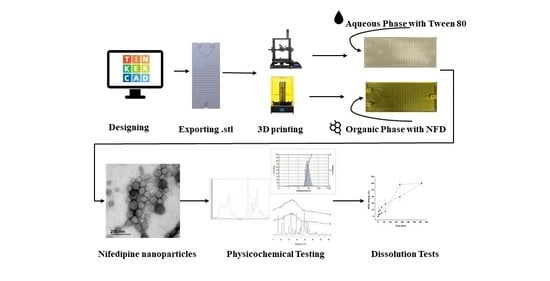
Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
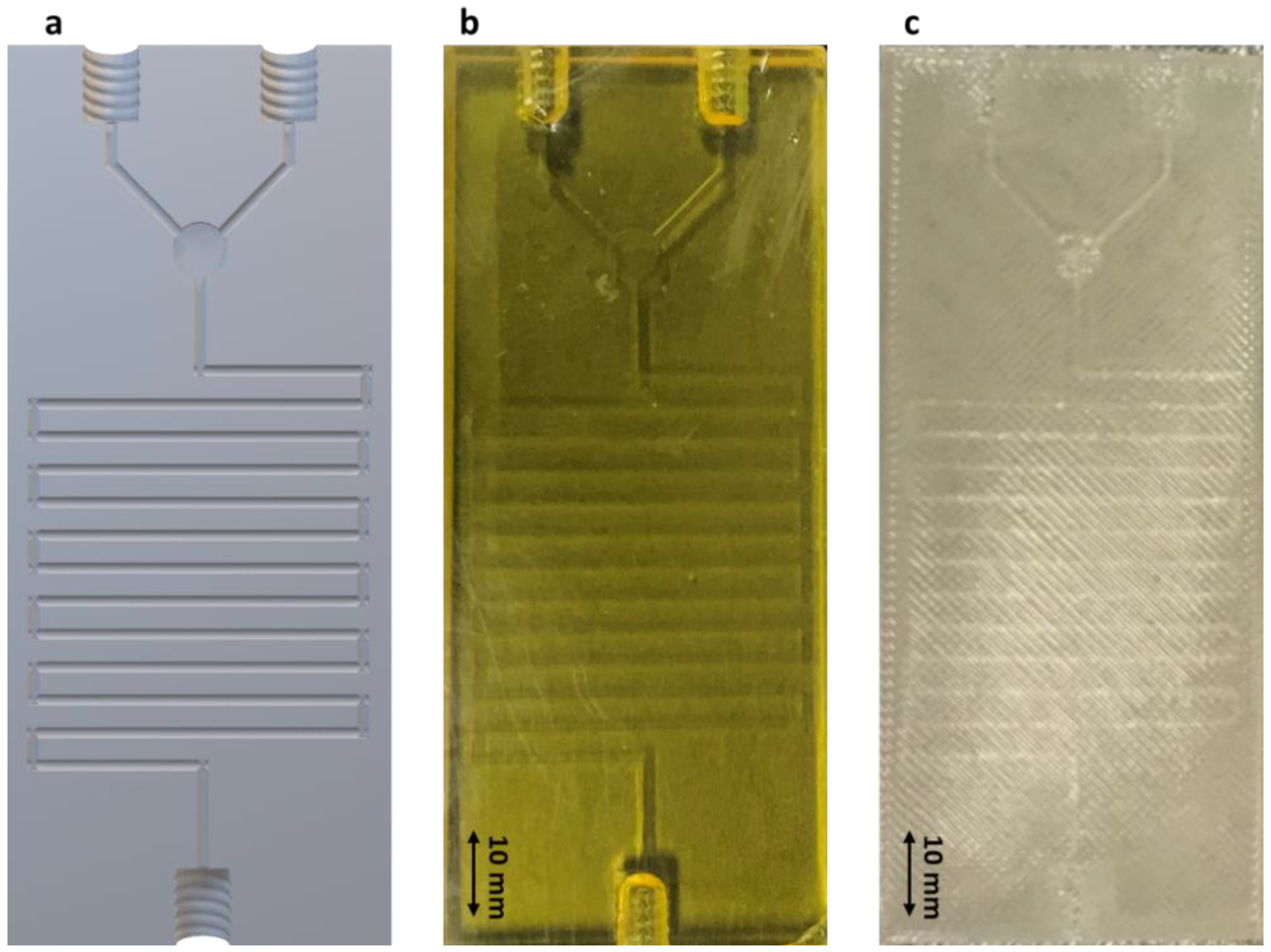
2.2. Microfluidic Chip Manufacturing and Characterization
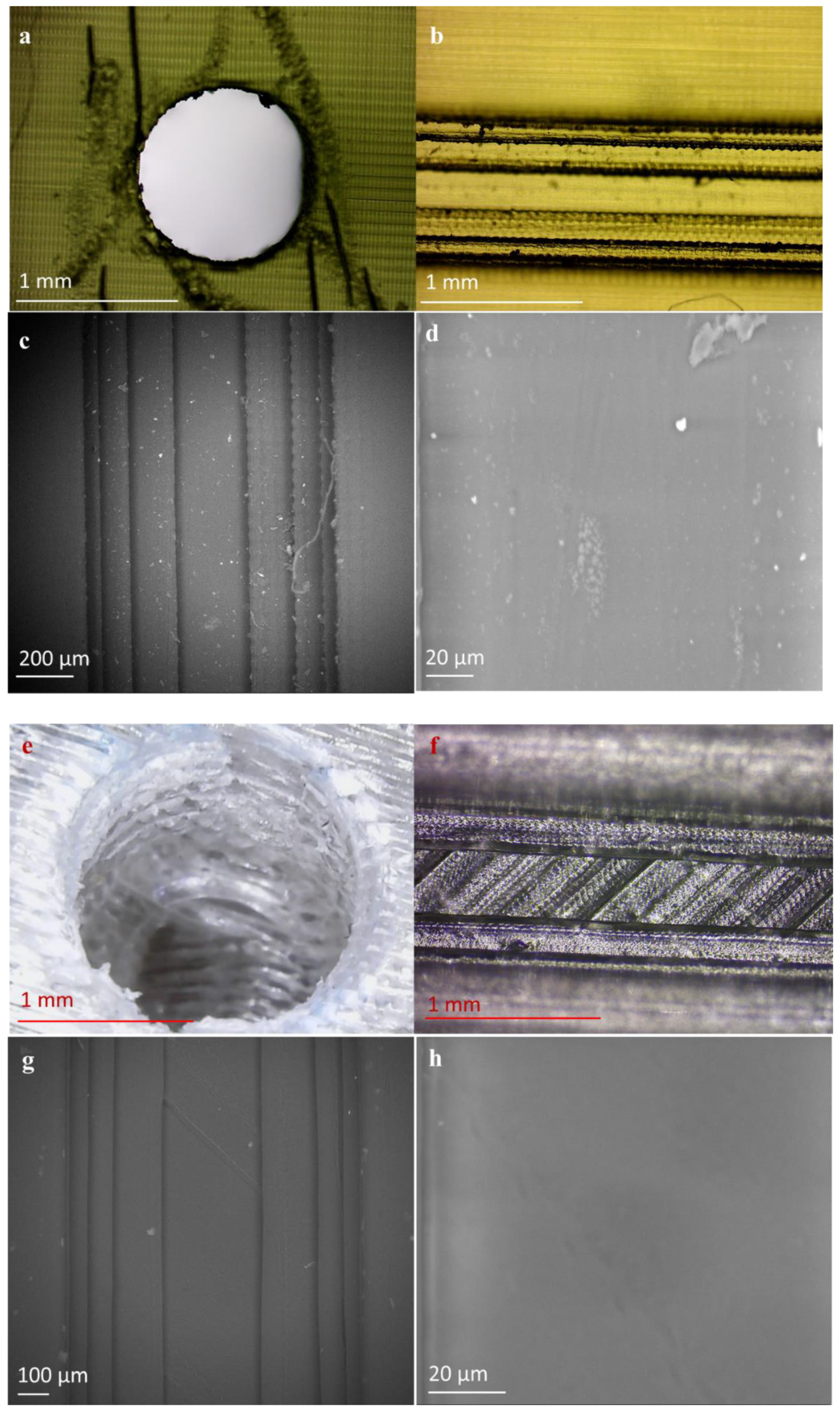
2.2.1. Imaging
2.2.2. Channel Surface Roughness
2.2.3. Manufacturing of Nifedipine Polymeric Nanoparticles with 3D Printed Microfluidic Chips
2.3. Preparation of Nifedipine Polymeric Nanoparticles with Solvent Evaporation
2.4. Drug Loading
2.5. Particle Size and Zeta Potential
2.6. Transmission Electron Microscopy (TEM)
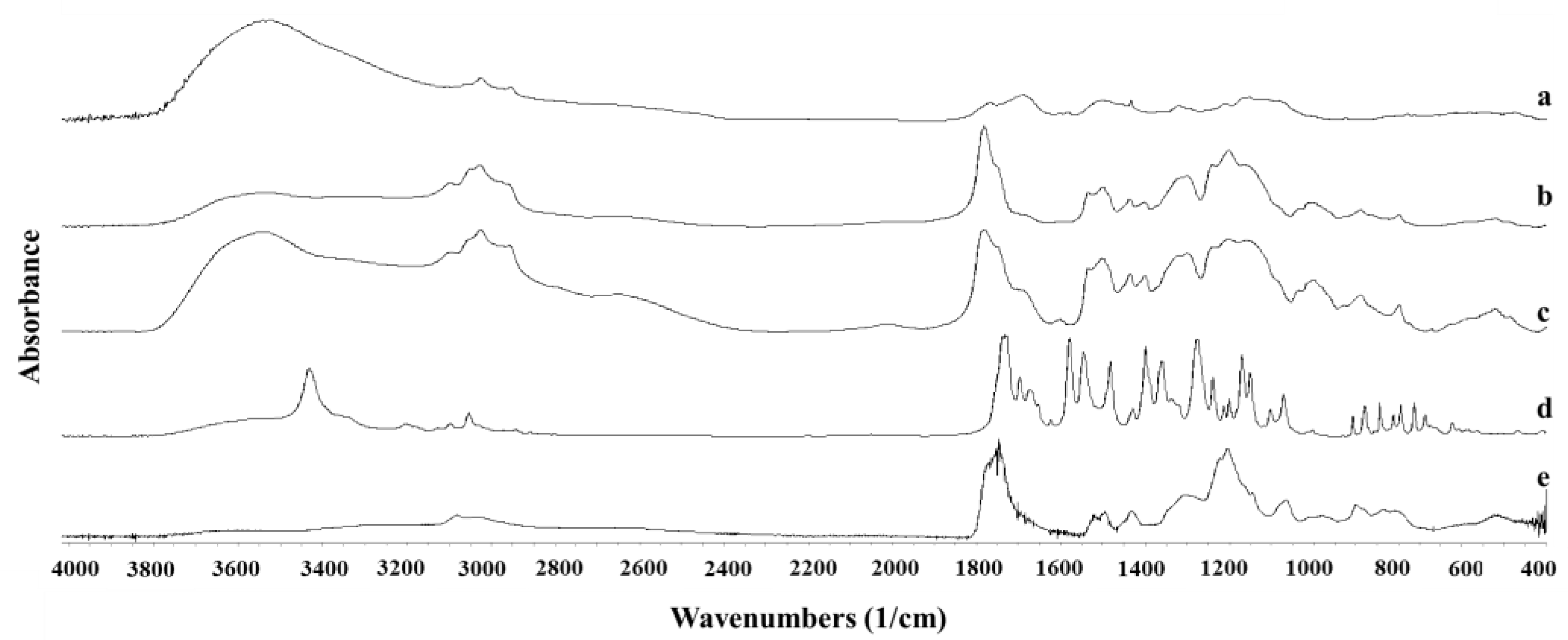
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.8. X-ray Powder Diffraction (pXRD)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Release Studies
3. Results
3.1. Design and Engineering of 3D Printed Microfluidic Chips
3.2. Microfluidic Preparation and Characterization of NFD Polymeric Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreyling, W. Nanomedicine: An ESF-European Medical Councils (EMRC) forword look report. Strasbg. Cedex 2005, 4, 5–25. [Google Scholar]
- Research, G.V. Nanomedicine Market Size Worth $350.8 Billion by 2025–GR: 11.2%. 2017. Available online: https://www.grandviewresearch.com/press-release/global-nanomedicine-market#:~:text=The%20global%20nanomedicine%20market%20is,more%20cost%2Deffective%20than%20traditional (accessed on 1 December 2021).
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; Steinmetz, N.F.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Ahmed, S.; Salmon, H.; Distasio, N.; Do, H.D.; Scherman, D.; Alhareth, K.; Tabrizian, M.; Mignet, N. Viscous Core Liposomes Increase siRNA Encapsulation and Provides Gene Inhibition When Slightly Positively Charged. Pharmaceutics 2021, 13, 479. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Vandamme, T.F.; Serra, C.A. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: An overview. Expert Opin. Drug Deliv. 2016, 13, 1447–1460. [Google Scholar] [CrossRef]
- Havel, H.A. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar]
- Lapworth, K.; Dawe, S.; Davis, P.; Kavanagh, D.; Young, R.; Saunders, J. Impulsivity and positive psychotic symptoms influence hostility in methamphetamine users. Addict. Behav. 2009, 34, 380–385. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Reflection Paper on Nanotechnology-Based Medicinal Products for Human Use. EMEA/CHMP/79769/2006, London. 2006. Available online: https://etp-nanomedicine.eu/wp-content/uploads/2018/10/reflection-paper-nanotechnology-based-medicinal-products-human-use_en-1.pdf (accessed on 1 December 2021).
- FDA, U. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. Guid. Ind. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/liposome-drug-products-chemistry-manufacturing-and-controls-human-pharmacokinetics-and (accessed on 1 December 2021).
- FDA. Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry; US Department of Health and Human Services: Silver Spring, MD, USA, 2017. [Google Scholar]
- Fornaguera, C.; García-Celma, M.J. Personalized nanomedicine: A revolution at the nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. Aaps. Pharm. Sci. Tech. 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Satalkar, P.; Elger, B.S.; Hunziker, P.; Shaw, D. Challenges of clinical translation in nanomedicine: A qualitative study. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The nanomedicine revolution: Part 1: Emerging concepts. Pharm. Ther. 2012, 37, 512–525. [Google Scholar]
- Au, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, D.; Li, Z. Grand challenges in nanomedicine. Mater. Sci. Eng. C 2020, 106, 110302. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, U.; Akhtar, J. 3D Printing in Pharmaceutical Sector: An Overview. Pharm. Formul. Des.-Recent Pract. 2020. Available online: https://www.intechopen.com/chapters/70715 (accessed on 1 December 2021).
- Yuste, I.; Luciano, F.C.; González-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar]
- Shah, D.M.; Morris, J.; Plaisted, T.A.; Amirkhizi, A.V.; Hansen, C.J. Highly filled resins for DLP-based printing of low density, high modulus materials. Addit. Manuf. 2021, 37, 101736. [Google Scholar] [CrossRef]
- Lepowsky, E.; Tasoglu, S. 3D printing for drug manufacturing: A perspective on the future of pharmaceuticals. Int. J. Bioprinting 2018, 4, 119. [Google Scholar] [CrossRef]
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596, 120290. [Google Scholar] [CrossRef]
- Pereira, T.; Kennedy, J.V.; Potgieter, J. A comparison of traditional manufacturing vs. additive manufacturing, the best method for the job. Procedia Manuf. 2019, 30, 11–18. [Google Scholar]
- Hwang, H.H.; Zhu, W.; Victorine, G.; Lawrence, N.; Chen, S. 3D-printing of functional biomedical microdevices via light-and extrusion-based approaches. Small Methods 2018, 2, 1700277. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A. How 3D Printing Will Change the Future of Borrowing Lending and Spending? In Handbook of Blockchain, Digital Finance, and Inclusion; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 493–520. [Google Scholar]
- Choonara, Y.E.; Toit, L.C.d.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharm. Outcomes Res. 2016, 16, 23–32. [Google Scholar] [CrossRef]
- Ayyoubi, S.J.C.; Cerda, R.; Fernández-García, P.; Knief, A.; Lalatsa, A.M.; Healy, D.R. Serrano3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Kief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D printed medicines: Optimising material properties for successful passive diffusion loading of filaments for fused deposition modelling of solid dosage forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.D.R.; Serrano, M.; Mauger, F.; Bolas-Fernández, M.A.; Dea-Ayuela, A. Lalatsa Orally bioavailable and effective buparvaquone lipid-based nanomedicines for visceral leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Guirales, S.A.; Jurado, N.; Kara, A.; Lalatsa, A.; Serrano, D.R. Understanding direct powder extrusion for fabrication of 3D printed personalised medicines: A case study for nifedipine minitablets. Pharmaceutics 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Lao, L.L.; Peppas, N.A.; Chiang BOey, F.Y.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga, M.D. Matrix tablets: The effect of hydroxypropyl methylcellulose/anhydrous dibasic calcium phosphate ratio on the release rate of a water-soluble drug through the gastrointestinal tract I. In Vitro tests. Aaps. Pharm. Sci. Tech. 2012, 13, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, Y.; Wigent, R.J.; Malick, W.A.; Sandhu, H.K.; Singhal, D.; Shah, N.H. Interplay of formulation and process methodology on the extent of nifedipine molecular dispersion in polymers. Int. J. Pharm. 2011, 420, 59–67. [Google Scholar] [CrossRef]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roces, C.B.; Khadke, S.; Christensesn, D.; Perrie, Y. Scale-Independent Microfluidic Production of Cationic Liposomal Adjuvants and Development of Enhanced Lymphatic Targeting Strategies. Mol. Pharm. 2019, 16, 4372–4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wuthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic Olefin Copolymer Microfluidic Devices for Forensic Applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Nunes, P.S.; Ohlsson, P.D.; Ordeig, O.; Kutter, J.P. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluidics 2010, 9, 145–161. [Google Scholar] [CrossRef]
- Chen, Z.; Han, J.Y.; Shumate, L.; Fedak, R.; Devoe, D.L. High Throughput Nanoliposome Formation Using 3D Printed Microfluidic Flow Focusing Chips. Adv. Mater. Technol. 2019, 4, 1800511. [Google Scholar] [CrossRef]
- Ballacchino, G.; Weaver, E.; Mathew, E.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Manufacturing of 3D-Printed Microfluidic Devices for the Synthesis of Drug-Loaded Liposomal Formulations. Int. J. Mol. Sci. 2021, 22, 8064. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Benedetti, S.; Skouras, A.; Curzi, G.; Perinelli, D.R.; Palmieri, G.F.; Casettari, L. 3D-printed microfluidic chip for the preparation of glycyrrhetinic acid-loaded ethanolic liposomes. Int. J. Pharm. 2020, 584, 119436. [Google Scholar] [CrossRef]
- Lee, J.M.; Zhang, M.; Yeong, W.Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid. Nanofluidics 2016, 20, 5. [Google Scholar] [CrossRef]
- Jog, R.; Unachukwu, K.; Burgess, D.J. Formulation design space for stable, pH sensitive crystalline nifedipine nanoparticles. Int. J. Pharm. 2016, 514, 81–92. [Google Scholar] [CrossRef] [PubMed]




| Parameters | FDM | SLA | Conventional Method | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| D10 (nm) | 46 | 3 | 41 | 1 | 32 | 1 |
| D50 (nm) | 75 | 3 | 68 | 2 | 52 | 2 |
| D90 (nm) | 131 | 12 | 119 | 5 | 91 | 4 |
| PDI | <0.1 | - | <0.1 | - | <0.1 | - |
| Span | 1.134 | 0.054 | 1.153 | 0.003 | 1.134 | 0.002 |
| Zeta Potential (mV) | −35.5 | 8.1 | −32.5 | 2.7 | −35.4 | 1.8 |
| Encapsulation efficiency (%) | 42.3 | 1.3 | 49.6 | 3.2 | 53.0 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hing, R.; Lalatsa, A.; Serrano, D.R. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. https://doi.org/10.3390/pharmaceutics13122134
Kara A, Vassiliadou A, Ongoren B, Keeble W, Hing R, Lalatsa A, Serrano DR. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics. 2021; 13(12):2134. https://doi.org/10.3390/pharmaceutics13122134
Chicago/Turabian StyleKara, Aytug, Athina Vassiliadou, Baris Ongoren, William Keeble, Richard Hing, Aikaterini Lalatsa, and Dolores R. Serrano. 2021. "Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines" Pharmaceutics 13, no. 12: 2134. https://doi.org/10.3390/pharmaceutics13122134
APA StyleKara, A., Vassiliadou, A., Ongoren, B., Keeble, W., Hing, R., Lalatsa, A., & Serrano, D. R. (2021). Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics, 13(12), 2134. https://doi.org/10.3390/pharmaceutics13122134