Advanced Hydrogels for the Controlled Delivery of Insulin
Abstract
:1. Introduction
2. Biocompatibility and Biodegradability of Hydrogels as Insulin Carriers
2.1. Biocompatibility
2.2. Biodegradability
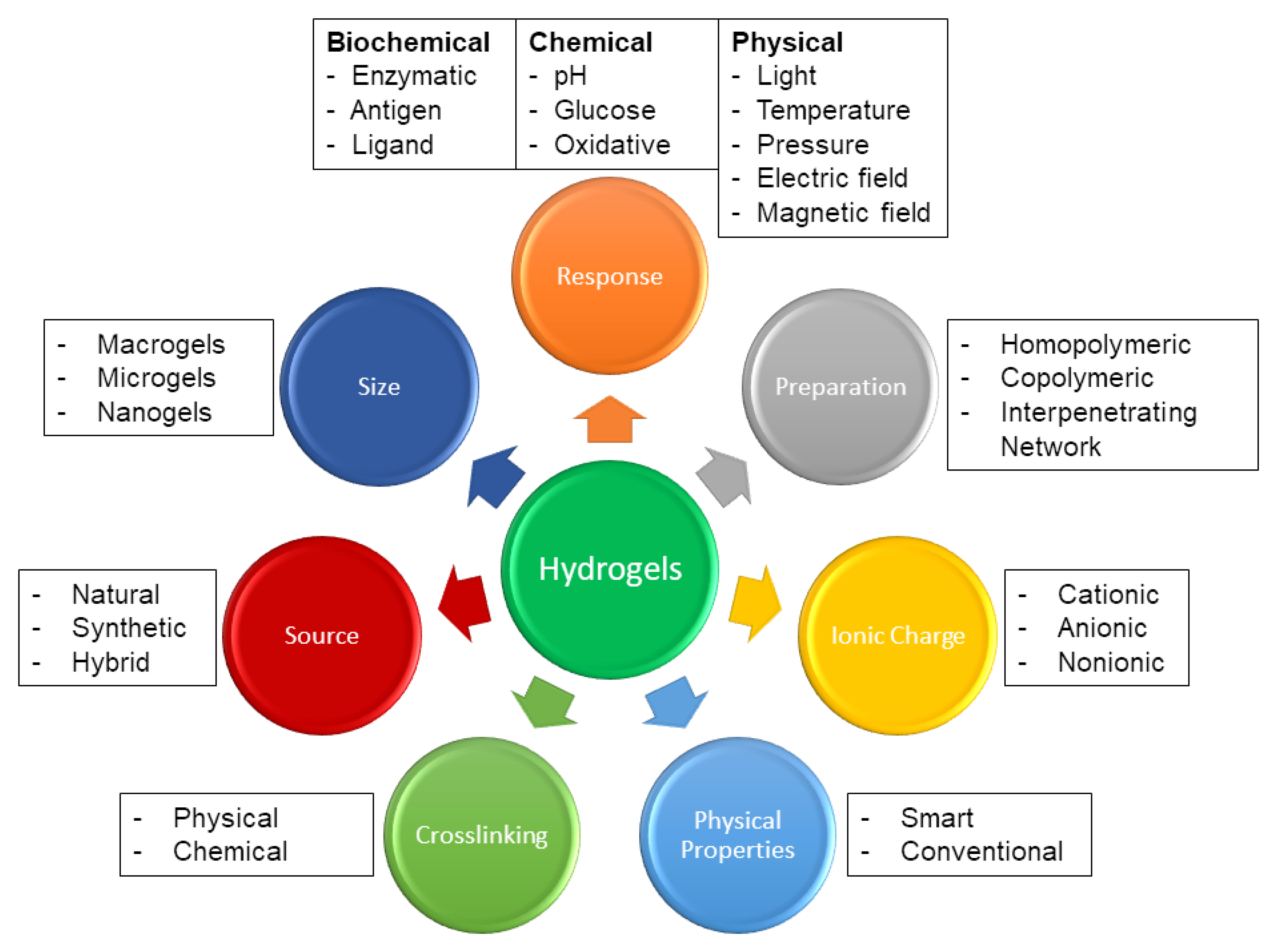
3. Hydrogel Synthesis, Morphology, and Properties
3.1. Physical and Chemical Crosslinking
3.2. Morphological Components
3.3. Molecular Characteristics
3.3.1. Viscoelasticity
3.3.2. Pore Size
3.3.3. Swelling and Drug Release
4. Modified Hydrogel Platforms for Controlled Insulin Delivery
4.1. Injectable Hydrogel Systems for the Regulated Delivery of Insulin
4.2. The Use of Microgels for the Controlled Delivery of Insulin
4.3. The Use of Nanogels for the Controlled Delivery of Insulin
5. Stimuli-Responsive Hydrogels for Controlled Insulin Delivery
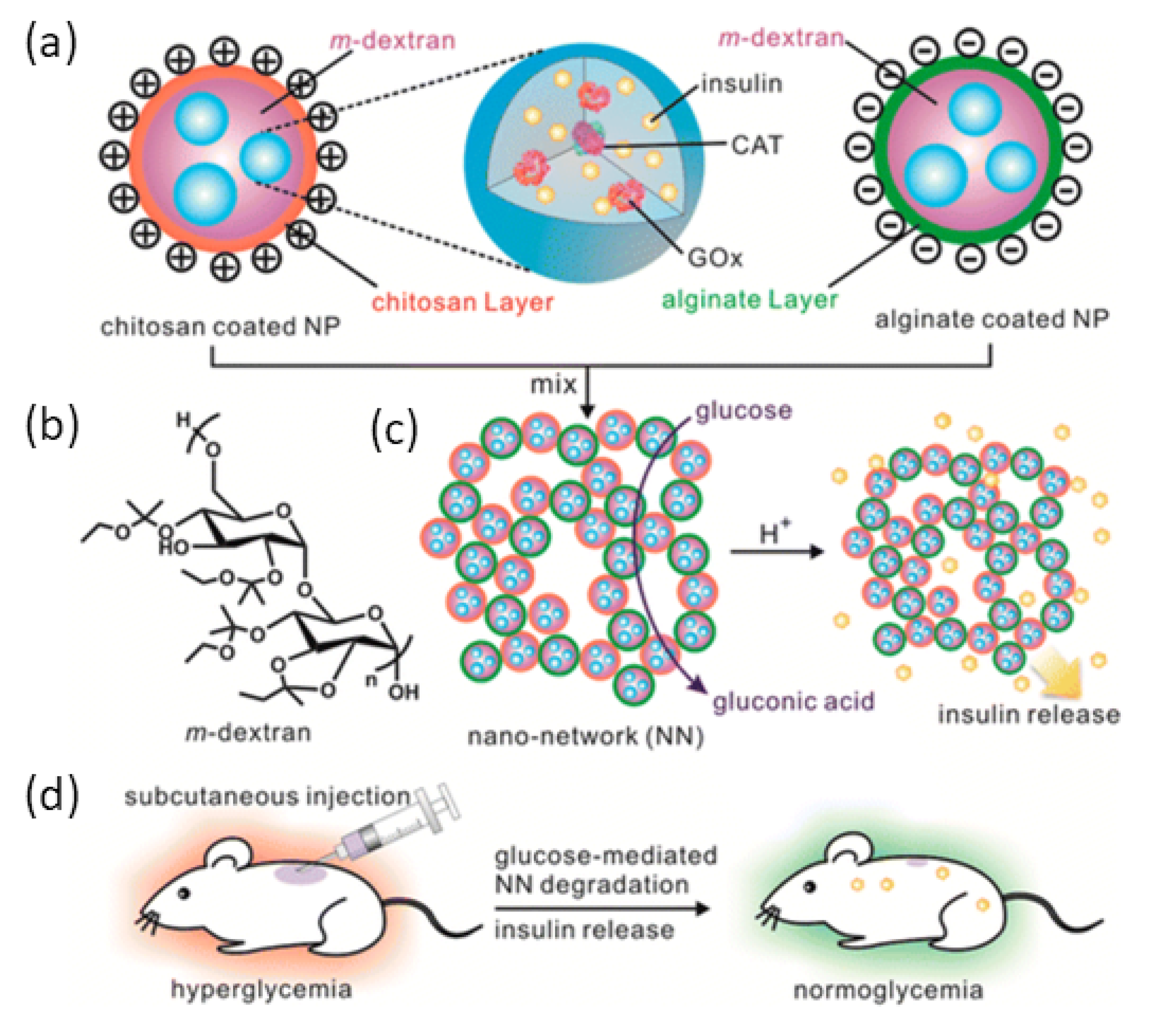
5.1. Glucose Oxidase Stimuli Release Systems
5.2. Glucose-Responsive Stimuli Release Systems
5.3. Temperature/Thermo-Responsive Stimuli Release of Insulin
5.4. Metal-Conjugated Platforms for Potential Insulin Delivery
6. The Use of 3D Bio-Printing to Engineer an Artificial Pancreas and Other 21st Century Techniques
7. Future Perspectives
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Diabetes Epidemiology, Genetics, Pathogenesis, Diagnosis, Prevention, and Treatment; Bonora, E., DeFronzo, R.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–253. [Google Scholar] [CrossRef]
- Sarbacker, G.B.; Urteaga, E.M. Adherence to insulin therapy. Diabetes Spectr. 2016, 29, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.M.; Gran, M.P.; Peppas, N.A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm. Sin. B 2018, 8, 147–164. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, J.; Peng, Q.; Dong, J.; Wang, Y.; Gong, T.; Zhang, Z. Injectable and biodegradable phospholipid-based phase separation gel for sustained delivery of insulin. Colloids Surf. B Biointerfaces 2019, 176, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Cho, K.-H.; Uthaman, S.; Cho, C.-S.; Park, I.-K. Stimuli-regulated smart polymeric systems for gene therapy. Polymers 2017, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A review: Tailor-made hydrogel structures (classifications and synthesis parameters). Polym.-Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 10671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Protein/peptide drug delivery systems: Practical considerations in pharmaceutical product development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–684. [Google Scholar]
- Doostmohammadi, M.; Ameri, A.; Mohammadinejad, R.; Dehghannoudeh, N.; Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Hydrogels For Peptide Hormones Delivery: Therapeutic And Tissue Engineering Applications. Drug Des. Dev. Ther. 2019, 13, 3405. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels… A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Chen, Y.; Li, Y.; Zhou, Z.; Cheng, Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials 2017, 112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Stanislawska, I.; Liwinska, W.; Lyp, M.; Stojek, Z.; Zabost, E. Recent Advances in Degradable Hybrids of Biomolecules and NGs for Targeted Delivery. Molecules 2019, 24, 1873. [Google Scholar] [CrossRef] [Green Version]
- Kondiah, P.P.; Choonara, Y.E.; Tomar, L.K.; Tyagi, C.; Kumar, P.; du Toit, L.C.; Marimuthu, T.; Modi, G.; Pillay, V. Development of a gastric absorptive, immediate responsive, oral protein-loaded versatile polymeric delivery system. AAPS PharmSciTech 2017, 18, 2479–2493. [Google Scholar] [CrossRef]
- Matsumoto, N.M.; González-Toro, D.C.; Chacko, R.T.; Maynard, H.D.; Thayumanavan, S. Synthesis of nanogel–protein conjugates. Polym. Chem. 2013, 4, 2464–2469. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, M.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. In situ forming reduction-sensitive degradable nanogels for facile loading and triggered intracellular release of proteins. Biomacromolecules 2013, 14, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Xiong, W.; Pei, Y.; Li, B.; Chen, Y. Nanogels fabricated from bovine serum albumin and chitosan via self-assembly for delivery of anticancer drug. Colloids Surf. B Biointerfaces 2016, 146, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nukolova, N.V.; Oberoi, H.S.; Cohen, S.M.; Kabanov, A.V.; Bronich, T.K. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 2011, 32, 5417–5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, M.; Ueda, S.; Nishikawa, H.; Kitano, S.; Hirayama, M.; Ikeda, H.; Toyoda, H.; Tanaka, K.; Kanai, M.; Takabayashi, A. Antibody responses against NY-ESO-1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine 2009, 27, 6854–6861. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.; Lambrecht, B.N. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Park, K.-H. Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels. Carbohydr. Polym. 2016, 136, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv. Sci. 2018, 5, 1700821. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Y.; Song, X.; Zhang, Z.; Zhuang, X.; He, C.; Chen, X. Biodegradable, pH-Responsive Carboxymethyl Cellulose/Poly(Acrylic Acid) Hydrogels for Oral Insulin Delivery. Macromol. Biosci. 2014, 14, 565–575. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.-L.; Teng, B. Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef]
- Park, M.J.; Hur, S.M.; Rhee, H.K. Online estimation and control of polymer quality in a copolymerization reactor. AIChE J. 2002, 48, 1013–1021. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Kondiah, P.P.; Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Pillay, V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int. J. Pharm. 2013, 456, 459–472. [Google Scholar] [CrossRef]
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Igarzabal, C.I.A.; Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Xue, K. Injectable Supramolecular Hydrogels for Insulin Delivery; Massachusetts Institute of Technology: Boston, MA, USA, 2017. [Google Scholar]
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.-W.; Weir, G.C.; Langer, R.; et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Wu, D.; Yao, D.; Guo, R.; Wang, W.; Dong, A.; Kong, D.; Zhang, J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017, 64, 334–345. [Google Scholar] [CrossRef]
- Lee, J.; Ko, J.H.; Mansfield, K.M.; Nauka, P.C.; Bat, E.; Maynard, H.D. Glucose-Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery. Macromol. Biosci. 2018, 18, e1700372. [Google Scholar] [CrossRef]
- Maity, B.; Samanta, S.; Sarkar, S.; Alam, S.; Govindaraju, T. Injectable Silk Fibroin-Based Hydrogel for Sustained Insulin Delivery in Diabetic Rats. ACS Appl. Bio Mater. 2020, 3, 3544–3552. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Facklam, A.L.; Cortinas, A.B.; Lu, Y.-C.; Matranga, M.A.; MacIsaac, C.; Hill, M.C.; Langer, R.; Anderson, D.G. Microgel encapsulated nanoparticles for glucose-responsive insulin delivery. Biomaterials 2021, 267, 120458. [Google Scholar] [CrossRef] [PubMed]
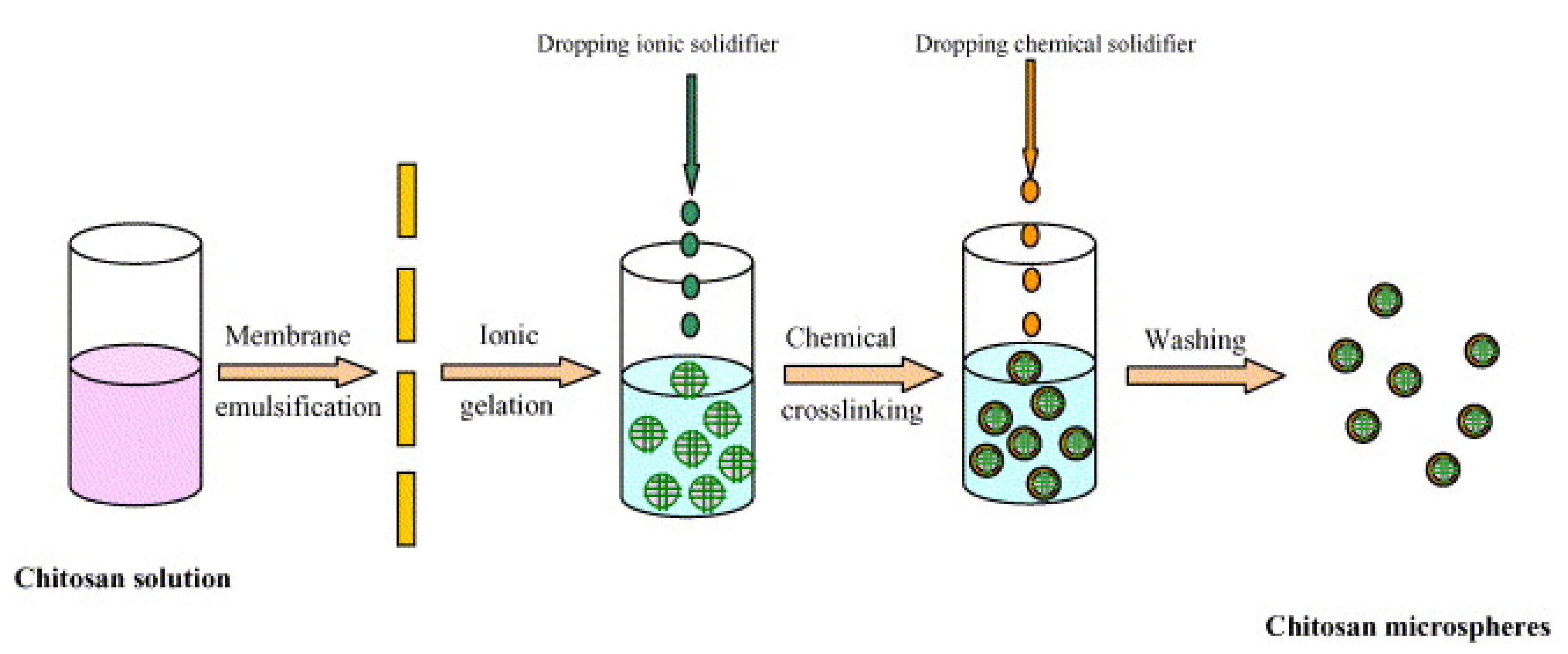
- Wang, L.-Y.; Gu, Y.-H.; Zhou, Q.-Z.; Ma, G.-H.; Wan, Y.-H.; Su, Z.-G. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf. B Biointerfaces 2006, 50, 126–135. [Google Scholar] [CrossRef]
- Di, J.; Yu, J.; Wang, Q.; Yao, S.; Suo, D.; Ye, Y.; Pless, M.; Zhu, Y.; Jing, Y.; Gu, Z. Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Res. 2017, 10, 1393–1402. [Google Scholar] [CrossRef]
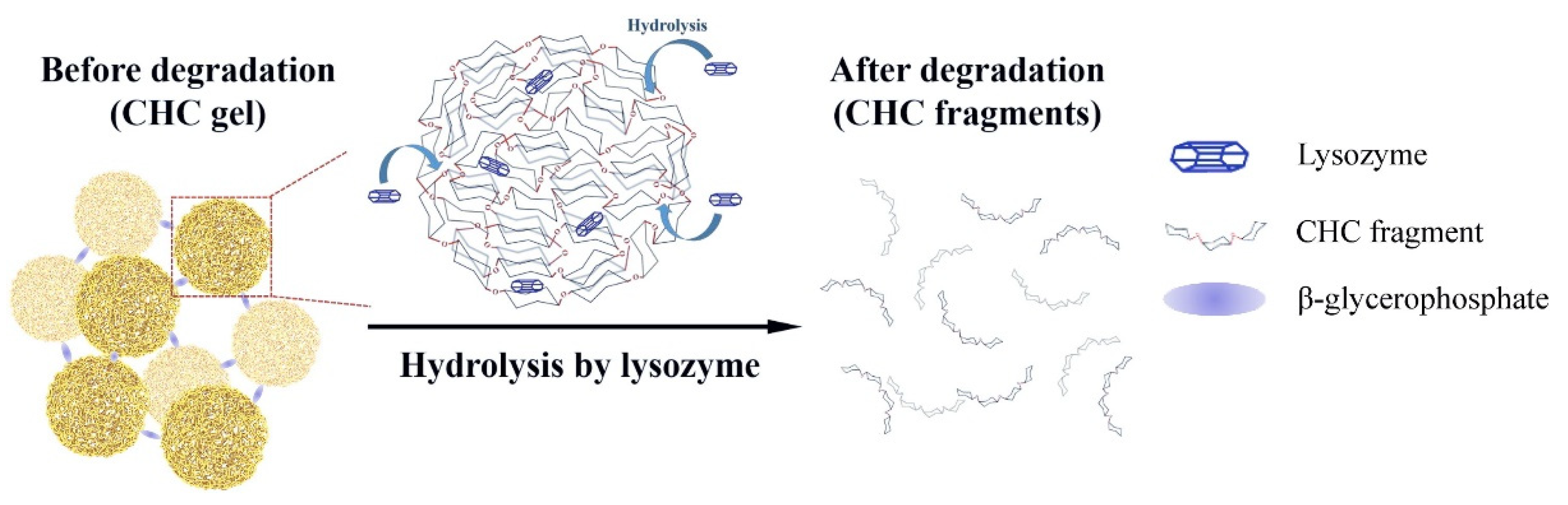
- Chou, H.-S.; Larsson, M.; Hsiao, M.-H.; Chen, Y.-C.; Röding, M.; Nydén, M.; Liu, D.-M. Injectable insulin-lysozyme-loaded nanogels with enzymatically-controlled degradation and release for basal insulin treatment: In vitro characterization and in vivo observation. J. Control. Release 2016, 224, 33–42. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Guo, H.; Li, C.; Yu, D. An injectable and glucose-sensitive nanogel for controlled insulin release. J. Mater. Chem. 2012, 22, 22788–22796. [Google Scholar] [CrossRef]
- Lee, D.; Choe, K.; Jeong, Y.; Yoo, J.; Lee, S.M.; Park, J.-H.; Kim, P.; Kim, Y.-C. Establishment of a controlled insulin delivery system using a glucose-responsive double-layered nanogel. RSC Adv. 2015, 5, 14482–14491. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Sengel, S.B.; Rees, D.J.; Reed, W.F.; Sahiner, N. Responsive biopolymer-based microgels/nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 453–500. [Google Scholar]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, tunable constructs for tissue regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Namdeo, M.; Murali Mohan, Y.; Bajpai, S.; Bajpai, M. Review on polymer, hydrogel and microgel metal nanocomposites: A facile nanotechnological approach. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 45, 107–119. [Google Scholar] [CrossRef]
- Pepe, A.; Podesva, P.; Simone, G. Tunable uptake/release mechanism of protein microgel particles in biomimicking environment. Sci. Rep. 2017, 7, 6014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Askari, V.R. Thermosensitive Chitosan-β-Glycerophosphate Hydrogels as Targeted Drug Delivery Systems: An Overview on Preparation and Their Applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6640893. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, I. Stimuli-Controlled Hydrogels and Their Applications; ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and characterization of chemically cross-linked acrylic acid/gelatin hydrogels: Effect of pH and composition on swelling and drug release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Deen, G.R.; Loh, X.J. Stimuli-responsive cationic hydrogels in drug delivery applications. Gels 2018, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, K.; Ganguly, K.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric hydrogels for oral insulin delivery. J. Control. Release 2013, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Q.; Wang, C.-C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
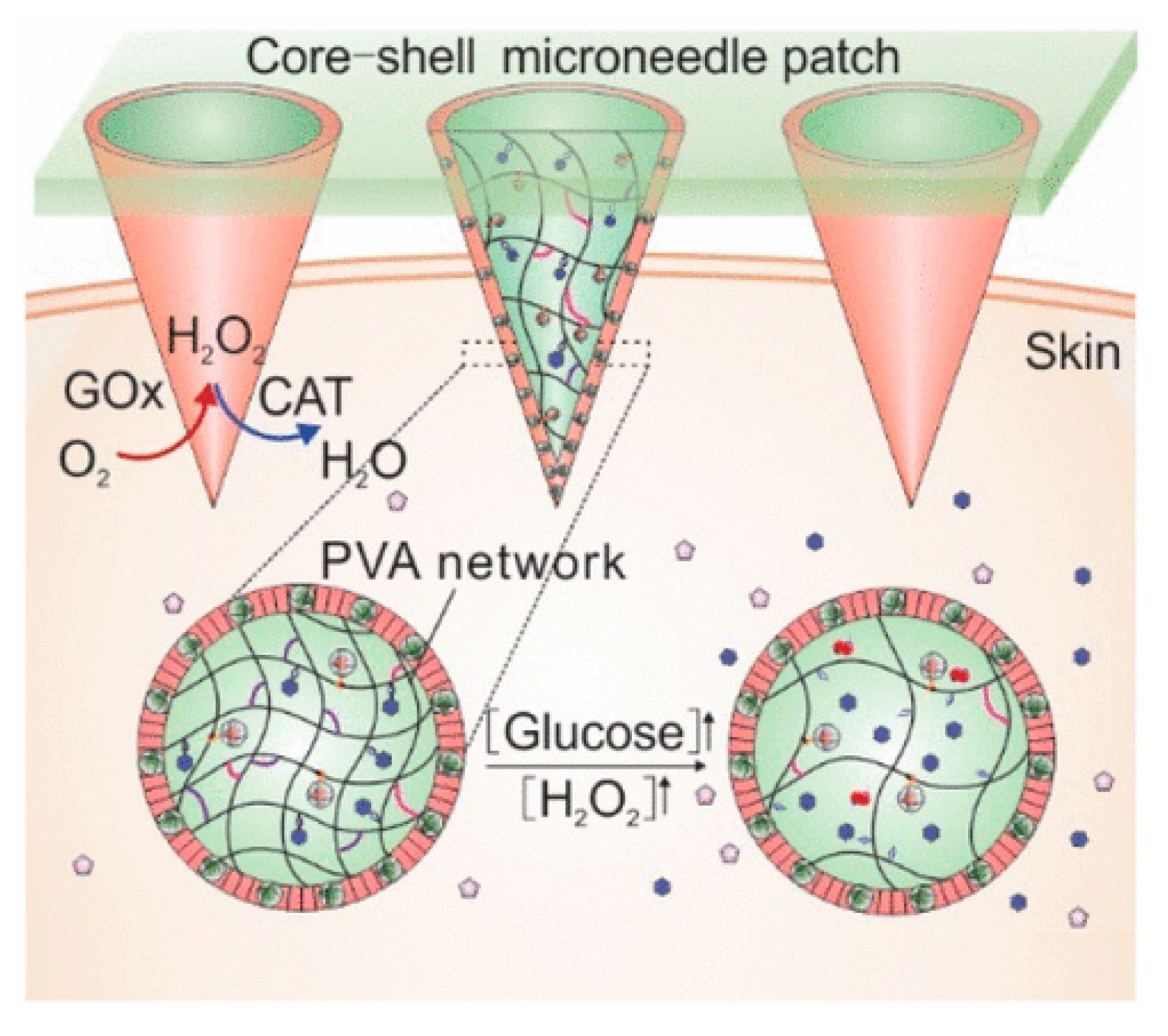
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef]
- Di, J.; Yu, J.; Ye, Y.; Ranson, D.; Jindal, A.; Gu, Z. Engineering Synthetic Insulin-Secreting Cells Using Hyaluronic Acid Microgels Integrated with Glucose-Responsive Nanoparticles. Cell. Mol. Bioeng. 2015, 8, 445–454. [Google Scholar] [CrossRef]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, K.; Du, S.; Chen, L.; Nie, J.; Zhang, W. Design of genipin-crosslinked microgels from concanavalin A and glucosyloxyethyl acrylated chitosan for glucose-responsive insulin delivery. Carbohydr. Polym. 2014, 103, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Li, M.; Ge, S.; Yang, J.; Sun, Q.; Xiong, L. Glucose-responsive biopolymer nanoparticles prepared by co-assembly of concanavalin A and amylopectin for insulin delivery. Ind. Crop. Prod. 2018, 112, 98–104. [Google Scholar] [CrossRef]
- Taylor, M.; Gregory, R.; Tomlins, P.; Jacob, D.; Hubble, J.; Sahota, T. Closed-loop glycaemic control using an implantable artificial pancreas in diabetic domestic pig (Sus scrofa domesticus). Int. J. Pharm. 2016, 500, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yi, J.; Mao, X.; Wu, H.; Zhang, L.-M.; Yang, L. Glucose-sensitive hydrogels from covalently modified carboxylated pullulan and concanavalin A for smart controlled release of insulin. React. Funct. Polym. 2019, 139, 112–119. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Choi, J.S.; Kim, J.S.; Choi, Y.C.; Cho, Y.W. Injectable and Thermosensitive Soluble Extracellular Matrix and Methylcellulose Hydrogels for Stem Cell Delivery in Skin Wounds. Biomacromolecules 2016, 17, 4–11. [Google Scholar] [CrossRef]
- Lee, D.S.; Huynh, D.P.; Thai Minh, D.L.; Trinh, T.A.; Ho, H.G.V.; To, T.C.T.; Nguyen, V.V.L. A Novel Injectable pH-Temperature Sensitive Hydrogel Contained Chitosan-Insulin Electrosprayed Nanospheres Composite For Insulin Delivery System In Type I Diabetes Treatment. Biomater. Sci. 2020, 8, 3830–3843. [Google Scholar] [CrossRef]
- Ghadban, A.; Ahmed, A.S.; Ping, Y.; Ramos, R.; Arfin, N.; Cantaert, B.; Ramanujan, R.V.; Miserez, A. Bioinspired pH and magnetic responsive catechol-functionalized chitosan hydrogels with tunable elastic properties. Chem. Commun. 2016, 52, 697–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karzyński, K.; Kosowska, K.; Ambrożkiewicz, F.; Berman, A.; Cichoń, J.; Klak, M.; Serwańska-Świętek, M.; Wszoła, M. Use of 3D bioprinting in biomedical engineering for clinical application. Med. Stud. Studia Med. 2018, 34, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Kim, J.; Shim, I.K.; Hwang, D.G.; Lee, Y.N.; Kim, M.; Kim, H.; Kim, S.-W.; Lee, S.; Kim, S.C.; Cho, D.-W.; et al. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J. Mater. Chem. B 2019, 7, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel Blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.; Choonara, Y.; Kondiah, P.; Marimuthu, T.; Kumar, P.; du Toit, L.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [Green Version]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; Saenz del Burgo, L.; Pedraz, J.L. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar] [CrossRef]





| Linker Type | Chemical Structure | Degradation Conditions | References |
|---|---|---|---|
| Acetalic linker | 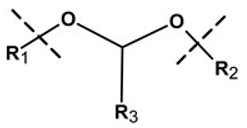 | Hydrolysis in acidic medium, pH = 5 | [22] |
| Ketal linker | 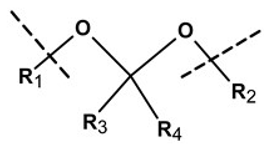 | Hydrolysis in acidic medium, pH = 5.5 | [23] |
| Ester linker | 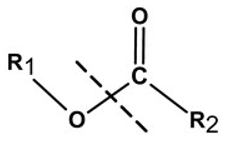 | Hydrolysis below physiological pH | [24] |
| Vinyl ether linker | 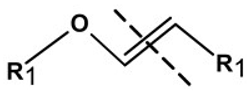 | Hydrolysis in acidic medium, pH < 5 | [25] |
| Linker based on ortho-nitrobenzyl ester |  | Hydrolysis under the influence of UV 315–390 nm | [26] |
| Linker based on disulfide or diselenide bridges |  | Hydrolysis in the presence of GSH carboxyethylphosphine tris (TCEP), and dithiothreitol (DTT) | [27,28,29] |
| Phosphoester linker |  | Hydrolysis in the presence of phosphatase or phospholipase enzyme | [30] |
| System Employed | Polymers Utilized | Insulin Encapsulation Efficiency (EE)/Loading Capacity (LC) (%) | Insulin Release Time | In Vitro or In Vivo Studies Carried Out | References |
|---|---|---|---|---|---|
| Nanoparticles within microgel | -Alginate -Acetylated dextran | 39% -EE 6.5% -LC | 22 days (2 doses) in vivo | In vitro and in vivo studies carried out | [45] |
| Microgel encapsulated microspheres | -CHC (Chitosan) | 62.96 ± 0.68% -EE | 7 days in vitro | In vitro studies | [46] |
| Ultrasound triggered nanocapsules within microgel | -PLGA -CHC | 71.3 ± 1.8% -EE 11.9 ± 0.6% -LC | 10 days in vitro 7 days in vivo | In vitro and in vivo studies carried out | [47] |
| Self-assembled nanoparticles in gel (nanogel) | -Carboxymethyl-hexanoyl CHC | Insulin loaded 5 mg/mL | 10 days in vivo | In vitro and in vivo studies carried out | [48] |
| Monodispersed nanogels | -Poly(N-isopropylacrylamide) -Dextran -Poly(3-acrylamidophenylboronic acid) | 80.6% -EE 16.2% -LC | 2 h in vivo | In vitro and in vivo studies carried out | [49] |
| Double-layered nanogel | -Glycol CHC -Sodium alginate poly (L-glutmate-co-N-3-L-glutamylphenylboronic acid) | 71 ± 3.5% -LC | 3 h in vivo | In vitro and in vivo studies carried out. | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Hydrogels for the Controlled Delivery of Insulin. Pharmaceutics 2021, 13, 2113. https://doi.org/10.3390/pharmaceutics13122113
Mansoor S, Kondiah PPD, Choonara YE. Advanced Hydrogels for the Controlled Delivery of Insulin. Pharmaceutics. 2021; 13(12):2113. https://doi.org/10.3390/pharmaceutics13122113
Chicago/Turabian StyleMansoor, Shazia, Pierre P. D. Kondiah, and Yahya E. Choonara. 2021. "Advanced Hydrogels for the Controlled Delivery of Insulin" Pharmaceutics 13, no. 12: 2113. https://doi.org/10.3390/pharmaceutics13122113
APA StyleMansoor, S., Kondiah, P. P. D., & Choonara, Y. E. (2021). Advanced Hydrogels for the Controlled Delivery of Insulin. Pharmaceutics, 13(12), 2113. https://doi.org/10.3390/pharmaceutics13122113









