Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gentian Extract Preparation
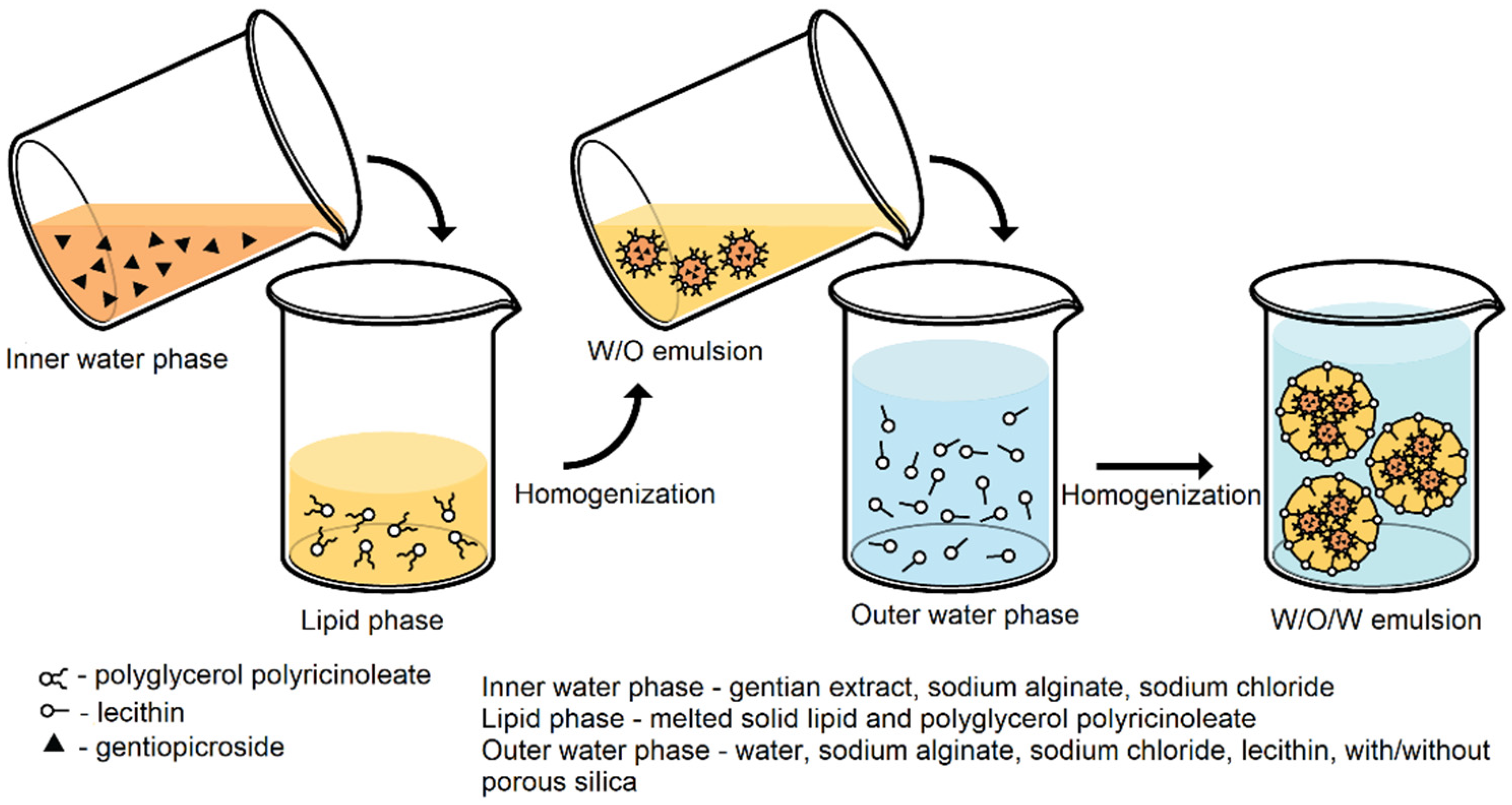
2.3. Preparation of Double W/O/W Emulsion
2.4. Characterization of Double Emulsions
2.4.1. Conductometric Analysis
2.4.2. Centrifugation Test
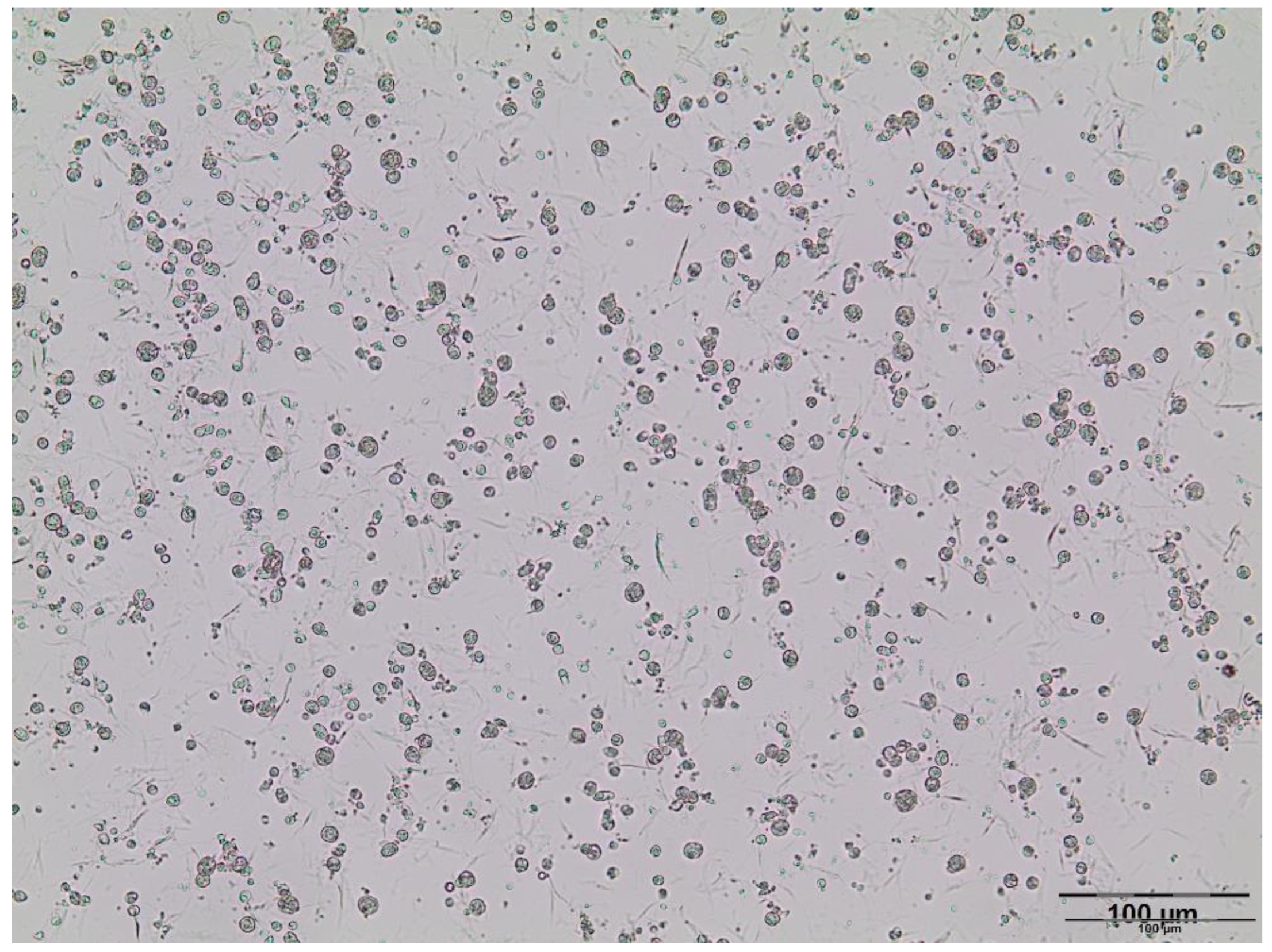
2.4.3. Microscopic Analysis
2.5. Preparation of Solid Lipid Microparticles
2.6. Characterization of Solid Lipid Microparticles
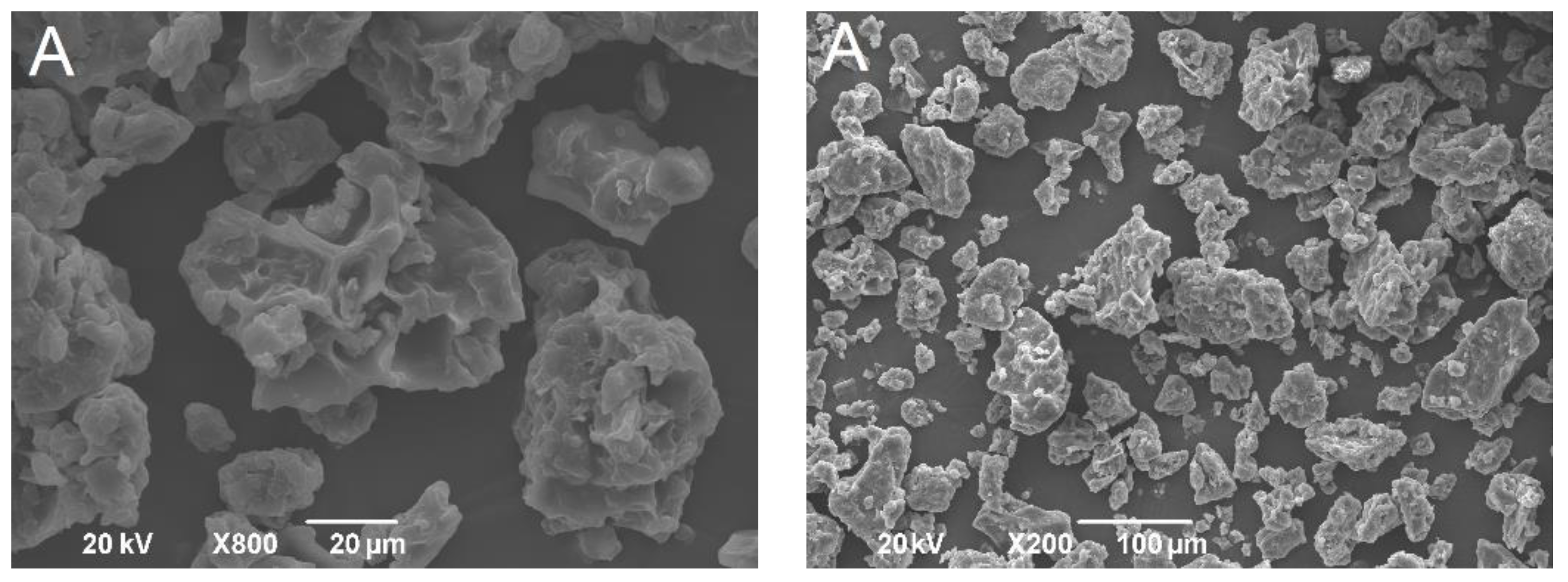
2.6.1. Scanning Electron Microscopy
2.6.2. Determination of the Encapsulation Efficiency
2.6.3. High-Performance Liquid Chromatography
2.6.4. Determination of Yield
2.6.5. Mercury Intrusion Porosimetry
2.6.6. Flow Properties Evaluation
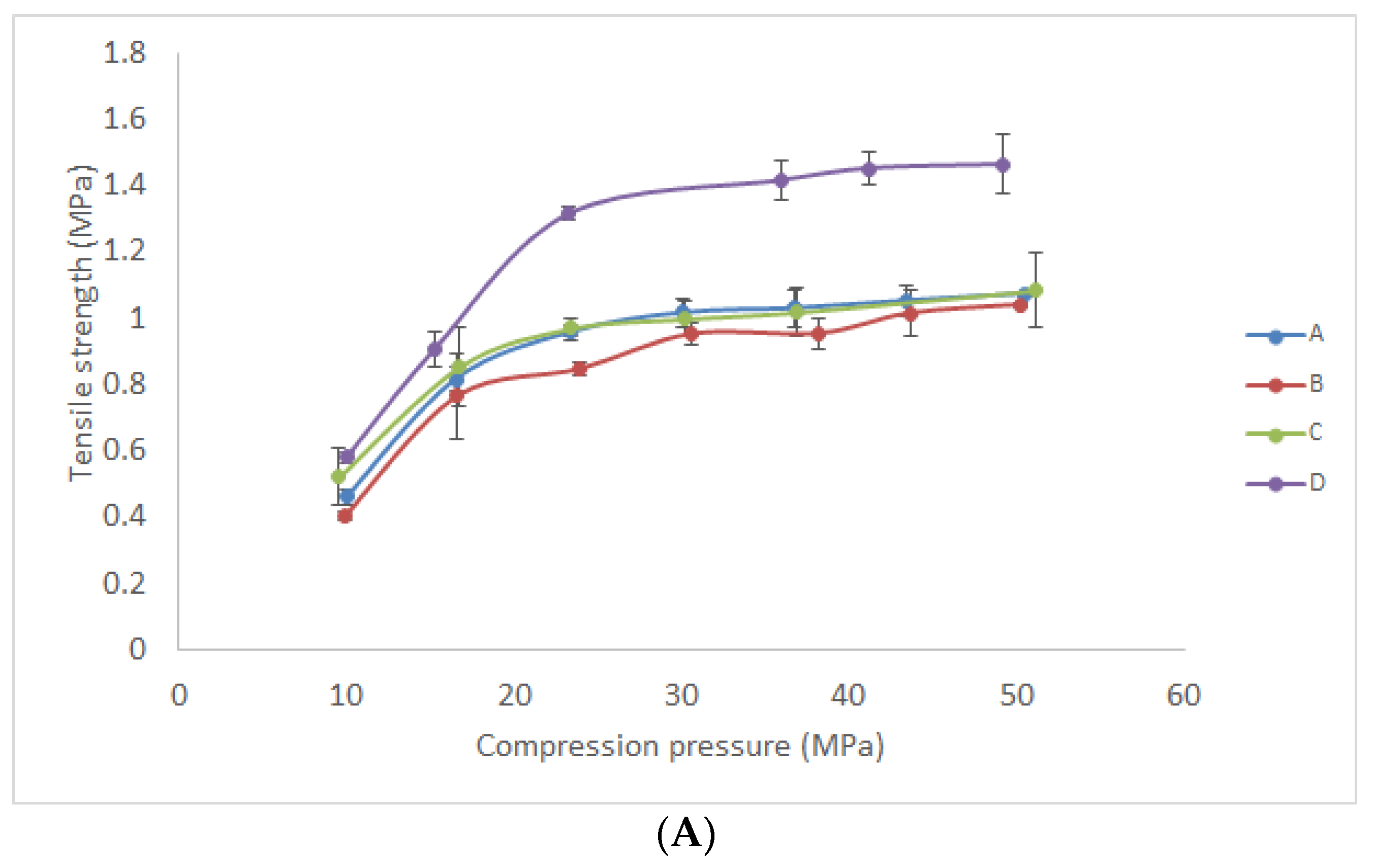
2.6.7. Tablets Preparation and Determination of Mechanical Properties
2.6.8. In Vitro Gentiopicroside Dissolution Testing
2.6.9. Kinetic Modeling of Gentiopicroside Release
2.6.10. Assessment of Dispersibility during In Vitro Dissolution
2.6.11. Mucoadhesion Evaluation
3. Results and Discussion
3.1. Double Emulsions Development
3.2. Solid Lipid Microparticles Characterization
3.2.1. Morphology
3.2.2. Yield and Encapsulation Efficiency
3.2.3. Porosity
3.2.4. Flowability
3.2.5. Mechanical Properties
3.2.6. Mucoadhesivity Evaluation
3.2.7. In Vitro Gentiopicroside Release
3.2.8. Dispersibility during In Vitro Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council of Europe. European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines & Health Care of the Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Tasić, S. Ethnobotany in SEE-WB countries; traditional uses of indigenous plants. Lek. Sirovine 2012, 32, 71–81. [Google Scholar]
- Šavikin, K.; Aljančić, I.; Vajs, V.; Milosavljević, S.; Jadranin, M.; Ðordević, I.; Menković, N. Bioactive secondary metabolites in several genera of Gentianaceae species from the central regions of the Balkan Peninsula. In The Gentianaceae Volume 2: Biotechnology and Applications; Rybczynski, J., Davey, M., Mikuła, A., Eds.; Springer: Berlin, Germany, 2015; Volume 3, pp. 319–347. [Google Scholar]
- Jiang, M.; Cui, B.W.; Wu, Y.L.; Nan, J.X.; Lian, L.H. Genus Gentiana: A review on phytochemistry, pharmacology and molecular mechanism. J. Ethnopharmacol. 2021, 264, 113391. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, Z.-T.; Annie Bligh, W.W.; White, K.N.; White, C.J.B. Pharmacokinetics and tissue distribution of gentiopicroside following oral and intravenous administration in mice. Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 199–203. [Google Scholar] [CrossRef]
- European Medicines Agency, Assessment Report on Gentiana lutea L., Radix. Ref.: EMA/HMPC/607863/2017. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-gentiana-lutea-l-radix-revision-1_en.pdf (accessed on 28 October 2021).
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J. Pharm. Pharmacol. 2009, 61, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Young, P.M.; Traini, D. Solid lipid microparticles as an approach to drug delivery. Expert Opin. Drug Deliv. 2015, 12, 583–599. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Molet-Rodríguez, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Formation of double (W 1/O/W 2) emulsions as carriers of hydrophilic and lipophilic active compounds. Food Bioprocess Technol. 2019, 12, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Bodmeier, R.; Wang, J.; Bhagwatwar, H. Process and formulation variables in the preparation of wax microparticles by a melt dispersion technique. II. W/O/W multiple emulsion technique for water-soluble drugs. J. Microencapsul. 1992, 9, 99–107. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the stability of double emulsions—A review of the measurement techniques for the systematic investigation of instability mechanisms. Colloids Interfaces 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, B.; Shimpi, S.H.Y.A.M.; Mahadik, K.R.; Paradkar, A. Preparation and evaluation of floating risedronate sodium Gelucire® 39/01 matrices. Acta. Pharm. 2004, 54, 205–214. [Google Scholar]
- Irshad, S.; Khan, I.U.; Khalid, S.H.; Asghar, S.; Irfan, M.; Khalid, I.; Shahzad, Y. Probing the effect of various lipids and polymer blends on clopidogrel encapsulated floating microcarriers. DARU J. Pharm. Sci. 2019, 27, 571–582. [Google Scholar] [CrossRef]
- Dhawan, V.; Sutariya, B.; Lokras, A.; Thamm, J.; Saraf, M.; Warawdekar, U.; Nagarsenker, M. Lipid nanoconstructs for superior hepatoprotection: In vitro assessments as predictive tool for in vivo translation. Int. J. Pharm. 2020, 579, 119176. [Google Scholar] [CrossRef]
- Seljak, K.B.; Ilić, I.G.; Gašperlin, M.; Pobirk, A.Z. Self-microemulsifying tablets prepared by direct compression for improved resveratrol delivery. Int. J. Pharm. 2018, 548, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Balijagić, J.; Janković, T.; Zdunić, G.; Bosković, J.; Šavikin, K.; Gođevac, D.; Stanojković, T.; Jovančić, M.; Menković, N. Chemical profile, radical scavenging and cytotoxic activity of yellow gentian leaves (Genitaneae luteae folium) grown in northern regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1487–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanovic, A.; Aleksic, I.; Ibric, S.; Parojcic, J.; Cvijic, S. Tableting of hot-melt coated paracetamol granules: Material tableting properties and quality characteristics of the obtained tablets. Eur. J. Pharm. Sci. 2020, 142, 105121. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. Disintegration and Dissolution of Dietary Supplements. In The United States Pharmacopeia and National Formulary USP 36–NF 31; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2013; pp. 1111–1116. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Peña, J.; Veiga, M.D. Tenofovir hot-melt granulation using Gelucire® to develop sustained-release vaginal systems for weekly protection against sexual transmission of HIV. Pharmaceutics 2019, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 69–79. [Google Scholar] [CrossRef]
- Neumann, S.M.; Scherbej, I.; Van Der Schaaf, U.S.; Karbstein, H.P. Investigations on the influence of osmotic active substances on the structure of water in oil emulsions for the application as inner phase in double emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 56–62. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Li, X.; Zhang, Z.; Dai, X.; Wang, X.; Xin, Y.; Liu, K.; Gao, L.; Du, D.; et al. The phase inversion mechanism of the pH-sensitive reversible invert emulsion from w/o to o/w. Open Phys. 2020, 18, 380–390. [Google Scholar] [CrossRef]
- Florence, A.T.; Whitehill, D. The formulation and stability of multiple emulsions. Int. J. Pharm. 1982, 11, 277–308. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control Release 2019, 295, 31–49. [Google Scholar] [CrossRef]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S.; Scherer, R.; Castilho, R.O.; Faraco, A.A.G.; Ruas, R.G.; Oliveira, J.P.; Cunegundes Guimarães, M.C.; de Andrade, T.U.; et al. Maltodextrin and gum Arabic-based microencapsulation methods for anthocyanin preservation in Juçara palm (Euterpe edulis Martius) fruit pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef]
- Dolly, P.; Anishaparvin, A.; Joseph, G.S.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (mtcc 5422) by spray-freeze-drying method and evaluation of survival in simulated gastrointestinal conditions. J. Microencapsul. 2011, 28, 568–574. [Google Scholar] [CrossRef]
- El Assasy, A.E.H.I.; Younes, N.F.; Makhlouf, A.I. Enhanced oral absorption of amisulpride via a nanostructured lipid carrier-based capsules: Development, optimization applying the desirability function approach and in vivo pharmacokinetic study. AAPS PharmSciTech 2019, 20, 1–14. [Google Scholar]
- Peres, L.B.; Peres, L.B.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Aberham, A.; Pieri, V.; Croom Jr, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef] [Green Version]
- Graham, D. Characterization of physical adsorption systems. III. The separate effects of pore size and surface acidity upon the adsorbent capacities of activated carbons. J. Phys. Chem. 1955, 59, 896–900. [Google Scholar] [CrossRef]
- Dastidar, D.G.; Saha, S.; Chowdhury, M. Porous microspheres: Synthesis, characterisation and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar]
- Kovačič, B.; Vrečer, F.; Planinšek, O. Solid dispersions of carvedilol with porous silica. Chem. Pharm. Bull. 2011, 59, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudrić, J.; Arsenijević, J.; Maksimović, Z.; Ibrić, S.; Gopčević, K.; Đuriš, J. Tablet and capsule formulations incorporating high doses of a dry optimized herbal extract: The case of Satureja kitaibelii. J. Drug Deliv. Sci. Technol. 2021, 66, 102776. [Google Scholar] [CrossRef]
- Panda, T.; Das, D.; Panigrahi, L. Formulation development of solid dispersions of bosentan using Gelucire 50/13 and Poloxamer 188. J. Appl. Pharm. Sci. 2016, 6, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.R.; Teixeira, C.C.C.; Freitas, L.A.P. The preparation of ternary solid dispersions of an herbal drug via spray drying of liquid feed. Dry. Technol. 2010, 28, 412–421. [Google Scholar] [CrossRef]
- Madgulkar, A.R.; Bhalekar, M.R.; Padalkar, R.R. Formulation design and optimization of novel taste masked mouth-dissolving tablets of tramadol having adequate mechanical strength. AAPS PharmSciTech 2009, 10, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, V.T.; Shah, P.A.; Soni, T.G.; Parmar, M.Y.; Gohel, M.C.; Gandhi, T.R. Goodness-of-fit model-dependent approach for release kinetics of levofloxacin hemihydrates floating tablet. Dissolution Technol. 2009, 16, 35–39. [Google Scholar] [CrossRef]
- Amidon, G.E.; Secreast, P.J.; Mudie, D. Developing Solid Oral Dosage Forms; Qiu, Y., Zhang, G., Chen, Y., Liu, L., Porter, W., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 8, pp. 163–186. [Google Scholar]
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Kurćubić, I.; Cvijić, S.; Filipčev, B.; Ignjatović, J.; Ibrić, S.; Đuriš, J. Development of propranolol hydrochloride bilayer mucoadhesive buccal tablets supported by in silico physiologically-based modeling. React. Funct. Polym. 2020, 151, 104587. [Google Scholar] [CrossRef]
- Sharma, O.P.; Shah, M.V.; Parikh, D.C.; Mehta, T.A. Formulation optimization of gastroretentive drug delivery system for allopurinol using experimental design. Expert Opin. Drug Deliv. 2015, 12, 513–524. [Google Scholar] [CrossRef]
- Md, S.; Ahuja, A.; Khar, R.K.; Baboota, S.; Chuttani, K.; Mishra, A.K.; Ali, J. Gastroretentive drug delivery system of acyclovir-loaded alginate mucoadhesive microspheres: Formulation and evaluation. Drug Deliv. 2011, 18, 255–264. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Alginate-based composite sponges as gastroretentive carriers for curcumin-loaded self-microemulsifying drug delivery systems. Sci. Pharm. 2017, 85, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, F.; Mosafa, F.; Afrasiabi, G.H. Effect of particle size, compaction force and presence of Aerosil 200 on the properties of matrices prepared from physical mixture of propranolol hydrochloride and eudragit RS or RL. Iran. J. Basic Med. Sci. 2007, 10, 197–205. [Google Scholar]
- Fuji Silysia Chemical Ltd. Available online: https://www.fujisilysia.com/products/sylysia/ (accessed on 28 October 2021).
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-ionic surfactants for stabilization of polymeric nanoparticles for biomedical uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]




| A | B | C | D | |
|---|---|---|---|---|
| Gentian extract (%) | 66.5 | 66.5 | 66.5 | 66.5 |
| Sodium alginate (%) | 1.3 | 1.3 | 1.3 | 1.3 |
| Sodium chloride (M) | 0.05 | 0.05 | 0.05 | 0.05 |
| Gelucire® 39/01 (%) * | 26.9 | 26.9 | / | / |
| Gelucire® 43/01 (%) ** | / | / | 26.9 | 26.9 |
| Lipophilic emulsifier (%) *** | 5.0 | 5.0 | 5.0 | 5.0 |
| A | B | C | D | |
|---|---|---|---|---|
| Primary emulsion with PGPR (%) * | 19.8 | 19.8 | 19.8 | 19.8 |
| Sodium alginate (%) | 2 | 2 | 2 | 2 |
| Sodium chloride (%) | 0.05 | 0.2 | 0.2 | 0.2 |
| Hydrophilic emulsifier (%) ** | 1.6 | 1.6 | 1.6 | 1.6 |
| Trehalose (%) | 7.9 | 7.9 | 7.9 | 7.9 |
| Sylysia® 350 (%) | / | 1.0 | / | 1.0 |
| Purified water to (%) | 100.0 | 100.0 | 100.0 | 100.0 |
| Sample | A | B | C | D |
|---|---|---|---|---|
| EE (%) *,** | 98.92 ± 1.06 a | 103.02 ± 0.15 ab | 98.77 ± 4.28 a | 104.32 ± 0.16 b |
| Yield (%) ** | 92.05 ± 1.48 a | 95.17 ± 1.03 a | 91.85 ± 2.03 a | 93.57 ± 1.87 a |
| Sample | Run | Dav (µm) | BD (g/cm3) | P (%) |
|---|---|---|---|---|
| A | I | 9.78 | 1.03 | 24.7 |
| II | 9.78 | 1.03 | 16.2 | |
| B | I | 9.78 | 0.95 | 32.8 |
| II | 9.00 | 0.95 | 16.7 | |
| C | I | 6.29 | 0.93 | 29.9 |
| II | 9.78 | 0.93 | 17.3 | |
| D | I | 9.0 | 0.93 | 32.4 |
| II | 9.0 | 0.93 | 21.3 | |
| Dry gentian extract | I | 9.8 | 1.22 | 20.2 |
| II | 0.01 | 1.22 | 3.1 |
| Sample | Hausner Ratio | Carr Index (%) | Flowability |
|---|---|---|---|
| A | 1.13 ± 0.04 | 11.84 ± 3.11 | good |
| B | 1.23 ± 0.02 | 18.67 ± 1.26 | fair |
| C | 1.10 ± 0.02 | 9.73 ± 1.48 | excellent |
| D | 1.12 ± 0.03 | 10.64 ± 2.18 | good |
| Dry gentian extract | 1.28 ± 0.08 | 21.95 ± 5.26 | passable |
| Sample | A | B | C | D |
|---|---|---|---|---|
| The force of adhesion (N) * | 1.73 ± 0.66 a | 2.02 ± 0.46 a | 2.08 ± 0.64 a | 2.46 ± 0.12 a |
| Sample | Correlation Coefficients (r2) | |||
|---|---|---|---|---|
| Zero-order | First-order | Higuchi | Korsmeyer–Peppas | |
| A | 0.6241 | 0.4783 | 0.8422 | 0.8524 |
| B | 0.7433 | 0.7319 | 0.9423 | 0.9819 |
| C | 0.7268 | 0.6628 | 0.9339 | 0.9601 |
| D | 0.7279 | 0.6600 | 0.9339 | 0.9601 |
| Time | 15 min | 6 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak | Peak 1 | Peak 2 | Peak 1 | Peak 2 | ||||
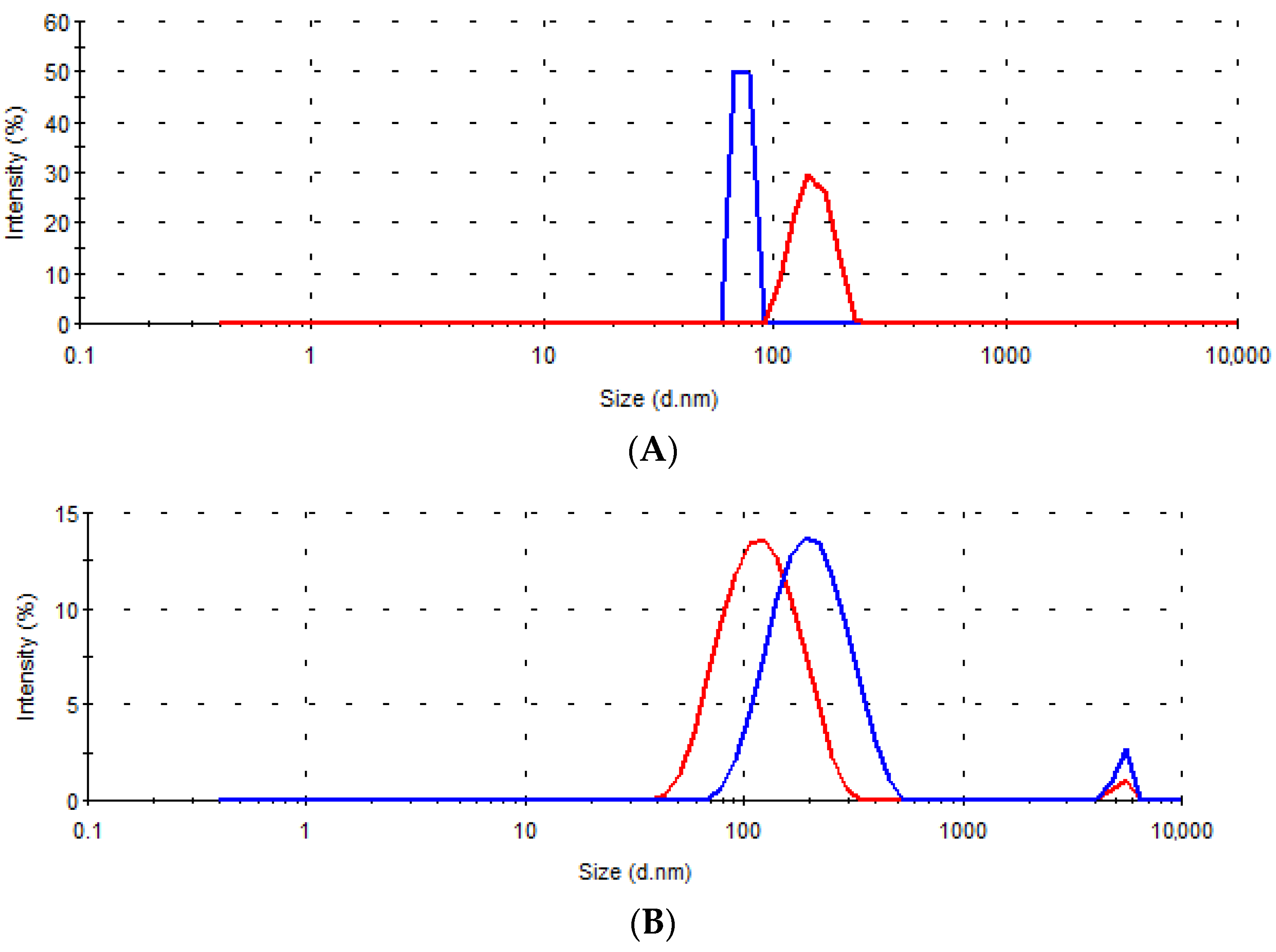
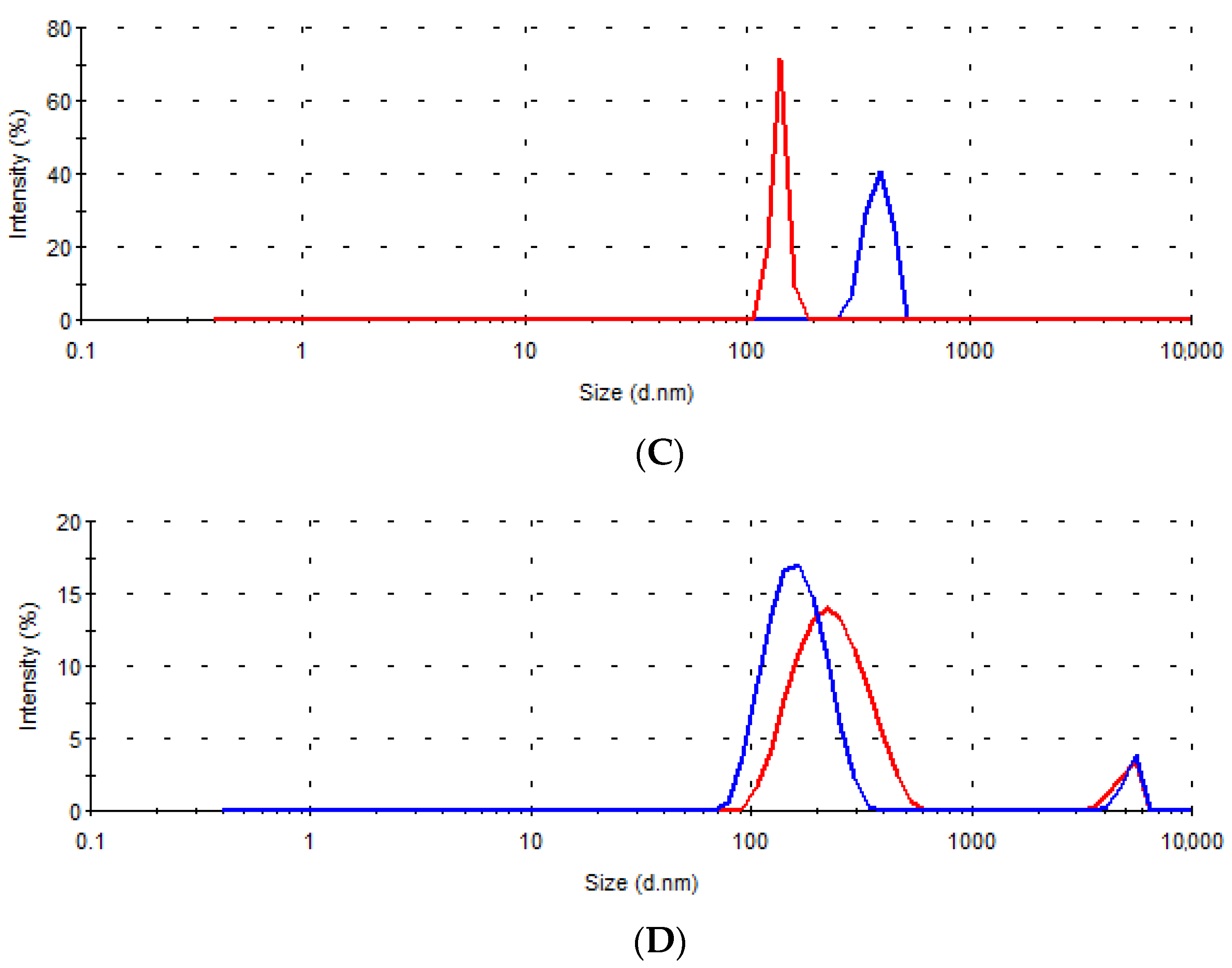
| Sample | D (nm) | I (%) | D (nm) | I (%) | D (nm) | I (%) | D (nm) | I (%) |
| A | 73.4 | 100.0 | / | / | 147.4 | 100.0 | / | / |
| B | 210.2 | 96.3 | 5319.0 | 3.7 | 126.4 | 98.2 | 5236.0 | 1.8 |
| C | 389.6 | 100.0 | / | / | 140.1 | 100.0 | / | / |
| D | 165.5 | 94.1 | 5271.0 | 5.9 | 239.3 | 93.0 | 5007.0 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudrić, J.; Šavikin, K.; Đekić, L.; Pavlović, S.; Kurćubić, I.; Ibrić, S.; Đuriš, J. Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique. Pharmaceutics 2021, 13, 2095. https://doi.org/10.3390/pharmaceutics13122095
Mudrić J, Šavikin K, Đekić L, Pavlović S, Kurćubić I, Ibrić S, Đuriš J. Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique. Pharmaceutics. 2021; 13(12):2095. https://doi.org/10.3390/pharmaceutics13122095
Chicago/Turabian StyleMudrić, Jelena, Katarina Šavikin, Ljiljana Đekić, Stefan Pavlović, Ivana Kurćubić, Svetlana Ibrić, and Jelena Đuriš. 2021. "Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique" Pharmaceutics 13, no. 12: 2095. https://doi.org/10.3390/pharmaceutics13122095
APA StyleMudrić, J., Šavikin, K., Đekić, L., Pavlović, S., Kurćubić, I., Ibrić, S., & Đuriš, J. (2021). Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique. Pharmaceutics, 13(12), 2095. https://doi.org/10.3390/pharmaceutics13122095












