New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent
Abstract
:1. Introduction
2. Materials and Methods
3. Results
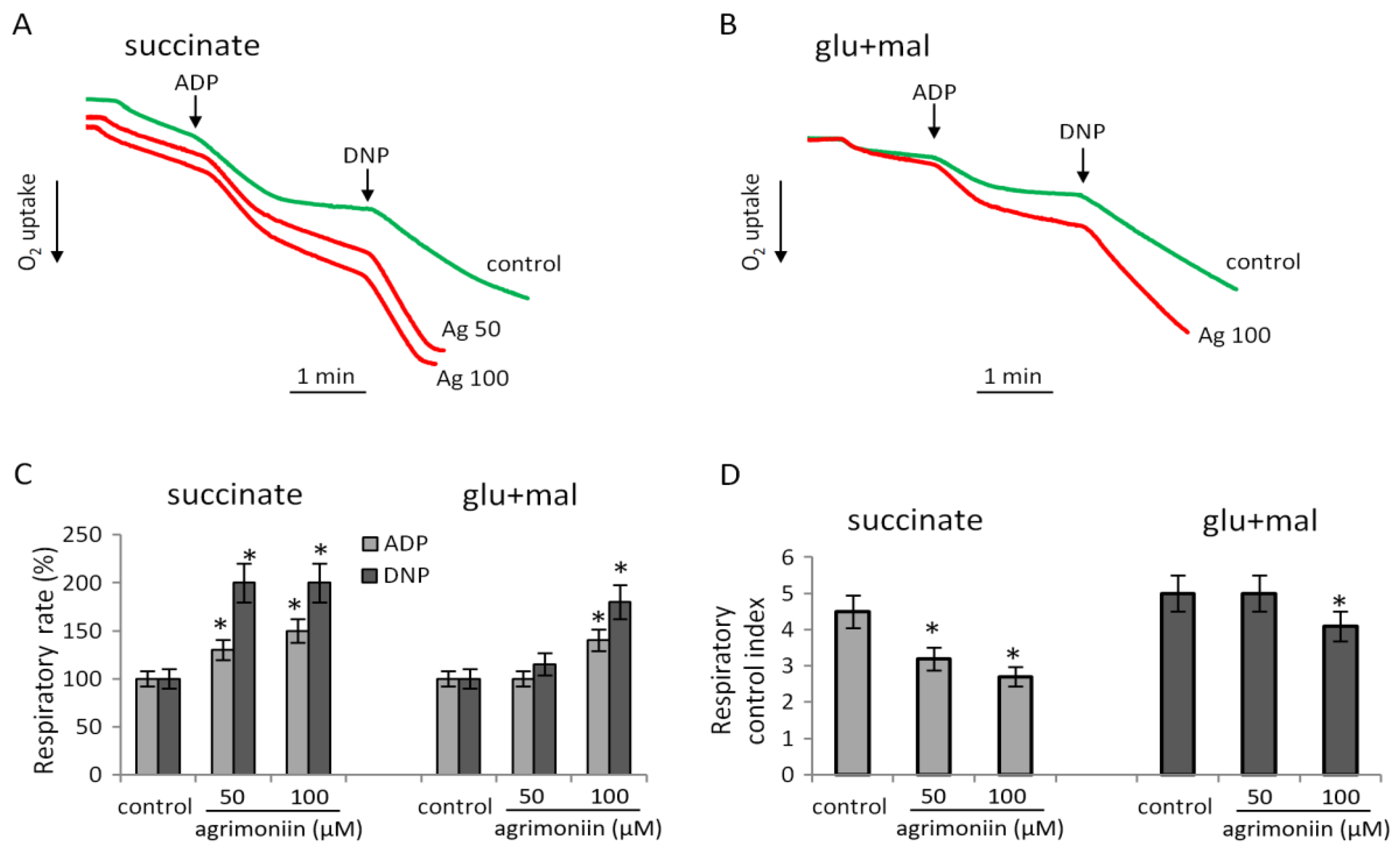
3.1. Influence of Agrimoniin on Mitochondrial Respiration and SDH Activity
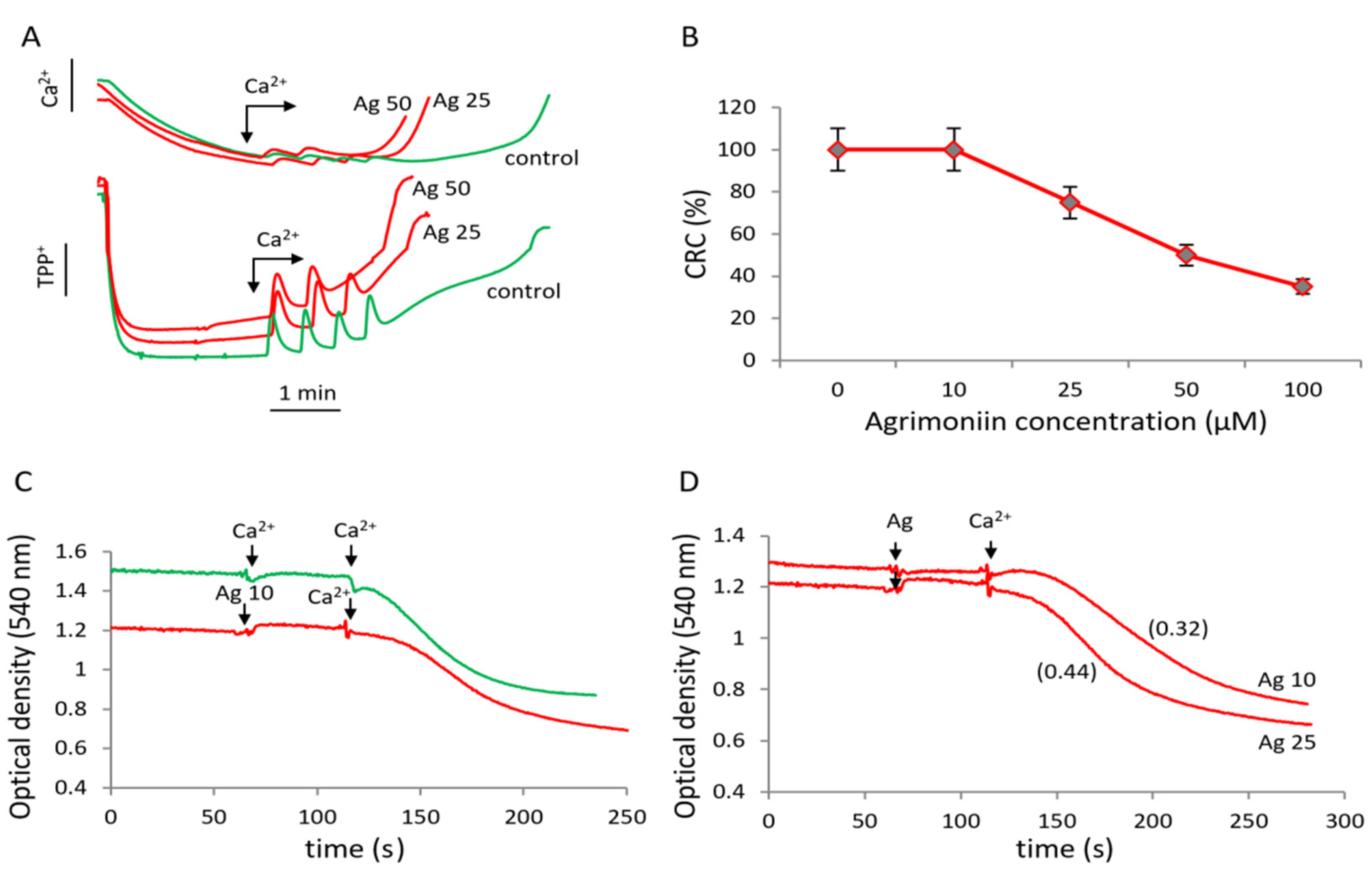
3.2. Influence of Agrimoniin on Calcium-Induced MPTP Opening
3.3. Effect of Agrimoniin on the Mitochondrial Swelling
3.4. Effect of Agrimoniin on the Mitochondrial Swelling in the Presence of Iron Ions
3.5. Cytotoxic Effect of Agrimoniin on Cancer Cell Lines
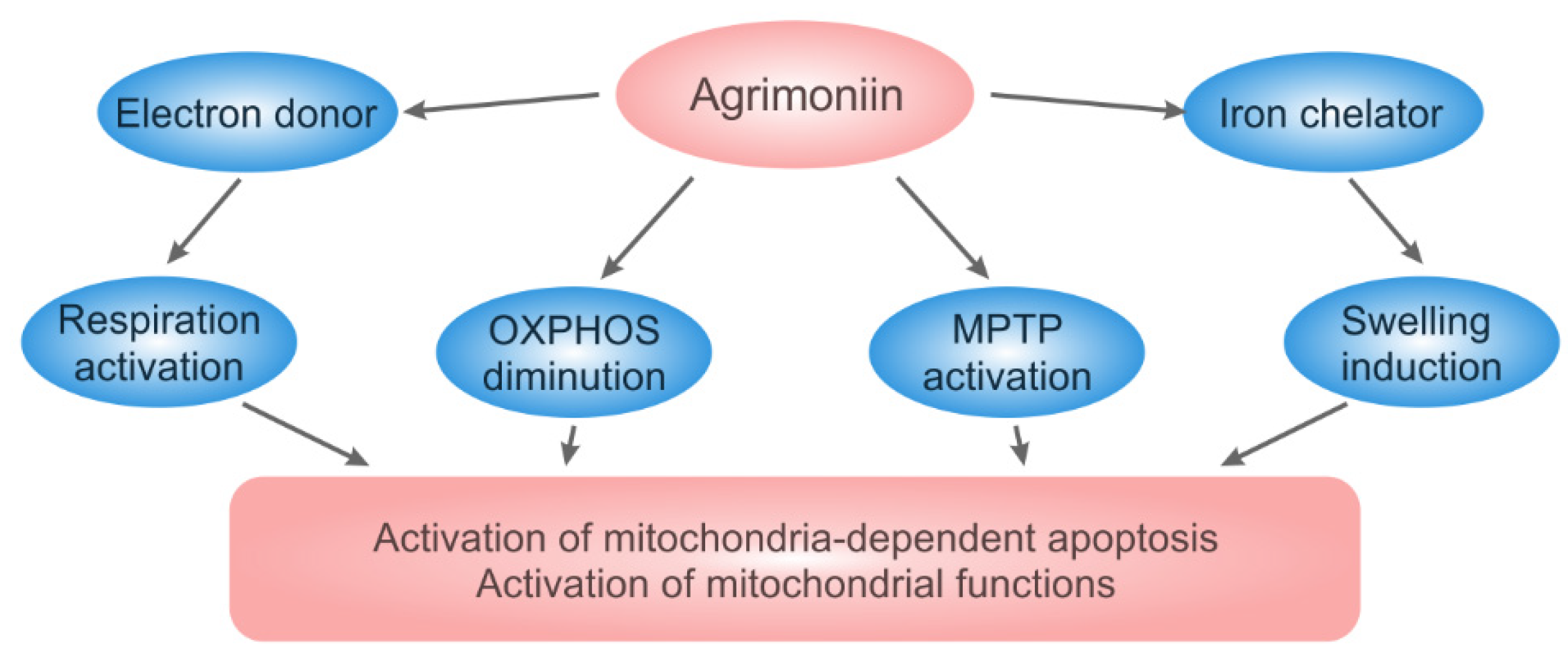
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Ag | agrimoniin |
| ANT | adenylate translocase |
| BHT | butylhydroxytoluene |
| CRC | calcium retention capacity |
| Cs A | cyclosporin A |
| DNP | 2,4-Dinitrophenol |
| DCPIP | 2,6-Dichlorophenolindophenol |
| MPTP | mitochondrial permeability transition pore |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide |
| PMS | phenazinemethosulfate |
| SDH | succinate dehydrogenase |
| TPP+ | tetraphenylphosphonium |
References
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T. Pharmacologically Active Tannins Isolated from Medicinal Plants. Plant Polyphen. 1992, 59, 539–569. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Kónya, Z.; Bécsi, B.; Kiss, A.; Horváth, D.; Raics, M.; Kövér, K.E.; Lontay, B.; Erdődi, F. Inhibition of protein phosphatase-1 and -2A by ellagitannins: Structure-inhibitory potency relationships and influences on cellular systems. J. Enzym. Inhib. Med. Chem. 2019, 34, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Ding, G.-B.; Liu, W.; Fu, R.; Sajid, A.; Li, Z. Tannic acid directly targets pyruvate kinase isoenzyme M2 to attenuate colon cancer cell proliferation. Food Funct. 2018, 9, 5547–5559. [Google Scholar] [CrossRef]
- Ribeiro, M.; De Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, J.; Zhang, J.; Yang, J.; Wang, X.; Peng, X. Protective effects of three structurally similar polyphenolic compounds against oxidative damage and their binding properties to human serum albumin. Food Chem. 2021, 349, 129118. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Järvenpää, J.; Huovinen, M.; Schroderus, A.-M.; Ihantola, E.-L.; Küblbeck, J.; Khadeer, M.; Moaddel, R.; Lahtela-Kakkonen, M. Effects of galloflavin and ellagic acid on sirtuin 6 and its anti-tumorigenic activities. Biomed. Pharmacother. 2020, 131, 110701. [Google Scholar] [CrossRef]
- Darvin, P.; Joung, Y.H.; Kang, D.Y.; Sp, N.; Byun, H.J.; Hwang, T.S.; Sasidharakurup, H.; Lee, C.H.; Cho, K.H.; Park, K.D.; et al. Tannic acid inhibits EGFR/STAT1/3 and enhances p38/STAT1 signalling axis in breast cancer cells. J. Cell Mol. Med. 2017, 21, 720–734. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, A.; Pattarayan, D.; Bethunaickan, R.; Kar, A.; Mahapatra, S.K.; Thimmulappa, R.K.; Palanichamy, R.; Rajasekaran, S. Tannic acid protects against experimental acute lung injury through downregulation of TLR4 and MAPK. J. Cell. Physiol. 2019, 234, 6463–6476. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Ueyama, M.; Tanaka, S.; Tsukayama, I.; Mega, T.; Konoike, Y.; Tamenobu, A.; Bastian, F.; Akai, I.; Ito, H.; et al. Ellagitannins from Punicagranatum leaves suppress microsomal prostaglandin E synthase-1 expression and induce lung cancer cells to undergo apoptosis. Biosci. Biotechnol. Biochem. 2020, 84, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Yang, Y.; Liu, D.; Luo, Y.; Ye, X.; Liu, Y.; Chen, X.; Wang, S.; Wu, H.; Wang, Y.; et al. Flavonoids and Tannins from Smilax china L. Rhizome Induce Apoptosis Via Mitochondrial Pathway and MDM2-p53 Signaling in Human Lung Adenocarcinoma Cells. Am. J. Chin. Med. 2017, 45, 369–384. [Google Scholar] [CrossRef]
- Geng, N.; Zheng, X.; Wu, M.; Yang, L.; Li, X.; Chen, J. Tannic acid synergistically enhances the anticancer efficacy of cisplatin on liver cancer cells through mitochondria-mediated apoptosis. Oncol. Rep. 2019, 42, 2108–2116. [Google Scholar] [CrossRef]
- Wang, B.-Q.; Jin, Z.-X. Agrimoniin induced SGC7901 cell apoptosis associated mitochondrial transmembrane potential and intracellular calcium concentration. J. Med. Plants Res. 2011, 5, 3513–3519. [Google Scholar]
- Wang, J.; Xiao, H.; Zhu, Y.; Liu, S.; Yuan, Z.; Wu, J.; Wen, L. Tannic Acid Induces the Mitochondrial Pathway of Apoptosis and S Phase Arrest in Porcine Intestinal IPEC-J2 Cells. Toxins 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.; Su, Y.; Li, K.; Jin, D.; Li, Q.; Li, Y.; Zhou, B. Gallic Acid Inhibits Bladder Cancer T24 Cell Progression Through Mitochondrial Dysfunction and PI3K/Akt/NF-κB Signaling Suppression. Front. Pharmacol. 2020, 11, 1222. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Wang, C.-P.; Huang, S.-S.; Hsu, F.-L. The Inhibitory Effect of Tannins on Lipid Peroxidation of Rat Heart Mitochondria. J. Pharm. Pharmacol. 2011, 47, 138–142. [Google Scholar] [CrossRef]
- Hong, C.; Wang, C.; Lo, Y.; Hsu, F. Effect of Flavan-3-ol Tannins Purified from Camellia sinensis on Lipid Peroxidation of Rat Heart Mitochondria. Am. J. Chin. Med. 1994, 22, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Savickas, A.; Vetchý, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct Effects of (−)-Epicatechin and Procyanidin B2 on the Respiration of Rat Heart Mitochondria. BioMed Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef] [Green Version]
- Koshiura, R.; Miyamoto, K.; Taguchi, H. Antitumor activity of methanol extract from roots of Agrimonia pilosa Ledeb. Jpn. J. Pharmacol. 1985, 38, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Muroyama, T.; Kishio, N.; Koshimura, R.; Takagi, K.; Furukawa, T.; Miyamoto, K. Agrimoniin antitumor tannin of Agrimonia pilosa L., induces interleukin. Anticancer Res. 1992, 12, 1471–1474. [Google Scholar]
- Miyamoto, K.-I.; Kishi, N.; Murayama, T.; Furukawa, T.; Koshiura, R. Induction of cytotoxicity of peritoneal exudate cells by agrimoniin, a novel immunomodulatory tannin of Agrimonia pilosa Ledeb. Cancer Immunol. Immunother. 1988, 27, 59–62. [Google Scholar]
- Kwon, D.H.; Kwon, H.Y.; Kim, H.J.; Chang, E.J.; Kim, M.B.; Yoon, S.K.; Song, E.Y.; Yoon, D.Y.; Lee, Y.H.; Choi, I.S.; et al. Inhibition of hepatitis B virus by an aqueous extract of Agrimoniaeu patoria L. Phytotherapy Res. 2005, 19, 355–358. [Google Scholar] [CrossRef]
- Wang, J.P.; Hsu, M.F.; Teng, C.M. Antigemostatic effect of Hsien-Ho-T’sao (Agrimoniapilosa). Am. J. Chin. Med. 1984, 12, 116–123. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Skalicka-Woźniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Piwowarski, J.P.; Granica, S.; Tomczyk, M. A comprehensive review of agrimoniin. Ann. N. Y. Acad. Sci. 2017, 1401, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Kuwahara, M.; Memon, U.; Shingu, T. Agrimoniin and Potentillin, an Ellagitannin Dimer and Monomer having anα-Glucose Core. J. Chem. Commun. 1982, 163–164. [Google Scholar] [CrossRef]
- Beloborodova, N.; Pautova, A.; Sergeev, A.; Fedotcheva, N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dynnik, V.V.; Grishina, E.V.; Fedotcheva, N.I. The mitochondrial NO-synthase/guanylate cyclase/protein kinase G signaling system underpins the dual effects of nitric oxide on mitochondrial respiration and opening of the permeability transition pore. FEBS J. 2019, 287, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.; Olenin, A.; Beloborodova, N. Influence of Microbial Metabolites on the Nonspecific Permeability of Mitochondrial Membranes under Conditions of Acidosis and Loading with Calcium and Iron Ions. Biomedicines 2021, 9, 558. [Google Scholar] [CrossRef]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.; Sveshnikova, E.; Sheina, N.; Sokolov, M.; Kudryavtsev, K.; Fedotcheva, N.; Shimanovskii, N. Synthesis and cytostatic activity of new derivatives of mepregenol 17-acetate against hela cancer cell culture. Pharm. Chem. J. 2020, 54, 119–125. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I. Protectors of the Mitochondrial Permeability Transition Pore Activated by Iron and Doxorubicin. Curr. Cancer Drug Targets 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Gogvadze, V.; Walter, P.B.; Ames, B.N. The role of Fe2+-induced lipid peroxidation in the initiation of the mitochondrial permeability transition. Arch. Biochem. Biophys. 2003, 414, 255–260. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, M.; Bode, A.M.; Liao, W.; Cao, Y. ANTs and cancer: Emerging pathogenesis, mechanisms, and perspectives. Biochim. Biophys. Acta-Bioenerg. 2021, 1875, 188485. [Google Scholar] [CrossRef]
- Gan, B. Mitochondrial regulation of ferroptosis. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Ta, N.; Zhang, T.; Gao, W. The Multifaceted Regulation of Mitochondria in Ferroptosis. Life 2021, 11, 222. [Google Scholar] [CrossRef]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, G.; Veuger, S. Reversing oncogenic transformation with iron chelation. Oncotarget 2021, 12, 106–124. [Google Scholar] [CrossRef]
- Ma, L.; Azad, M.G.; Dharmasivam, M.; Richardson, V.; Quinn, R.; Feng, Y.; Pountney, D.; Tonissen, K.; Mellick, G.; Yanatori, I.; et al. Parkinson’s disease: Alterations in iron and redox biology as a key to unlock therapeutic strategies. Redox Biol. 2021, 41, 101896. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Amit, T.; Mandel, S.; Kupershmidt, L.; Youdim, M.B. Neuroprotective Multifunctional Iron Chelators: From Redox-Sensitive Process to Novel Therapeutic Opportunities. Antioxidants Redox Signal. 2010, 13, 919–949. [Google Scholar] [CrossRef]
- Mellican, R.I.; Li, J.; Mehansho, H.; Nielsen, S.S. The Role of Iron and the Factors Affecting Off-Color Development of Polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.J.T.; Diosady, L.L. Development of Spectrophotometric Quantification Method of Iron-Polyphenol Complex in Iron-Fortified Black Tea at Relevant pH Levels. Food Anal. Methods 2018, 11, 1645–1655. [Google Scholar] [CrossRef]
- Horniblow, R.D.; Henesy, D.; Iqbal, T.H.; Tselepis, C. Modulation of iron transport, metabolism and reactive oxygen status by quercetin-iron complexes in vitro. Mol. Nutr. Food Res. 2016, 61. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine 2021, 153807. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef]
- Lytovchenko, O.; Kunji, E.R. Expression and putative role of mitochondrial transport proteins in cancer. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 641–654. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Choi, Y.; Jeon, Y.-K.; Aung, K.C.A.; Kim, C.-W. Over-expression of Adenine Nucleotide Translocase 1 (ANT1) Induces Apoptosis and Tumor Regression in vivo. BMC Cancer 2008, 8, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallerne, C.; Touat, Z.; Chen, Z.X.; Martel, C.; Mayola, E.; El Dein, O.S.; Buron, N.; Le Bras, M.; Jacotot, E.; Borgne-Sanchez, A. The fourth isoform of the adenine nucleotide translocator inhibits mitochondrial apoptosis in cancer cells. Int. J. Biochem. Cell Biol. 2010, 42, 623–629. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Kim, Y.-G.; Nam, S.J.; Keam, B.; Kim, T.M.; Jeon, Y.K.; Kim, C.W. Targeting Adenine Nucleotide Translocase-2 (ANT2) to Overcome Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2016, 15, 1387–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepien, G.; Torroni, A.; Chung, A.B.; Hodge, J.A.; Wallace, D.C. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J. Biol. Chem. 1992, 267, 14592–14597. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotcheva, T.A.; Sheichenko, O.P.; Fedotcheva, N.I. New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics 2021, 13, 2089. https://doi.org/10.3390/pharmaceutics13122089
Fedotcheva TA, Sheichenko OP, Fedotcheva NI. New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics. 2021; 13(12):2089. https://doi.org/10.3390/pharmaceutics13122089
Chicago/Turabian StyleFedotcheva, Tatiana A., Olga P. Sheichenko, and Nadezhda I. Fedotcheva. 2021. "New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent" Pharmaceutics 13, no. 12: 2089. https://doi.org/10.3390/pharmaceutics13122089
APA StyleFedotcheva, T. A., Sheichenko, O. P., & Fedotcheva, N. I. (2021). New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics, 13(12), 2089. https://doi.org/10.3390/pharmaceutics13122089





