Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research
Abstract
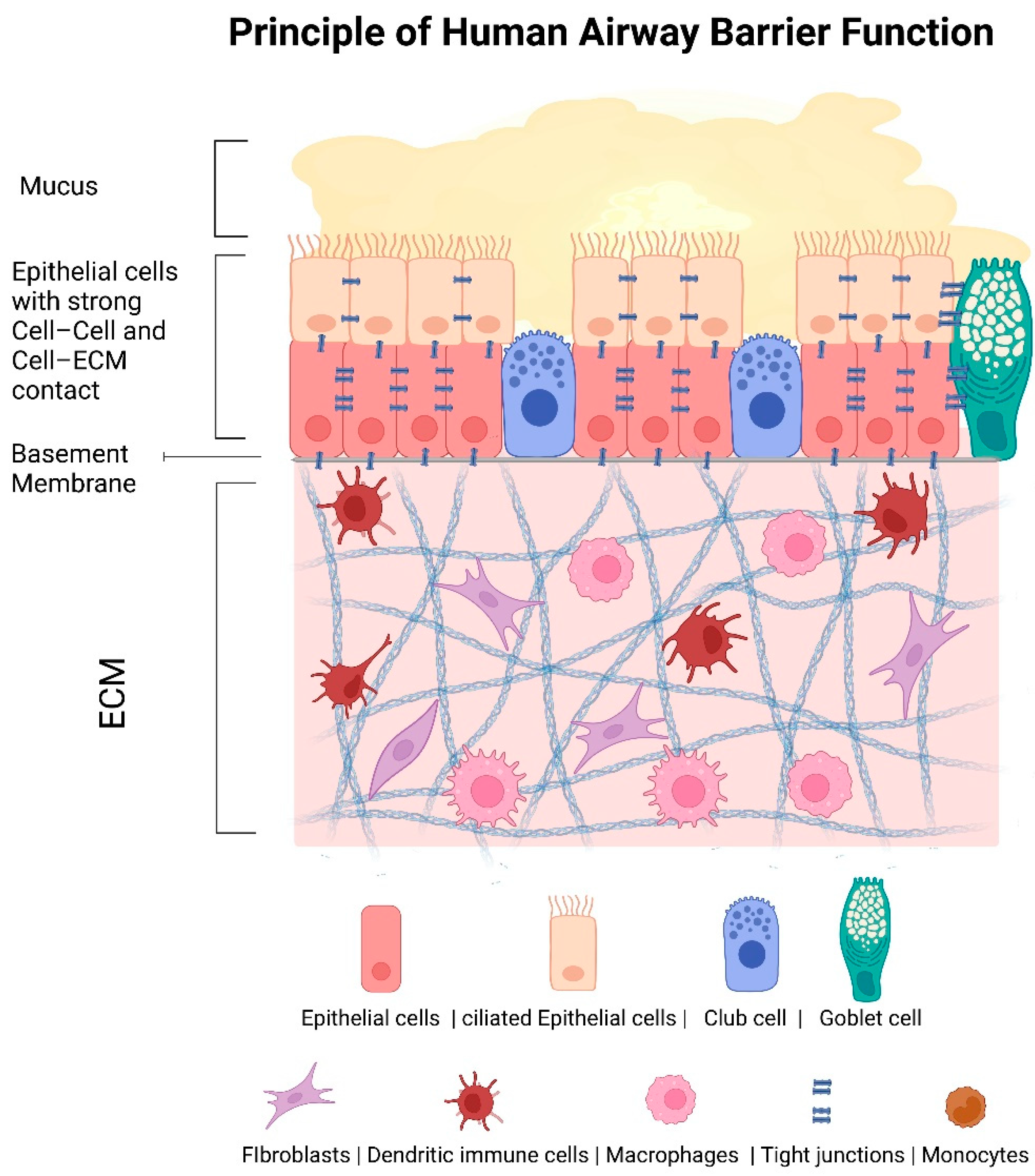
:1. Introduction
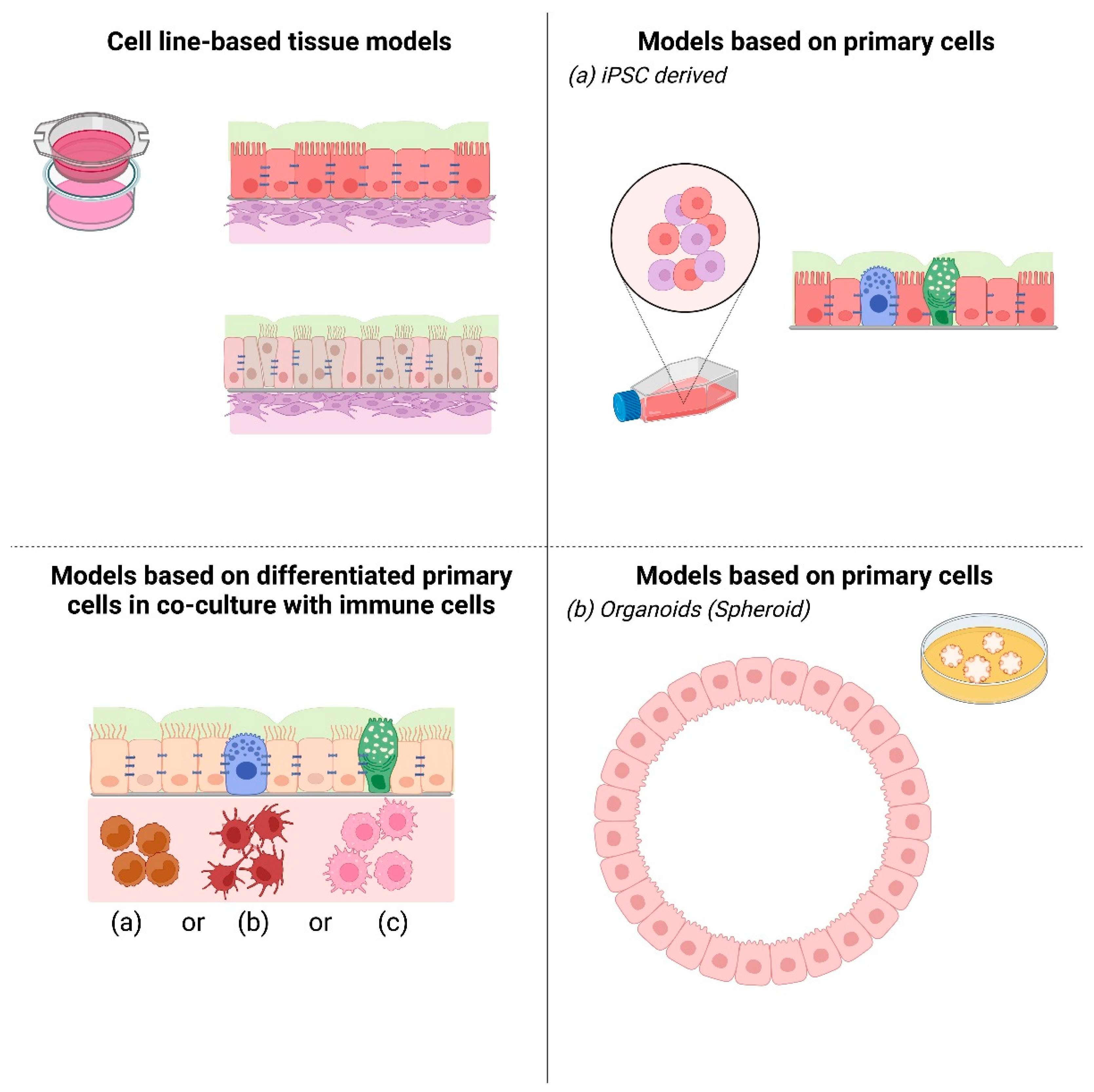
2. Cell Line-Based 3D Airway Tissue Models
3. Primary Cells Derived 3D Lower Airway Models
3.1. In Vitro 3D Cultures of Biopsies
3.2. iPS Differentiated Cells, Human Pluripotent Stem Cells
3.3. Organoids
4. Challenges
4.1. Determine the Barrier Function by Measuring the Electrical Resistance (TEER)
4.2. Involvement of Immune Cells
4.3. Live Cell Microscopy
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ivanov, A.I. Welcome to Tissue Barriers. Tissue Barriers 2013, 1, e24240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuong, C.; Nickoloff, B.; Elias, P.; Goldsmith, L.; Macher, E.; Maderson, P.; Sundberg, J.; Tagami, H.; Plonka, P.; Thestrup-Pederson, K.; et al. What is the “true” Function of Skin? Exp. Dermatol. 2002, 11, 159–187. [Google Scholar] [CrossRef]
- Pierce, R.; Worsnop, C. Upper Airway Function and Dysfunction in Respiration. Clin. Exp. Pharmacol. Physiol. 1999, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, C. Differences between Men and Women with Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2021, 42, 443–456. [Google Scholar] [CrossRef]
- Meehan, J.; Gray, M.; Martínez-Pérez, C.; Kay, C.; McLaren, D.; Turnbull, A.K. Tissue- and Liquid-Based Biomarkers in Prostate Cancer Precision Medicine. J. Pers. Med. 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Houser, S.R.; Margulies, K.B.; Murphy, A.M.; Spinale, F.G.; Francis, G.S.; Prabhu, S.D.; Rockman, H.A.; Kass, D.A.; Molkentin, J.D.; Sussman, M.A.; et al. Animal Models of Heart Failure: A Scientific Statement from the American Heart Association. Circ. Res. 2012, 111, 131–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selo, M.A.; Sake, J.A.; Kim, K.-J.; Ehrhardt, C. In Vitro and Ex Vivo Models in Inhalation Biopharmaceutical Research—Advances, Challenges and Future Perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113862. [Google Scholar] [CrossRef]
- Shen, B.Q.; Finkbeiner, W.E.; Wine, J.J.; Mrsny, R.J.; Widdicombe, J.H. Calu-3: A Human Airway Epithelial Cell Line That Shows CAMP-Dependent Cl- Secretion. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1994, 266, L493–L501. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Mustafa, F.; Bai, S.; Ahsan, F. Pulmonary Delivery of Low Molecular Weight Heparins. Pharm. Res. 2004, 21, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Grainger, C.I.; Greenwell, L.L.; Lockley, D.J.; Martin, G.P.; Forbes, B. Culture of Calu-3 Cells at the Air Interface Provides a Representative Model of the Airway Epithelial Barrier. Pharm. Res. 2006, 23, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Harrington, H.; Cato, P.; Salazar, F.; Wilkinson, M.; Knox, A.; Haycock, J.W.; Rose, F.; Aylott, J.W.; Ghaemmaghami, A.M. Immunocompetent 3D Model of Human Upper Airway for Disease Modeling and in Vitro Drug Evaluation. Mol. Pharm. 2014, 11, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Hevir-Kene, N.; Rižner, T.L.; Peternel, L.; Kristan, K. The Characterization of the Human Cell Line Calu-3 under Different Culture Conditions and Its Use as an Optimized in Vitro Model to Investigate Bronchial Epithelial Function. Eur. J. Pharm. Sci. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for in Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.; Sivarajan, R.; Walles, T.; Hackenberg, S.; Steinke, M. Susceptibility of Primary Human Airway Epithelial Cells to Bordetella Pertussis Adenylate Cyclase Toxin in Two- and Three-Dimensional Culture Conditions. Innate Immun. 2021, 27, 89–98. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumors in Nude Mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Winton, H.L.; Soeller, C.; Stewart, G.A.; Thompson, P.J.; Gruenert, D.C.; Cannell, M.B.; Garrod, D.R.; Robinson, C. Tight Junction Properties of the Immortalized Human Bronchial Epithelial Cell Lines Calu-3 and 16HBE14o. Eur. Respir. J. 2000, 15, 1058–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Reenstra, W.W.; Chidekel, A. Antibacterial Activity of Apical Surface Fluid from the Human Airway Cell Line Calu-3: Pharmacologic Alteration by Corticosteroids and β2-Agonists. Am. J. Respir. Cell Mol. Biol. 2001, 25, 196–202. [Google Scholar] [CrossRef]
- Finkbeiner, W.E.; Carrier, S.D.; Teresi, C.E. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Phenotypic Analysis of Cell Cultures of Human Tracheal Epithelium, Tracheobronchial Glands, and Lung Carcinomas. Am. J. Respir. Cell Mol. Biol. 1993, 9, 547–556. [Google Scholar] [CrossRef]
- Steinke, M.; Gross, R.; Walles, H.; Gangnus, R.; Schütze, K.; Walles, T. An Engineered 3D Human Airway Mucosa Model Based on an SIS Scaffold. Biomaterials 2014, 35, 7355–7362. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Skibinski, G.; Elborn, J.S.; Ennis, M. Bronchial Epithelial Cell Growth Regulation in Fibroblast Cocultures: The Role of Hepatocyte Growth Factor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L69–L76. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the Tumor Microenvironment: Cancer Associated Fibroblast Gene Expression Patterns Identify Both Common and Unique Features of Tumor-Stroma Crosstalk across Cancer Types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef]
- Manninen, A. Epithelial Polarity—Generating and Integrating Signals from the ECM with Integrins. Exp. Cell Res. 2015, 334, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Sandilands, E.; Mostov, K.E.; Bryant, D.M. Fibroblast-Derived HGF Drives Acinar Lung Cancer Cell Polarization through Integrin-Dependent RhoA-ROCK1 Inhibition. Cell. Signal. 2017, 40, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Wiese-Rischke, C.; (Otto-von-Guericke-University Magdeburg, University Clinic for Cardio and Thoracic Surgery, Magdeburg, Germany); Walles, H.; (Otto-von-Guericke-University Magdeburg, Magdeburg, Germany). Human 3D Airway Tissue Models for Real-Time Monitoring of Respiratory Virus Spreading. Personal communication, 2021. [Google Scholar]
- Pezron, I.; Mitra, R.; Pal, D.; Mitra, A.K. Insulin Aggregation and Asymmetric Transport across Human Bronchial Epithelial Cell Monolayers (Calu-3). J. Pharm. Sci. 2002, 91, 1135–1146. [Google Scholar] [CrossRef]
- Patel, J.; Pal, D.; Vangala, V.; Gandhi, M.; Mitra, A.K. Transport of HIV-Protease Inhibitors across 1 Alpha,25di-Hydroxy Vitamin D3-Treated Calu-3 Cell Monolayers: Modulation of P-Glycoprotein Activity. Pharm. Res. 2002, 19, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Florea, B.I.; Cassara, M.L.; Junginger, H.E.; Borchard, G. Drug Transport and Metabolism Characteristics of the Human Airway Epithelial Cell Line Calu-3. J. Control. Release 2003, 87, 131–138. [Google Scholar] [CrossRef]
- Lodes, N.; Seidensticker, K.; Perniss, A.; Nietzer, S.; Oberwinkler, H.; May, T.; Walles, T.; Hebestreit, H.; Hackenberg, S.; Steinke, M. Investigation on Ciliary Functionality of Different Airway Epithelial Cell Lines in Three-Dimensional Cell Culture. Tissue Eng. Part A 2020, 26, 432–440. [Google Scholar] [CrossRef]
- Foster, K.A.; Avery, M.L.; Yazdanian, M.; Audus, K.L. Characterization of the Calu-3 Cell Line as a Tool to Screen Pulmonary Drug Delivery. Int. J. Pharm. 2000, 208, 1–11. [Google Scholar] [CrossRef]
- Rojanasakul, Y.; Wang, L.; Bhat, M.; Glover, D.D.; Malanga, C.J.; Ma, J.K.H. The Transport Barrier of Epithelia: A Comparative Study on Membrane Permeability and Charge Selectivity in the Rabbit. Pharm. Res. 1992, 9, 1029–1034. [Google Scholar] [CrossRef]
- Leung, C.; Wadsworth, S.J.; Yang, S.J.; Dorscheid, D.R. Structural and Functional Variations in Human Bronchial Epithelial Cells Cultured in Air-Liquid Interface Using Different Growth Media. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1063–L1073. [Google Scholar] [CrossRef] [PubMed]
- Elbrecht, D.H.; Long, C.J.; Hickman, J. Transepithelial/Endothelial Electrical Resistance (TEER) Theory and Applications for Microfluidic Body-on-a-Chip Devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Lieber, M.; Todaro, G.; Smith, B.; Szakal, A.; Nelson-Rees, W. A Continuous Tumor-Cell Line from a Human Lung Carcinoma with Properties of Type II Alveolar Epithelial Cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Fiegel, J.; Fuchs, S.; Abu-Dahab, R.; Schaefer, U.F.; Hanes, J.; Lehr, C.-M. Drug Absorption by the Respiratory Mucosa: Cell Culture Models and Particulate Drug Carriers. J. Aerosol Med. 2002, 15, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Göttlich, C.; Müller, L.C.; Kunz, M.; Schmitt, F.; Walles, H.; Walles, T.; Dandekar, T.; Dandekar, G.; Nietzer, S.L. A Combined 3D Tissue Engineered In Vitro/In Silico Lung Tumor Model for Predicting Drug Effectiveness in Specific Mutational Backgrounds. J. Vis. Exp. 2016, 110, e53885. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.G.; Neufurth, M.; Wang, S.; Tan, R.; Schröder, H.C.; Wang, X. Morphogenetic (Mucin Expression) as Well as Potential Anti-Corona Viral Activity of the Marine Secondary Metabolite Polyphosphate on A549 Cells. Mar. Drugs 2020, 18, 639. [Google Scholar] [CrossRef]
- Endter, S.; Francombe, D.; Ehrhardt, C.; Gumbleton, M. RT-PCR Analysis of ABC, SLC and SLCO Drug Transporters in Human Lung Epithelial Cell Models. J. Pharm. Pharmacol. 2009, 61, 583–591. [Google Scholar] [CrossRef]
- Liu, H.; Pu, Y.; Amina, Q.; Wang, Q.; Zhang, M.; Song, J.; Guo, J.; Mardan, M. Prognostic and Therapeutic Potential of Adenylate Kinase 2 in Lung Adenocarcinoma. Sci. Rep. 2019, 9, 17757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Birch, N.P.; Suresh, V. An Optimised Human Cell Culture Model for Alveolar Epithelial Transport. PLoS ONE 2016, 11, e0165225. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Shi, Y.-F.; Yang, J.-J.; Hsiao, Y.-C.; Tzang, B.-S.; Hsu, T.-C. Effects of Human Parvovirus B19 and Bocavirus VP1 Unique Region on Tight Junction of Human Airway Epithelial A549 Cells. PLoS ONE 2014, 9, e107970. [Google Scholar] [CrossRef]
- Walles, H.; (Otto-von-Guericke-University Magdeburg, Magdeburg, Germany). 3D Model to Study Bacterial Infection Mechanisms. Personal communication, 2021. [Google Scholar]
- Dvorak, A.; Tilley, A.E.; Shaykhiev, R.; Wang, R.; Crystal, R.G. Do Airway Epithelium Air–Liquid Cultures Represent the in Vivo Airway Epithelium Transcriptome? Am. J. Respir. Cell Mol. Biol. 2011, 44, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Rackley, C.R.; Stripp, B.R. Building and Maintaining the Epithelium of the Lung. J. Clin. Invest. 2012, 122, 2724–2730. [Google Scholar] [CrossRef] [Green Version]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in Vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef]
- Wohnhaas, C.T.; Gindele, J.A.; Kiechle, T.; Shen, Y.; Leparc, G.G.; Stierstorfer, B.; Stahl, H.; Gantner, F.; Viollet, C.; Schymeinsky, J.; et al. Cigarette Smoke Specifically Affects Small Airway Epithelial Cell Populations and Triggers the Expansion of Inflammatory and Squamous Differentiation Associated Basal Cells. Int. J. Mol. Sci. 2021, 22, 7646. [Google Scholar] [CrossRef]
- Huang, C.-G.; Lee, L.-A.; Wu, Y.-C.; Hsiao, M.-J.; Horng, J.-T.; Kuo, R.-L.; Huang, C.-H.; Lin, Y.-C.; Tsao, K.-C.; Chen, M.-C.; et al. A Pilot Study on Primary Cultures of Human Respiratory Tract Epithelial Cells to Predict Patients’ Responses to H7N9 Infection. Oncotarget 2018, 9, 14492–14508. [Google Scholar] [CrossRef] [Green Version]
- Touret, F.; Driouich, J.-S.; Cochin, M.; Petit, P.R.; Gilles, M.; Barthélémy, K.; Moureau, G.; Mahon, F.-X.; Malvy, D.; Solas, C.; et al. Preclinical Evaluation of Imatinib Does Not Support Its Use as an Antiviral Drug against SARS-CoV-2. Antivir. Res. 2021, 193, 105137. [Google Scholar] [CrossRef]
- Wong, A.P.; Bear, C.E.; Chin, S.; Pasceri, P.; Thompson, T.O.; Huan, L.-J.; Ratjen, F.; Ellis, J.; Rossant, J. Directed Differentiation of Human Pluripotent Stem Cells into Mature Airway Epithelia Expressing Functional CFTR Protein. Nat. Biotechnol. 2012, 30, 876–882. [Google Scholar] [CrossRef] [Green Version]
- Jacob, A.; Morley, M.; Hawkins, F.; McCauley, K.B.; Jean, J.C.; Heins, H.; Na, C.-L.; Weaver, T.E.; Vedaie, M.; Hurley, K.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 2017, 21, 472–488.e10. [Google Scholar] [CrossRef] [PubMed]
- Tamò, L.; Hibaoui, Y.; Kallol, S.; Alves, M.P.; Albrecht, C.; Hostettler, K.E.; Feki, A.; Rougier, J.-S.; Abriel, H.; Knudsen, L.; et al. Generation of an Alveolar Epithelial Type II Cell Line from Induced Pluripotent Stem Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L921–L932. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, S.; Ninaber, D.; Rottier, R.; Freund, C.; Hiemstra, P. Generation of Alveolar Epithelial Cells from Human Induced Pluripotent Stem Cells for a Model of Alveolar Wound Repair. In Proceedings of the Mechanisms of Lung Injury and Repair. Eur. Respir. J. 2018, 52, LSC-1122. [Google Scholar] [CrossRef]
- Kanagaki, S.; Ikeo, S.; Suezawa, T.; Yamamoto, Y.; Seki, M.; Hirai, T.; Hagiwara, M.; Suzuki, Y.; Gotoh, S. Directed Induction of Alveolar Type I Cells Derived from Pluripotent Stem Cells via Wnt Signaling Inhibition. Stem Cells 2021, 39, 156–169. [Google Scholar] [CrossRef]
- Benali, R.; Tournier, J.M.; Chevillard, M.; Zahm, J.M.; Klossek, J.M.; Hinnrasky, J.; Gaillard, D.; Maquart, F.X.; Puchelle, E. Tubule Formation by Human Surface Respiratory Epithelial Cells Cultured in a Three-Dimensional Collagen Lattice. Am. J. Physiol. Lung Cell. Mol. Physiol. 1993, 264, L183–L192. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Huch, M. Disease Modelling in Human Organoids. Dis. Model. Mech. 2019, 12, dmm039347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, B.S.; Zheng, D.; Li Yeo, J.S.; Yang, H.H.; Ng, S.Y.; Wong, L.H.; Zhang, W.; Li, P.; Nichane, M.; Asmat, A.; et al. CD166pos Subpopulation From Differentiated Human ES and IPS Cells Support Repair of Acute Lung Injury. Mol. Ther. 2012, 20, 2335–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.-H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In Vitro Generation of Human Pluripotent Stem Cell Derived Lung Organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef]
- Anderson, J.D.; Liu, Z.; Odom, L.V.; Kersh, L.; Guimbellot, J.S. CFTR Function and Clinical Response to Modulators Parallel Nasal Epithelial Organoid Swelling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L119–L129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Huang, S.X.; de Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A Three-Dimensional Model of Human Lung Development and Disease from Pluripotent Stem Cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef]
- Porotto, M.; Ferren, M.; Chen, Y.-W.; Siu, Y.; Makhsous, N.; Rima, B.; Briese, T.; Greninger, A.L.; Snoeck, H.-W.; Moscona, A. Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids. Mbio 2019, 10, e00723-19. [Google Scholar] [CrossRef] [Green Version]
- Lamers, M.M.; Vaart, J.; Knoops, K.; Riesebosch, S.; Breugem, T.I.; Mykytyn, A.Z.; Beumer, J.; Schipper, D.; Bezstarosti, K.; Koopman, C.D.; et al. An Organoid-derived Bronchioalveolar Model for SARS-CoV-2 Infection of Human Alveolar Type II-like Cells. EMBO J. 2021, 40, e105912. [Google Scholar] [CrossRef]
- Dye, B.R.; Dedhia, P.H.; Miller, A.J.; Nagy, M.S.; White, E.S.; Shea, L.D.; Spence, J.R. A Bioengineered Niche Promotes in Vivo Engraftment and Maturation of Pluripotent Stem Cell Derived Human Lung Organoids. eLife 2016, 5, e19732. [Google Scholar] [CrossRef]
- Schweinlin, M.; Rossi, A.; Lodes, N.; Lotz, C.; Hackenberg, S.; Steinke, M.; Walles, H.; Groeber, F. Human Barrier Models for the in Vitro Assessment of Drug Delivery. Drug Deliv. Transl. Res. 2017, 7, 217–227. [Google Scholar] [CrossRef]
- Kiesewetter, L.; Littau, L.; Walles, H.; Boccaccini, A.R.; Groeber-Becker, F. Reepithelialization in Focus: Non-Invasive Monitoring of Epidermal Wound Healing in Vitro. Biosens. Bioelectron. 2019, 142, 111555. [Google Scholar] [CrossRef] [PubMed]
- Zoio, P.; Lopes-Ventura, S.; Oliva, A. Barrier-on-a-Chip with a Modular Architecture and Integrated Sensors for Real-Time Measurement of Biological Barrier Function. Micromachines 2021, 12, 816. [Google Scholar] [CrossRef]
- Marin, T.M.; de Indolfo, N.C.; Rocco, S.A.; de Carvalho, M.; Dias, M.M.; Bento, G.I.V.; Bortot, L.O.; Schuck, D.C.; Lorencini, M.; Pagani, E. An Intestine/Liver Microphysiological System for Drug Pharmacokinetic and Toxicological Assessment. J. Vis. Exp. 2020, 166, 60184. [Google Scholar] [CrossRef]
- Holthaus, D.; Delgado-Betancourt, E.; Aebischer, T.; Seeber, F.; Klotz, C. Harmonization of Protocols for Multi-Species Organoid Platforms to Study the Intestinal Biology of Toxoplasma Gondii and Other Protozoan Infections. Front. Cell. Infect. Microbiol. 2021, 10, 610368. [Google Scholar] [CrossRef]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Interaktionen Zwischen Respiratorischem Mikrobiom Und Epithelzellen Formen Immunität in Der Lunge. Kompass Pneumol. 2020, 8, 240–250. [Google Scholar] [CrossRef]
- Zeis, P.; Lian, M.; Fan, X.; Herman, J.S.; Hernandez, D.C.; Gentek, R.; Elias, S.; Symowski, C.; Knöpper, K.; Peltokangas, N.; et al. In Situ Maturation and Tissue Adaptation of Type 2 Innate Lymphoid Cell Progenitors. Immunity 2020, 53, 775–792.e9. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and Characterization of a Human Acute Monocytic Leukemia Cell Line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Sadofsky, L.R.; Hayman, Y.A.; Vance, J.; Cervantes, J.L.; Fraser, S.D.; Wilkinson, H.N.; Williamson, J.D.; Hart, S.P.; Morice, A.H. Characterisation of a New Human Alveolar Macrophage-Like Cell Line (Daisy). Lung 2019, 197, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Montefusco-Pereira, C.V.; Horstmann, J.C.; Ebensen, T.; Beisswenger, C.; Bals, R.; Guzmán, C.A.; Schneider-Daum, N.; de Carvalho-Wodarz, C.S.; Lehr, C.-M.P. aeruginosa Infected 3D Co-Culture of Bronchial Epithelial Cells and Macrophages at Air-Liquid Interface for Preclinical Evaluation of Anti-Infectives. J. Vis. Exp. 2020, 160, e61069. [Google Scholar] [CrossRef] [PubMed]
- He, R.-W.; Braakhuis, H.M.; Vandebriel, R.J.; Staal, Y.C.M.; Gremmer, E.R.; Fokkens, P.H.B.; Kemp, C.; Vermeulen, J.; Westerink, R.H.S.; Cassee, F.R. Optimization of an Air-Liquid Interface in Vitro Cell Co-Culture Model to Estimate the Hazard of Aerosol Exposures. J. Aerosol Sci. 2021, 153, 105703. [Google Scholar] [CrossRef]
- Chandorkar, P.; Posch, W.; Zaderer, V.; Blatzer, M.; Steger, M.; Ammann, C.G.; Binder, U.; Hermann, M.; Hörtnagl, P.; Lass-Flörl, C.; et al. Fast-Track Development of an in Vitro 3D Lung/Immune Cell Model to Study Aspergillus Infections. Sci. Rep. 2017, 7, 11644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, J.; Koh, V.; So, J.B.Y.; Yong, W.P.; Zavros, Y. A Preclinical Human-Derived Autologous Gastric Cancer Organoid/Immune Cell Co-Culture Model to Predict the Efficacy of Targeted Therapies. J. Vis. Exp. 2021, 173, e61443. [Google Scholar] [CrossRef]
- Liu, J.; Cui, Z. Fluorescent Labeling of Proteins of Interest in Live Cells: Beyond Fluorescent Proteins. Bioconjug. Chem. 2020, 31, 1587–1595. [Google Scholar] [CrossRef]
- Jacquemet, G.; Carisey, A.F.; Hamidi, H.; Henriques, R.; Leterrier, C. The Cell Biologist’s Guide to Super-Resolution Microscopy. J. Cell Sci. 2020, 133, jcs240713. [Google Scholar] [CrossRef]
- Nonaka, P.N.; Uriarte, J.J.; Campillo, N.; Oliveira, V.R.; Navajas, D.; Farré, R. Lung Bioengineering: Physical Stimuli and Stem/Progenitor Cell Biology Interplay towards Biofabricating a Functional Organ. Respir. Res. 2016, 17, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaderer, V.; Hermann, M.; Lass-Flörl, C.; Posch, W.; Wilflingseder, D. Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model. Cells 2019, 8, 1292. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiese-Rischke, C.; Murkar, R.S.; Walles, H. Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research. Pharmaceutics 2021, 13, 2115. https://doi.org/10.3390/pharmaceutics13122115
Wiese-Rischke C, Murkar RS, Walles H. Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research. Pharmaceutics. 2021; 13(12):2115. https://doi.org/10.3390/pharmaceutics13122115
Chicago/Turabian StyleWiese-Rischke, Cornelia, Rasika S. Murkar, and Heike Walles. 2021. "Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research" Pharmaceutics 13, no. 12: 2115. https://doi.org/10.3390/pharmaceutics13122115
APA StyleWiese-Rischke, C., Murkar, R. S., & Walles, H. (2021). Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research. Pharmaceutics, 13(12), 2115. https://doi.org/10.3390/pharmaceutics13122115






