2007, 51, 2830–2836. [Google Scholar] [CrossRef] [Green Version]
- Fiegel, J.; Garcia-Contreras, L.; Thomas, M.; VerBerkmoes, J.; Elbert, K.; Hickey, A.; Edwards, D. Preparation and in vivo evaluation of a dry powder for inhalation of capreomycin. Pharm. Res. 2008, 25, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, A.S.; Kabadi, M.; Gerety, B.; Hickey, A.J.; Fourie, P.B.; Nardell, E. Phase I, Single-Dose, Dose-Escalating Study of Inhaled Dry Powder Capreomycin: A New Approach to Therapy of Drug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2613–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoubben, A.; Giovagnoli, S.; Tiralti, M.C.; Blasi, P.; Ricci, M. Capreomycin inhalable powders prepared with an innovative spray-drying technique. Int. J. Pharm. 2014, 469, 132–139. [Google Scholar] [CrossRef]
- Parumasivam, T.; Chang, R.Y.; Abdelghany, S.; Ye, T.T.; Britton, W.J.; Chan, H.K. Dry powder inhalable formulations for anti-tubercular therapy. Adv. Drug Deliv. Rev. 2016, 102, 83–101. [Google Scholar] [CrossRef]
- Schoubben, A.; Blasi, P.; Giontella, A.; Giovagnoli, S.; Ricci, M. Powder, capsule and device: An imperative ménage à trois for respirable dry powders. Int. J. Pharm. 2015, 494, 40–48. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Qiu, Y.; Lo, F.F.K.; Lin, H.H.S.; Chan, H.-K.; Kwok, P.C.L.; Lam, J.K.W. Inhaled powder formulation of naked siRNA using spray drying technology with l-leucine as dispersion enhancer. Int. J. Pharm. 2017, 530, 40–52. [Google Scholar] [CrossRef]
- Qiu, Y.; Man, R.C.H.; Liao, Q.; Kung, K.L.K.; Chow, M.Y.T.; Lam, J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release 2019, 314, 102–115. [Google Scholar] [CrossRef]
- Liao, Q.; Lam, I.C.H.; Lin, H.H.S.; Wan, L.T.L.; Lo, J.C.K.; Tai, W.; Kwok, P.C.L.; Lam, J.K.W. Effect of formulation and inhaler parameters on the dispersion of spray freeze dried voriconazole particles. Int. J. Pharm. 2020, 584, 119444. [Google Scholar] [CrossRef]
- Liang, W.; Chow, M.Y.T.; Chow, S.F.; Chan, H.-K.; Kwok, P.C.L.; Lam, J.K.W. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. Int. J. Pharm. 2018, 552, 67–75. [Google Scholar] [CrossRef]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Erdoğan, A.; Esen, M.; Simpson, R. Chemical Imaging of Human Fingermark by X-ray Photoelectron Spectroscopy (XPS). J. Forensic Sci. 2020, 65, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Corby, S.; Tecedor, M.-G.; Tengeler, S.; Steinert, C.; Moss, B.; Mesa, C.A.; Heiba, H.F.; Wilson, A.A.; Kaiser, B.; Jaegermann, W.; et al. Separating bulk and surface processes in NiOx electrocatalysts for water oxidation. Sustain. Energy Fuels 2020, 4, 5024–5030. [Google Scholar] [CrossRef]
- Qiu, Y.S.; Liao, Q.Y.; Chow, M.Y.T.; Lam, J.K.W. Intratracheal Administration of Dry Powder Formulation in Mice. Jove J. Vis. Exp. 2020, 161, e61469. [Google Scholar] [CrossRef]
- Whitesell, J.K. The Merck Index, 12th Edition, CD-ROM (Macintosh): An encyclopedia of chemicals, drugs & biologicals. J. Am. Chem. Soc. 1998, 120, 2209. [Google Scholar] [CrossRef]
- Rim, P.B.; Runt, J.P. Melting point depression in crystalline/compatible polymer blends. Macromolecules 1984, 17, 1520–1526. [Google Scholar] [CrossRef]
- Hulse, W.L.; Forbes, R.T.; Bonner, M.C.; Getrost, M. The characterization and comparison of spray-dried mannitol samples. Drug Dev. Ind. Pharm. 2009, 35, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Henck, J.O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stottner, H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
- Smith, R.R.; Shah, U.V.; Parambil, J.V.; Burnett, D.J.; Thielmann, F.; Heng, J.Y. The Effect of Polymorphism on Surface Energetics of D-Mannitol Polymorphs. AAPS J. 2017, 19, 103–109. [Google Scholar] [CrossRef]
- Hickey, A.J.; Durham, P.G.; Dharmadhikari, A.; Nardell, E.A. Inhaled drug treatment for tuberculosis: Past progress and future prospects. J. Control. Release 2016, 240, 127–134. [Google Scholar] [CrossRef]
- Muraoka, Y.; Hayashi, Y.; Minesita, T. Studies of capreomycin nephrotoxicity. Toxicol. Appl. Pharmacol. 1968, 12, 350–359. [Google Scholar] [CrossRef]
- Shibeshi, W.; Sheth, A.N.; Admasu, A.; Berha, A.B.; Negash, Z.; Yimer, G. Nephrotoxicity and ototoxic symptoms of injectable second-line anti-tubercular drugs among patients treated for MDR-TB in Ethiopia: A retrospective cohort study. BMC Pharmacol. Toxicol. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Arpagaus, C.; Meuri, M. Laboratory scale Spray drying of inhalable particles: A review. In Proceedings of the Respiratory Drug Delivery, Orlando, FL, USA, 25–29 April 2010; pp. 469–476. [Google Scholar]
- Momin, M.A.M.; Tucker, I.G.; Das, S.C. High dose dry powder inhalers to overcome the challenges of tuberculosis treatment. Int. J. Pharm. 2018, 550, 398–417. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; Grabarek, A.; Chow, M.Y.T.; Lan, Y.; Li, J.C.W.; Casettari, L.; Mason, A.J.; Lam, J.K.W. Inhalable spray-dried formulation of D-LAK antimicrobial peptides targeting tuberculosis. Int. J. Pharm. 2015, 491, 367–374. [Google Scholar] [CrossRef]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef]
- Weers, J.G.; Miller, D.P.; Tarara, T.E. Spray-Dried PulmoSphere Formulations for Inhalation Comprising Crystalline Drug Particles. AAPS PharmSciTech 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Saint-Lorant, G.; Leterme, P.; Gayot, A.; Flament, M.P. Influence of carrier on the performance of dry powder inhalers. Int. J. Pharm. 2007, 334, 85–91. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Kramek-Romanowska, K.; Odziomek, M.; Sosnowski, T.R.; Gradoń, L. Effects of Process Variables on the Properties of Spray-Dried Mannitol and Mannitol/Disodium Cromoglycate Powders Suitable for Drug Delivery by Inhalation. Ind. Eng. Chem. Res. 2011, 50, 13922–13931. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Young, P.M.; Price, R. The influence of humidity on the aerosolisation of micronised and SEDS produced salbutamol sulphate. Eur. J. Pharm. Sci. 2004, 22, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Littringer, E.M.; Paus, R.; Mescher, A.; Schroettner, H.; Walzel, P.; Urbanetz, N.A. The morphology of spray dried mannitol particles—The vital importance of droplet size. Powder Technol. 2013, 239, 162–174. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Nguyen, P.-A.; Sweeney, T.; Shire, S.J.; Hsu, C.C. Protein Inhalation Powders: Spray Drying vs Spray Freeze Drying. Pharm. Res. 1999, 16, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A. Particle size and density in spray drying—effects of carbohydrate properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A.; Alderborn, G.; Elofsson, U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 2003, 92, 900–910. [Google Scholar] [CrossRef]
- Grohganz, H.; Lee, Y.Y.; Rantanen, J.; Yang, M. The influence of lysozyme on mannitol polymorphism in freeze-dried and spray-dried formulations depends on the selection of the drying process. Int. J. Pharm. 2013, 447, 224–230. [Google Scholar] [CrossRef]
- Naini, V.; Byron, P.R.; Phillips, E.M. Physicochemical Stability of Crystalline Sugars and Their Spray-Dried Forms: Dependence upon Relative Humidity and Suitability for Use in Powder Inhalers. Drug Dev. Ind. Pharm. 1998, 24, 895–909. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A.V. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Donald, P.R.; McIlleron, H. Antituberculosis Drugs. In Tuberculosis; Schaaf, H.S., Zumla, A.I., Grange, J.M., Raviglione, M.C., Yew, W.W., Starke, J.R., Pai, M., Donald, P.R., Eds.; W.B. Saunders: Edinburgh, UK, 2009; pp. 608–617. [Google Scholar]
- Muttil, P.; Wang, C.; Hickey, A.J. Inhaled drug delivery for tuberculosis therapy. Pharm. Res. 2009, 26, 2401–2416. [Google Scholar] [CrossRef]
- Wolf, A.J.; Linas, B.; Trevejo-Nunez, G.J.; Kincaid, E.; Tamura, T.; Takatsu, K.; Ernst, J.D. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo. J. Immunol. 2007, 179, 2509–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]

| Sample Name | Capreomycin: Mannitol Ratio (w/w) | Capreomycin Percentage by Mass | Inlet Temperature (°C) |
|---|---|---|---|
| C20_T60 | 1:4 | 20.0 | 60 |
| C20_T90 | 90 | ||
| C20_T120 | 120 | ||
| C20_T150 | 150 | ||
| C25_T60 | 1:3 | 25.0 | 60 |
| C25_T90 | 90 | ||
| C25_T120 | 120 | ||
| C25_T150 | 150 | ||
| C33_T60 | 1:2 | 33.3 | 60 |
| C33_T90 | 90 | ||
| C33_T120 | 120 | ||
| C33_T150 | 150 | ||
| C50_T60 | 1:1 | 50.0 | 60 |
| C50_T90 | 90 | ||
| C50_T120 | 120 | ||
| C50_T150 | 150 |
| Sample Name | Outlet Temperature (°C) | Production Yield (%, w/w) | Drug Content (%, w/w) | Residual Moisture (%, w/w) |
|---|---|---|---|---|
| C20_T60 | 36–38 | 53.0 | 19.2 ± 0.4 | 2.2 |
| C20_T90 | 52–55 | 69.9 | 18.7 ± 1.4 | 1.2 |
| C20_T120 | 70–74 | 66.9 | 19.8 ± 0.3 | 1.6 |
| C20_T150 | 90–95 | 63.3 | 19.0 ± 0.6 | 1.4 |
| C25_T60 | 35–39 | 47.3 | 25.5 ± 0.3 | 2.3 |
| C25_T90 | 52–56 | 65.7 | 24.9 ± 0.6 | 0.7 |
| C25_T120 | 68–71 | 78.1 | 26.7 ± 0.4 | 1.5 |
| C25_T150 | 83–86 | 63.9 | 24.5 ± 0.8 | 0.5 |
| C33_T60 | 34–37 | 18.2 | 33.2 ± 0.6 | 3.1 |
| C33_T90 | 53–56 | 25.8 | 33.6 ± 0.4 | 2.8 |
| C33_T120 | 68–70 | 52.5 | 34.4 ± 0.8 | 2.0 |
| C33_T150 | 88–92 | 39.1 | 34.1 ± 0.2 | 2.8 |
| C50_T60 | 34–37 | 1.0 | N.A. | N.A. |
| C50_T90 | 52–57 | 6.9 | N.A. | N.A. |
| C50_T120 | 66–68 | 4.3 | N.A. | N.A. |
| C50_T150 | 84–87 | 7.2 | N.A. | N.A. |
| Sample | Volumetric Diameter | Aerodynamic Diameter | ||||
|---|---|---|---|---|---|---|
| D10 (μm) | D50 (μm) | D90 (μm) | Span Value | MMAD (μm) | GSD | |
| C20_T60 | 1.21 ± 0.02 | 2.67 ± 0.02 | 5.20 ± 0.14 | 1.49 ± 0.03 | 4.29 ± 0.77 | 3.09 ± 0.14 |
| C20_T90 | 1.20 ± 0.02 | 2.52 ± 0.00 | 4.59 ± 0.10 | 1.35 ± 0.05 | 4.28 ± 0.85 | 3.30 ± 0.28 |
| C20_T120 | 0.76 ± 0.03 | 1.82 ± 0.06 | 3.47 ± 0.04 | 1.49 ± 0.07 | 4.58 ± 0.37 | 3.89 ± 0.33 |
| C20_T150 | 1.66 ± 0.04 | 5.36 ± 0.17 | 10.67 ± 0.44 | 1.68 ± 0.03 | 9.85 ± 1.15 | 4.02 ± 0.63 |
| C25_T60 | 1.32 ± 0.08 | 3.07 ± 0.09 | 6.51 ± 0.37 | 1.69 ± 0.07 | 4.46 ± 0.21 | 2.45 ± 0.25 |
| C25_T90 | 1.16 ± 0.01 | 2.62 ± 0.05 | 5.06 ± 0.16 | 1.49 ± 0.03 | 3.38 ± 0.22 | 2.83 ± 0.14 |
| C25_T120 | 0.97 ± 0.03 | 2.44 ± 0.04 | 4.80 ± 0.06 | 1.57 ± 0.03 | 4.28 ± 0.36 | 2.70 ± 0.25 |
| C25_T150 | 1.28 ± 0.04 | 5.16 ± 0.14 | 11.73 ± 0.22 | 2.03 ± 0.03 | 8.79 ± 0.38 | 2.72 ± 0.20 |
| C33_T60 | 1.57 ± 0.03 | 3.33 ± 0.02 | 6.83 ± 0.15 | 1.58 ± 0.05 | 5.29 ± 0.93 | 3.06 ± 0.06 |
| C33_T90 | 1.49 ± 0.05 | 3.16 ± 0.05 | 6.51 ± 0.24 | 1.59 ± 0.05 | 4.74 ± 1.14 | 3.08 ± 0.26 |
| C33_T120 | 1.08 ± 0.05 | 2.60 ± 0.11 | 5.17 ± 0.37 | 1.57 ± 0.07 | 4.32 ± 0.34 | 2.35 ± 0.31 |
| C33_T150 | 2.16 ± 0.21 | 6.44 ± 0.54 | 15.74 ± 2.83 | 2.10 ± 0.25 | 16.59 ± 2.66 | 3.87 ± 0.31 |
| Element | Raw Capreomycin (as Sulfate) | Raw Mannitol | ||
|---|---|---|---|---|
| Theoretical | Experimental | Theoretical | Experimental | |
| Carbon | 47.1 | 57.2 ± 0.2 | 50.0 | 52.7 ± 0.2 |
| Oxygen | 23.5 | 19.0 ± 0.1 | 50.0 | 47.3 ± 0.2 |
| Nitrogen | 27.5 | 21.7 ± 0.1 | - | - |
| Sulphur | 2.0 | 2.1 ± 0.0 | - | - |
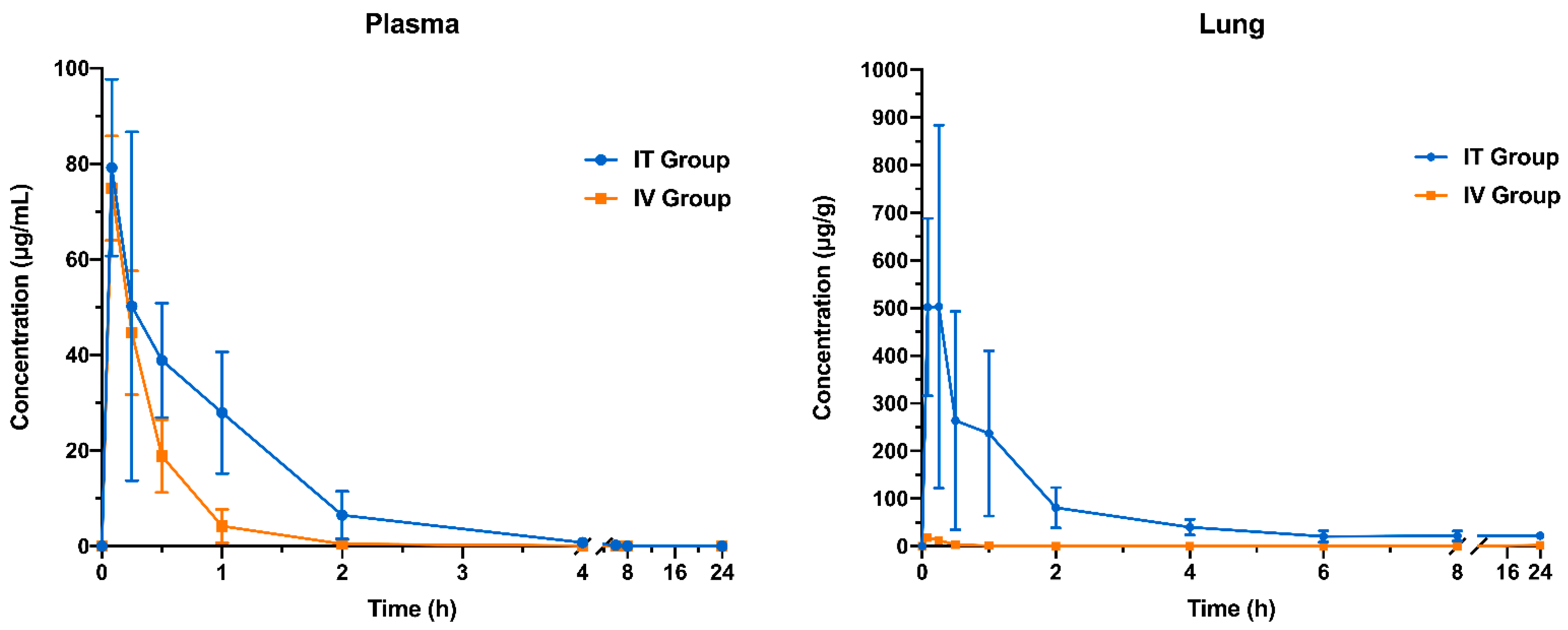
| Parameters a | Plasma | Lung | ||
|---|---|---|---|---|
| IT Group | IV Group | IT Group | IV Group | |
| Kel (h−1) | 1.15 ± 0.57 | 2.74 ± 1.67 | 0.07 ± 0.04 *** | 2.37 ± 0.23 b |
| t1/2 (h) | 0.73 ± 0.33 | 0.34 ± 0.20 | 18.94 ± 20.67 | 0.29 ± 0.03 b |
| CL (mL/h·kg) | 321.88 ± 81.65 *** | 612.91 ± 93.46 | 12.99 ± 3.66 *** | 2624.71 ± 157.19 b |
| AUC0–t (µg·h/mL) | 65.10 ± 16.37 ** | 32.55 ± 5.52 | 1061.88 ± 235.76 *** | 6.71 ± 0.51 |
| AUC0–∞ (µg·h/mL) | 67.26 ± 16.92 ** | 33.78 ± 5.54 | 1726.37 ± 658.83 ** | 7.78 ± 0.46 b |
| MRT (h) | 0.79 ± 0.22 ** | 0.30 ± 0.11 | 6.62 ± 1.25 *** | 0.23 ± 0.13 |
| Cmax (µg/mL) or (µg/g) c | 80.08 ± 18.84 | 74.89 ± 10.94 | 739.13 ± 180.66 *** | 18.23 ± 8.34 |
| Tmax (h) | 0.15 ± 0.09 | 0.08 ± 0 | 0.27 ± 0.20 | 0.12 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Tai, W.; Qiu, Y.; Man, R.C.H.; Liao, Q.; Chow, M.Y.T.; Kwok, P.C.L.; Lam, J.K.W. Spray-Dried Powder Formulation of Capreomycin Designed for Inhaled Tuberculosis Therapy. Pharmaceutics 2021, 13, 2044. https://doi.org/10.3390/pharmaceutics13122044
Shao Z, Tai W, Qiu Y, Man RCH, Liao Q, Chow MYT, Kwok PCL, Lam JKW. Spray-Dried Powder Formulation of Capreomycin Designed for Inhaled Tuberculosis Therapy. Pharmaceutics. 2021; 13(12):2044. https://doi.org/10.3390/pharmaceutics13122044
Chicago/Turabian StyleShao, Zitong, Waiting Tai, Yingshan Qiu, Rico C. H. Man, Qiuying Liao, Michael Y. T. Chow, Philip C. L. Kwok, and Jenny K. W. Lam. 2021. "Spray-Dried Powder Formulation of Capreomycin Designed for Inhaled Tuberculosis Therapy" Pharmaceutics 13, no. 12: 2044. https://doi.org/10.3390/pharmaceutics13122044
APA StyleShao, Z., Tai, W., Qiu, Y., Man, R. C. H., Liao, Q., Chow, M. Y. T., Kwok, P. C. L., & Lam, J. K. W. (2021). Spray-Dried Powder Formulation of Capreomycin Designed for Inhaled Tuberculosis Therapy. Pharmaceutics, 13(12), 2044. https://doi.org/10.3390/pharmaceutics13122044







