Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering
Abstract
:1. Introduction
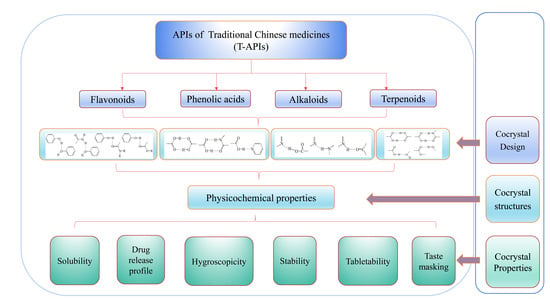
2. The Physicochemical Properties of Flavonoids, Terpenoids, Alkaloids, and Phenolic Acids in T-APIs
2.1. Flavonoids
2.2. Alkaloids
2.3. Phenolic Acids
2.4. Terpenoids
3. Design of T-API Cocrystals
3.1. Flavonoids
3.2. Alkaloids
3.3. Formation of Cocrystals of Phenolic Acids
3.4. Terpenoids
4. Preparation of Cocrystals
| Preparation Methods | Drug | Co-Former |
|---|---|---|
| Slow evaporation | Baicalein | Caffeine [123] |
| Berberine | Phthalic acid * [65] | |
| Curcumin | 2-Aminopyridine * [124], 2,5-Dihydroxybenzoic acid * [125] | |
| Rutin | Carbamide [112], polyethylene glycol * [112] | |
| Puerarin | Lornoxicam [86] | |
| Ferulic acid | Nicotinamide [100] | |
| Ursolic acid | Ethylenediamine * [107], piperazine [108] | |
| Oleanolic acid | Ethylenediamine * [126], piperazine [127] | |
| Ligustrazine | Saccharine [128], febuxostat [129] | |
| Rapid solvent removal | Curcumin | Isoniazid [90], hydroquinone * [91], phloroglucinol [92] |
| Slurry | Berberine chloride | Fumaric acid [62], myricetin [64], dihydromyricetin [64] |
| Recrystallization | Matrine | Salvianolic acid B [130] |
| Supercritical fluids | Resveratrol | Curcumin [104] |
| Antisolvent precipitation | Ursolic acid | Metformin [109], arginine [109], lysine [109], N-methylglucamine [109] |
| Neat grinding | Curcumin | Trimesic acid [95] |
| Solvent assisted grinding | Berberine chloride | Citric acid [60], ibuprofen [61], fumaric acid [62] |
| Ligustrazine | Ethinylestradiol [131] | |
| Baicalein | Nicotinamide [69] | |
| Celastrol | Threonine [132], phenylalanine [132], L-tyrosine [132] |
5. Modifications of Physicochemical Properties of API of TCMs through Cocrystal Engineering
5.1. Stability
5.1.1. Thermal Stability
5.1.2. Hygroscopicity
| Pharmaceutical Applications | Drug | Coformer |
|---|---|---|
| Enhanced solubility and dissolution rate | Andrographolide | Salicylic acid [110] |
| 11-Aza-artemisinin | Benzoic acid [54], salicylic acid [54], succinic acid [54], heptanedioic acid [54] | |
| Baicalein | Isoniazid [70], caffeine [70], isonicotinamide [70], theophylline [70], betaine [71] | |
| Berberine chloride | Fumaric acid [62], lactic acid * [63] | |
| Celastrol | Threonine [132], phenylalanine [132], L-tyrosine [12] | |
| Curcumin | Resorcinol [93], phloroglucinol [92], Hydroquinone * [91] | |
| Ligustrazine | Saccharine [128] | |
| Myricetin | Berberine chloride [64] | |
| Oleanolic acid | Ethylenediamine [126], piperazine [127] | |
| Puerarin | Lornoxicam [86] | |
| Quercetin | Isonicotinamide [7], caffeine [7], theobromine dihydrate [7], betaine [71] | |
| Ursolic acid | Piperazine [108], ethylenediamine [107] | |
| Hygroscopicity | Baicalein | Isoniazid [70], isonicotinamide [70], caffeine [70] |
| Berberine | Chrysin [66] | |
| Berberine chloride | Saccharin [114], acesulfame [114] | |
| Curcumin | Resorcinol [93], pyrogallol [93] | |
| Dihydromyricetin | Berberine chloride [64] | |
| Ferulic acid | Isonicotinamide [100] | |
| Myricetin | Berberine chloride [64] | |
| Oleanolic acid | Ethylenediamine [126], piperazine [127] | |
| Quercetin | Betaine [71] | |
| Ursolic acid | Piperazine [108], ethylenediamine [107] | |
| Extended release | Curcumin | Isoniazid [90] |
| Piperazine ferulate | Pyrazinamide [134] | |
| Improved tabletability | Baicalein | Nicotinamide [69], caffeine [69], isoniazid [69] |
| Berberine chloride | Saccharine [114], acesulfame [114] | |
| Puerarin | Lornoxicam [86] | |
| Improved thermal stability | Berberine chloride | Fumaric acid [62] |
| Ligustrazine | Saccharin [31], 1,4-cyclohexanedicarboxylic acid * [115], 2,6-dihydroxybenzoic acid * [115], 2,6-pyridinedicarboxylic acid * [115], 6-hydroxy-2-naphthoic acid * [115], 3-hydroxybenzoic acid * [115] | |
| Taste masking | Berberine chloride | Saccharin [114], acesulfame [114] |
| Ligustrazine | saccharine [128] | |
| Increased Chemical stability | Andrographolide | Salicylic acid [110] |
5.1.3. Chemical Stability
5.2. Organoleptic Properties
5.3. Solubility
5.4. Dissolution Performances
5.5. Bioavailability
5.6. Tabletability
5.7. Others
6. Mechanisms for Modifications of Physicochemical Properties
6.1. Solubility
6.2. Hygroscopicity
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, H.W. Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies, 1st ed.; John Wiley and Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Cao, Y.; Xuan, B.F.; Peng, B.; Li, C.; Chai, X.Y.; Tu, P.F. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Grazia, A.; Sabino, D.P.; Carmine, D.A. Nab-paclitaxel for the management of triple-negative metastatic breast cancer: A case study. Anti-Cancer Drugs 2015, 26, 117–122. [Google Scholar]
- Kumar, A.; Hoskins, P.J.; Tinker, A.V. Dose-dense paclitaxel in advanced ovarian cancer. Clin. Oncol 2015, 27, 40–47. [Google Scholar] [CrossRef]
- Banerjee, M.; Chattopadhyay, S.; Choudhuri, T.; Bera, R.; Mukherjee, S.K. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J. Biomed. Sci. 2016, 23, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef]
- Zhuang, C.; Xiao-Qin, M.A.; Zhu, B.L.; Chen, Q.W.; Lin, N.; Chen, Q. Progress on co-crystal of active ingredients of Chinese materia medica. Chem. Reagents 2018, 40, 943–952. [Google Scholar]
- Editorial Board of Chinese Materia Medica, National Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Scientific and Technical Publishers: Shanghai, China, 2009. [Google Scholar]
- Maryam, K.J.; Luis, P.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Gurav, N.P.; Dandagi, P.M.; Gadad, A.P.; Masthiholimath, V.S. Solubility enhancement of satranidazole using self- emulsified drug delivery systems. Indian J. Pharm. Educ. Res. 2016, 50 (Suppl. S3), 68–76. [Google Scholar]
- Kulthe, V.V.; Chaudhari, P.D. Solubility enhancement of etoricoxib by solid dispersions prepared by spray drying technique. Indian J. Pharm. Educ. Res. 2011, 45, 248–258. [Google Scholar]
- Gao, N.; Guo, M.; Fu, Q.; He, Z. Application of hot melt extrusion to enhance the dissolution and oral bioavailability of oleanolic acid. Asian J. Pharm. Sci. 2017, 12, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atipairin, A.; Sawatdee, S. Inclusion complexes between sildenafil citrate and cyclodextrins enhance drug solubility. Asian J. Pharm. Sci. 2016, 11, 104–105. [Google Scholar] [CrossRef]
- Burapapadh, K.; Takeuchi, H.; Sriamornsak, P. Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole. Asian J. Pharm. Sci. 2016, 11, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Lankalapalli, S.; Tenneti, V.S.V.K.; Nimmali, S.K. Design and development of vancomycin Liposomes. Indian J. Pharm. Educ. Res. 2015, 49, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Friši, T.; Fábián, L.; Jones, W. New solid forms of artemisinin obtained through cocrystallisation. CrystEngComm 2010, 12, 4038–4041. [Google Scholar] [CrossRef]
- Childs, S.L.; Hardcastle, K.I. Cocrystals of piroxicam with carboxylic acids. Cryst. Growth Des. 2007, 7, 1291–1304. [Google Scholar] [CrossRef]
- Hyun, S.M.; Lee, B.J.; Abuzar, S.M.; Lee, S.; Joo, Y.; Hong, S.H.; Kang, H.; Kwon, K.A.; Velaga, S.; Hwang, S.J. Preparation, characterization, and evaluation of celecoxib eutectic mixtures with adipic acid/saccharin for improvement of wettability and dissolution rate. Int. J. Pharm. 2019, 554, 61–71. [Google Scholar] [CrossRef]
- Bazzo, G.G.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixrtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Vemuri, V.D.; Lankalapalli, S. Insight into concept and progress on pharmaceutical co-crystals: An overview. Indian J. Pharm. Educ. Res. 2019, 53, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.F.; Sun, C.C.; Chow, S.F. Cocrystal engineering of pharmaceutical solids: Therapeutic potential and challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef]
- Khalaji, M.; Potrzebowski, M.J.; Dudek, M.K. Virtual cocrytal screening methods as tools to understand the formation of pharmaceutical cocrystals-a case study of linezolid, a wide-range antibacterial drug. Cryst. Growth Des. 2021, 21, 2301–2314. [Google Scholar] [CrossRef]
- Yuan, J.C.; Liu, X.T.; Wang, S.M.; Chang, C.; Zeng, Q.; Song, Z.T.; Jin, Y.D.; Zeng, Q.; Sun, G.X.; Ruan, S.G.; et al. Virtual conformer screening by a combined machine learning and physics-based approach. CrystEngComm 2021, 23, 6039–6044. [Google Scholar] [CrossRef]
- Przybylek, M.; Cysewski, P. Distinguishing cocrystals from simple eutectic mixtures: Phenolic acids as potential pharmaceutical coformers. Cryst. Growth Des. 2018, 18, 3524–3534. [Google Scholar] [CrossRef]
- Cysewski, P. In silico screening of dicarboxylic acids for cocrystallization with phenylpiperazine derivatives based on both cocrystallization propensity and solubility advantage. J. Mol. Model. 2017, 23, 136. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, C.; He, X.; Sun, C.C. Reducing sublimation tendency of ligustrazine through salt formation. Cryst. Growth Des. 2020, 20, 2057–2063. [Google Scholar] [CrossRef]
- Roy, P.; Ghosh, A. Progress on cocrystallization of poorly soluble NME’s in the last decade. CrystEngComm 2020, 22, 6958–6974. [Google Scholar] [CrossRef]
- Cambridge Crystallographic Data Centre. Cambridge Structural Database. Available online: http://webcsd.ccdc.cam.ac.uk/ (accessed on 9 November 2021).
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.J.; Wang, M.; Zhu, Y. Research progress of adverse reactions of traditional Chinese medicine injections. China J. Chin. Mater. Med. 2014, 39, 3889–3998. [Google Scholar]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Cocrystallization of nutraceuticals. Cryst. Growth Des. 2015, 15, 984–1009. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. Enhanced radical-scavenging activity of a planar catechin analogue. J. Am. Chem. Soc. 2002, 124, 5952–5953. [Google Scholar] [CrossRef] [PubMed]
- Shih-Yi, C.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar]
- Zhu, B.; Wang, J.R.; Mei, X. Insight into the phase transformation among various solid forms of baicalein. Cryst. Growth Des. 2015, 15, 4959–4968. [Google Scholar] [CrossRef]
- Dang, Y.; Lin, G.; Xie, Y.; Duan, J.; Ma, P.; Li, G.; Ji, G. Quantitative determination of myricetin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and its absolute bioavailability. Drug Res. 2013, 64, 516–522. [Google Scholar] [CrossRef]
- Xiang, L.U.; Jiang, C. Research progress on cocrystal of flavonoids. Her. Med. 2019, 38, 921–926. [Google Scholar]
- Klitou, P.; Rosbottom, I.; Simone, E. Synthonic modeling of quercetin and its hydrates: Explaining crystallization behavior in terms of molecular conformation and crystal packing. Cryst. Growth Des. 2019, 19, 4774–4783. [Google Scholar] [CrossRef]
- Peng, L.; Huang, X.W.; Lv, Q.J. Advances in studies on absorption, distribution, metabolism of flavonoids. China J. Chin. Mater. Med. 2007, 32, 1961–1964. [Google Scholar]
- Zhou, X.B.; Wu, S.X.; Sun, M.Y.; Hu, X.R. Research progress on co-crystal of insoluble active ingredients of Chinese materia medica. Chin. Tradit. Herb. Drugs 2016, 47, 336–343. [Google Scholar]
- Alan, W.B.; Cormac, T.T.; David, J.B. Non-antibiotic anti-diarrhoeal drugs: Factors affecting oral bioavailability of berberine and loperamide intestinal tissue. Adv. Drug Deliv. Rev. 1997, 23, 111–120. [Google Scholar]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form of oral delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromer, D.T.; Ihde, A.J.; Ritter, H.L. The crystal structure of tetramethylpyrazine. J. Am. Chem. Soc. 1951, 73, 5587–5590. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Xu, W.; Ma, X.; Zhen, J.; Zheng, Y.; You, S. Studies on the stability of salvianolic acid B as potential drug material. Phytochem. Anal. 2011, 22, 378–384. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Guru Row, T.N. Charge density analysis of ferulic acid: Robustness of a trifurcated C-H·O hydrogen bond. Cryst. Growth Des. 2012, 12, 6083–6091. [Google Scholar] [CrossRef]
- Cheng, G.L.; Deng, C.Y.; Jiang, C.J. Research progress on co-crystal of curcumin. Zhejiang Chem. Ind. 2017, 48, 12–17. [Google Scholar]
- Dai, B.; Zhang, H.; Dai, T.; Wang, J.; Cao, J. Determination of drug concentration in human plasma of Jiangzhi Tongluo soft capsule. Asia-Pac. Tradit. Med. 2018, 14, 63–66. [Google Scholar]
- Nan-Nan, L.I.; Lao, Y.S.; Dong, Z.Q. Advances in research on BCS classication of active constituents of Chinese wolfberry. World Latest Med. Inf. 2019, 19, 56–58. [Google Scholar]
- Nisar, M.; Wong, L.-Y.; Sung, H.-Y.; Haynes, R.K.; Williams, I.D. Cocrystals of the antimalarial drug 11-azaartemisinin with three alkenoic acids of 1:1 or 2:1 stoichiometry. Acta Cryst. 2018, 74, 742–751. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.Y.; Yang, L. Progress in new formulation studies of artemisinins. Chin. Pharm. J. 2015, 50, 189–193. [Google Scholar]
- Chan, K. Polymorphism of artemisinin from Artemisia annua. Phytochemistry 1997, 46, 1209–1214. [Google Scholar]
- Huang, L.H.; Xu, H.W.; Liu, G.Z.; Dai, G.F.; Liu, H.M. Synthesis, crystal structure and glucosidase inhibitory activities of (8R,13R)-8,12,13,17-tetrahydroandrographolide. Chin. Pharm. J. 2007, 7, 1304–1306. [Google Scholar]
- Kuminek, G.; Cao, F.; Alanny, B.; Cardoso, S.G.A.; Rodríguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagden, N.; Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Dun, J.; Chen, J.M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [PubMed]
- Cao, J.; Du, G.; Yang, L.; Yang, S.; Zhao, X. Berberine Hydrochloride and Ibuprofen Eutectic Substance as Well as Preparation Method, Composition and Application Thereof. Patent CN110041325A, 23 July 2019. [Google Scholar]
- Yang, D.; Cao, J.; Jiao, L.; Yang, S.; Du, G. Solubility and stability advantages of a new cocrystal of berberine chloride with fumaric acid. ACS Omega 2020, 5, 8283–8292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Lu, Q. Berberine Hydrochloride and Lactic Acid Eutectic, and Preparation Method and Application Thereof. Patent CN109400598A, 1 March 2019. [Google Scholar]
- Li, P.; Ramaiah, T.; Zhang, M.; Zhang, Y.; Lou, B. Two cocrystals of berberine chloride with myricetin and dihydromyricetin: Crystal structures, characterization, and antitumor activities. Cryst. Growth Des. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhang, Y. Berberine-Phthalic Acid Medicine Salt Single Crystal and Preparation Method Thereof. Patent CN 109081839 A, 18 September 2018. [Google Scholar]
- Rongjian, S.; Yanjie, Z.; Yanping, D.; Yali, H.; Mei, Z.; Benyong, L. Novel salt cocrystal of chrysin with berberine: Preparation, characterization, and oral bioavailability. Cryst. Growth Des. 2018, 18, 4724–4730. [Google Scholar]
- Ren, S.Y.; Jiao, L.T.; Yang, S.Y.; Zhang, L.; Song, J.K.; Yu, H.Y.; Wang, J.R.; Lv, T.T.; Sun, L.; Lu, Y.; et al. A novel co-crystal of bexarotene and ligustrazine improves pharmacokinetics and tissue distribution of bexarotene in SD rats. Pharmaceutics 2020, 12, 906. [Google Scholar] [CrossRef]
- Ma, X.Q.; Zhuang, C.; Wang, B.C.; Huang, Y.F.; Chen, Q.; Lin, N. Cocrystal of apigenin with higher soubility, enhanced oral bioavilability and anti-inflammatory effect. Cryst. Growth Des. 2019, 19, 5531–5537. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Dun, J.; Chow, A.; Sun, C.C. Lack of dependence of mechanical properties of baicalein cocrystals on those of the constituent components. CrystEngComm 2018, 20, 5486–5489. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Wang, J.R.; Mei, X. Cocrystals of baicalein with higher solubility and enhanced bioavailability. Cryst. Growth Des. 2017, 17, 1893–1901. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Luo, C.; Huang, C.; Zhang, H. Cocrystals of natural products: Improving the dissolution performance of flavonoids using betaine. Cryst. Growth Des. 2019, 19, 3851–3859. [Google Scholar] [CrossRef]
- Chadha, R.; Bhalla, Y.; Nandan, A.; Chadha, K.; Karan, M. Chrysin cocrystal: Characterization and evaluation. J. Pharm. Biomed. Anal. 2017, 134, 361–371. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.G.; Zhang, M.; Zhang, Y.J.; Lou, B.Y. A drug-drug cocrystal of dihydromyricetin and pentoxifylline. J. Pharm Sci. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Tong, Q.; Hou, X.L.; Hu, S.Y.; Fang, J.G.; Sun, C.Q. Enhancing bioavailability of dihydromyricetin through inhibiting precipitation of soluble cocrystals by a crystallization inhibitor. Cryst. Growth Des. 2016, 16, 5030–5039. [Google Scholar] [CrossRef]
- Sowa, M.; Slepokura, K.; Matczk-Jon, E. Cocrystals of fisetin, luteolin and genistein with pyridinecarboxamide coformers: Crystal structures, analysis of intermolecular interactions, spectral and thermal characterization. CrystEngComm 2013, 15, 7696–7708. [Google Scholar] [CrossRef]
- Sowa, M.; Slepokura, K.; Matczak-Jon, E. A 1:2 cocrystal of genistein with isonicotinamide: Crystal structure and Hirshfeld surface analysis. Acta Crystallogr C 2013, 69, 1267–1272. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yin, H.M.; Zhang, Y.; Zhang, D.J.; Su, X.; Kuang, H.X. Preparation of a 1:1 cocrystal of genistein with 4,4’-bipyridine. J. Cryst. Growth 2017, 458, 103–109. [Google Scholar] [CrossRef]
- Sowa, M.; Slepokura, K.; Matczak-Jon, E. Solid-state characterization and solubility of a genistein-caffeine cocrystal. J. Mol. Struct. 2014, 1076, 80–88. [Google Scholar] [CrossRef]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Cocrystals of hesperetin: Structural, pharmacokinetic, and pharmacodynamic evaluation. Cryst. Growth Des. 2017, 17, 2386–2405. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.L.; Lu, T.B.; Chen, J.M. Temozolomide-hesperetin drug-drug cocrystal with optimized performance in stability, dissolution, and tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar]
- Zhang, Y.N.; Yin, H.M.; Zhang, Y.; Zhang, D.J.; Su, X.; Kuang, H.X. Cocrystals of keampferol, quercetin and myricetin with 4’4-bipyridine: Crystal structures, analysese of intermolecular interactions and antibacterial properties. J. Mol. Struct. 2017, 1130, 199–207. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Zhou, L.; Hao, H.X.; Bao, Y.; Yin, Q.X.; Xie, C. Cocrystals of propylthiouracil and nutraceuticals toward sustained-release: Design, structure analysis, and solid-state characterization. Cryst. Grwoth Des. 2021, 21, 1202–1217. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Skorepova, E.; Sinha, A.S.; Bhogala, B.R.; Maguire, N.M.; Maguire, A.R.; Lawrence, S.E. Cocrsytals and a salt of the bioactive flavonoid: Naringenin. Cryst. Growth Des. 2018, 18, 4571–4577. [Google Scholar] [CrossRef]
- Luo, C.; Liang, W.D.; Chen, X.; Wang, J.M.; Deng, Z.W.; Zhang, H.L. Pharmaceutical cocrystals of naringenin with improved dissolution performance. CrystEngComm 2018, 20, 3025–3033. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Zhou, J.L.; Zhang, H.L.; Tong, H.Y.; Nie, J.J.; Li, L.; Zhang, Y.Y.; Du, J.; Ma, A.; Yang, X.M.; et al. Structure determination and in vitro/vivo study on carbamazepine-naringenin (1:1) cocrystal. J. Drug Deliv. Sci. Technol. 2019, 54, 101244. [Google Scholar] [CrossRef]
- Cheng, R.; Gao, Y.; Hua, Y.; Luo, M.; Xue, Y.; Zhang, J.; Zhu, H. Lornoxicam and Puerarin Eutectic Crystal and Preparation Method Thereof. Patent CN111004256A, 14 April 2020. [Google Scholar]
- Liu, F.; Wang, L.Y.; Yu, M.C.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. A new cocrystal of isoniazid-quercetin with hepatoprotective effect: The design, structure, and in vitro/in vivo performance evaluation. Eur. J. Pharm. Sci. 2020, 144, 105216. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Ren, J.; Zeng, A.G.; Liu, J.T. Preparation of quercetin-nicotinamide cocrystals and their evaluation under in vivo and in vitro conditions. RSC Adv. 2020, 10, 21852–21859. [Google Scholar] [CrossRef]
- Veverka, M.; Dubaj, T.; Gallovic, J.; Jorik, V.; Veverkova, E.; Danihelova, M.; Simon, P. Cocrystals of quercetin: Synthesis, characterization, and screening of biological activity. Monatsh. Chem. Chem. Mon. 2015, 146, 99–109. [Google Scholar] [CrossRef]
- Xuan, B.; Wong, S.; Zhang, Y.; Weng, J.; Tong, H.; Wang, C.; Sun, C.; Chow, S. Extended release of highly water soluble isoniazid attained through cocrystallization with curcumin. Cryst. Growth Des. 2020, 20, 1951–1960. [Google Scholar] [CrossRef]
- Nga, W.S.; Hu, S.; Wing, N.W.; Xu, X.; Lun, L.K.; Thomas, L.; Lum, C.; Sun, C.C.; Fung, C.S. Cocrystallization of curcumin with benzenediols and benzenetriols via rapid solvent removal. Cryst. Growth Des. 2018, 18, 5534–5546. [Google Scholar]
- Chow, S.F.; Shi, L.; Ng, W.W.; Leung, K.; Nagapudi, K.; Sun, C.; Chow, A. Kinetic entrapment of a hidden curcumin cocrystal with phloroglucinol. Cryst. Growth Des. 2014, 14, 5079–5089. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast dissolving curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel curcumin ascorbic acid cocrystal for improved solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Sathisaran, I.; Bhatia, D.D.; Dalvi, S.V. New curcumin-trimesic acid cocrystal and anti-invasion activity of curcumin multicomponent solids against 3D tumor models. Int. J. Pharm. 2020, 587, 119667. [Google Scholar] [CrossRef]
- Zheng, K.; Li, A.; Wu, W.W.; Qian, S.S.; Liu, B.H.; Pang, Q.X. Preparation, characterization, in vitro and in vivo evaluation of metronidazole-gallic acid cocrystal: A combined experimental and theoretical investigation. J. Mol. Struct. 2019, 1197, 727–735. [Google Scholar] [CrossRef]
- Song, J.X.; Chen, J.M.; Lu, T.B. Lenalidomide-gallic acid cocrystals with constant high solubility. Cryst. Growth Des. 2015, 15, 4869–4875. [Google Scholar] [CrossRef]
- Kaur, R.; Perumal., S.S.R.R.; Bhattacharyya, A.J.; Yashonath, S.; Row, T.N.G. Structural insights into proton conduction in gallic acid-isoniazid cocrystals. Cryst. Growth Des. 2014, 14, 423–426. [Google Scholar] [CrossRef]
- Al-Otaibi, J.S.; Mary, Y.S.; Mary, Y.S.; Panicker, C.Y.; Thomas., R. Cocrystals of pyrazinamide with p-tolunesulfonic and ferulic acids: DFT investigations and molecular docking studies. J. Mol. Struct. 2019, 1175, 916–926. [Google Scholar] [CrossRef]
- Aitipamula, S.; Das, S. Cocrystal Formulations: A case study of topical formulations consisting of ferulic acid cocrystals. Eur. J. Pharm. Biopharm. 2020, 149, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Fadipe, V.O.; Haruna, M.S.; Opoku, A.R. Isoniazid-oleanolic acid co-crystal system: Synthesis, anti-TB and toxicological effect on the human embryonic kidney (HEK293) and human hepatocellular carcinoma (HepG2) cell lines. J. Nanometer. Mol. NanoTechnol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Bofill, L.; Barbas, R.; Sande, D.; Font-Bardia, M.; Rafols, C.; Alberti, J.; Prohens, R. A novel, extremely bioavailable cocrystal of pterostilbene. Cryst. Growth Des. 2021, 21, 2315–2323. [Google Scholar] [CrossRef]
- Schultheiss, N.; Bethune, S.; Henck, J.O. Nutraceutical cocrystals: Utilizing pterostilbene as a cocrystal former. CrystEngComm 2010, 12, 2436–2442. [Google Scholar] [CrossRef]
- Magro., C.D.; Santos, A.E.; Ribas, M.M.; Aguiar, G.P.S.; Volfe, G.R.B.; Lopes, M.; Siebel, A.M.; Muller, L.G.; Bortoluzzi, A.J.; Lanza, M.; et al. Production of curcumin-resveratrol cocrystal using cocrystallization with supercritical solvent. J. Supercrit. Fluids 2021, 171, 105190. [Google Scholar] [CrossRef]
- Rosa, J.; Machado, T.C.; Silva, A.K.; Kuminek, G.; Bortolluzzi, A.J.; Caon, T.; Cardoso, S.G. Isoniazid-resveratrol cocrystal: A novel alternative for topical treatment of cutaneous tuberculosis. Cryst. Growth Des. 2019, 19, 5029–5036. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Li, W.Y.; Sun, W.J.; Lu, T.B.; Tong, H.; Sun, C.C.; Zheng, Y. Resveratrol cocrystals with enhanced solubility and tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, Z. Ursolate as Well as Preparation Method and Crystal Thereof. Patent CN102241723A, 12 November 2011. [Google Scholar]
- Shi, B.; Zhang, Z. Ursolic acid Salt, Preparation Method Thereof, and Crystal Thereof. Patent CN102234304A, 9 November 2011. [Google Scholar]
- Mylari, B.L.; Fleming, G.A. Ursolic Acid Salts for Treating Diabetes and Obesity. U.S. Patent Application No 13/864,509, 23 January 2014. [Google Scholar]
- Suresh, K.; Goud, N.R.; Nangia, A. Andrographolide: Solving chemical instability and poor solubility by means of cocrystals. Chem. Asian J. 2013, 8, 3032–3041. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, D.; Sari, R.; Yusuf, H.; Primaharinastiti, R. Preparation and characterization of artesunate-nicotinamide cocrystal by solvent evaporation and slurry method. Asian J. Pharm. Clin. Res. 2014, 7, 62–65. [Google Scholar]
- Ni, G.; Wang, H.; Ren, H. Water-Solubility Rutoside and Method of Manufacturing the Same and Oral Preparation. Patent CN101108193 A, 23 January 2008. [Google Scholar]
- Jie, L.; Rohani, S. Preparation and characterization of theophylline—Nicotinamide cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar]
- Wang, C.; Perumalla, R.S.; Lu, R.; Fang, J.; Sun, C.C. Sweet berberine. Cryst. Growth Des. 2016, 16, 933–939. [Google Scholar]
- Wang, L.; Xue, R.; Li, Y.; Zhao, Y.; Liu, F.; Huang, K. Hydrogen-bonding patterns in a series of multi-component molecular solids formed by 2,3,5,6-tetramethylpyrazine with selected carboxylic acids. CrystEngComm 2014, 16, 7074–7089. [Google Scholar] [CrossRef]
- Hui, X.; Zhang, F.; Chi, Z.; Cai, B. Preparation of curcumin-lysine cocrystal and solubility comparison of different crystal forms. China Pharm. 2017, 20, 208–212. [Google Scholar]
- Madiha, N.; Sung, H.H.-Y.; Horst, P.; Richard, L. 11-Azaartemisinin cocrystals with preserved lactam: Acid heterosynthons. CrystEngComm 2018, 20, 1205–1219. [Google Scholar]
- Wong, S.N.; Chan, S.W.S.; Peng, X.X.; Xuan, B.F.; Lee, H.W.; Tong, H.H.Y.; Chow, S.F. Effects of the glass-forming ability and annealing conditions on cocrystallization behaviors via rapid solvent removal: A case study of voriconazole. Pharmaceutics 2020, 12, 1209. [Google Scholar] [CrossRef]
- Cambridge structural database analysis of molecular complementarity in cocrystals. Cryst. Growth Des. 2009, 9, 1436–1443. [CrossRef]
- Padrela, L.; Azevedo, E.D.; Velaga, S.P. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev. Ind. Pharm. 2012, 38, 923–929. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef]
- Weng, J.W.; Wong, S.N.; Xu, X.Y.; Xuan, B.F.; Wang, C.G.; Chen, R.P.; Sun, C.C.; Lakerveld, R.; Kwok, P.C.L.; Chow, S.F. Cocrystal engineering of itraconazole with suberic acid via rotary evaporation and spray drying. Cryst. Growth Des. 2019, 19, 2736–2745. [Google Scholar]
- Mei, X.; Wang, J.; Zhu, B.; Zhu, L. Baicalein Caffeine Eutectic Crystal, Preparation Method Therefor, Pharmaceutical Composition, and Application Thereof. Patent WO2017076169A1, 11 May 2017. [Google Scholar]
- Jin, Q.; Li, J.; Liu, X.; Pan, R.; Zheng, H. Curcumin-2-Aminopyridine Eutectic Crystal and Preparation Method Thereof. Patent CN107721916A, 23 February 2018. [Google Scholar]
- Jin, Q.; Li, J.; Liu, X.; Pan, R.; Zheng, H. Curcumin-2,5-Dihydroxy-Benzoic Acid Eutectic Crystal and Preparation Method Thereof. Patent CN107827724A, 23 March 2018. [Google Scholar]
- Shi, B.; Zhang, Z. Oleanolic Acid Salt and its Preparation Method and Crystal. Patent CN102241724A, 16 November 2011. [Google Scholar]
- Shi, B.; Li, Y. Oleanolic Acid Piperazine Salt and Preparation Method Thereof. Patent CN101987863A, 23 March 2011. [Google Scholar]
- Zhao, X.; Li, Q.; Wang, C.; Hu, S.; He, X.; Sun, C.C. Simultaneous taste-masking and oral bioavailability enhancement of Ligustrazine by forming sweet salts. Int. J. Pharm. 2020, 577, 119089. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, L.; Zhang, L. Febuxostat-Ligustrazine Eutectic and Preparation and Application Methods Thereof. Patent CN108530382A, 14 September 2018. [Google Scholar]
- Li, Y. Matrine Salviol Acid B Complex Salt and Kuh-Seng Native Salviol Acid B Complex Salt, Preparation Method and Application Thereof. Patent CN101367799A, 18 February 2009. [Google Scholar]
- Chen, X.; Li, P.; Ling, L.; Ning, L.; Wang, H.; Xu, J. Pharmaceutical Co-Crystal of Ethinylestradiol and Ligustrazine and Application of Pharmaceutical Co-Crystal. Academy of Science and Tech Research National Health Commission, China Patent CN110003122A, 12 July 2019. [Google Scholar]
- Chen, K.; Ma, Q.; Miao, M.; Tian, Q.; Wu, X.; Zeng, H.; Zhu, X. Amino Acid Eutectics of Celastrol, Preparation Method and Application Thereof. Patent CN110229210A, 13 September 2019. [Google Scholar]
- Zhou, Z.Z.; Chan, H.M.; Sung, H.H.; Tong, H.; Zheng, Y. Identification of new cocrystal systems with stoichiometric diversity of salicylic acid using thermal methods. Pharm. Res. 2016, 33, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Wang, L.Y.; Liu, F.; Li, Y.T.; Yan, C.W. A sustained-release dual-drug ternary salt cocrystal of piperazine ferulate with pyrazinamide: The synthesis, structure and Hirshfeld surface analysis. Cryst. Growth Des. 2020, 20, 2064–2073. [Google Scholar] [CrossRef]
- Hong, C.; Xie, Y.; Yao, Y.; Li, G.; Yuan, X. A novel strategy for pharmaceutical cocrystal generation without knowledge of stoichiometric ratio: Myricetin cocrystals and a ternary phase diagram. Pharm. Res. 2015, 32, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72. [Google Scholar] [CrossRef]
- Xuan, B.F.; Chen, Y.C.S.; Wong, K.C.; Chen, R.P.; Lo, P.S.; Lakerveld, R.; Tong, H.; Chow, S.F. Impact of cocrytal solution-state stability on cocrytal dissociation and polymorphic drug recrystallization during dissolution. Int. J. Pharm. 2021, 610, 121239. [Google Scholar] [CrossRef]
- Vasisht, K.; Chadha, K.; Karan, M.; Bhalla, Y.; Jena, A.K.; Chadha, R. Enhancing biopharmaceutical parameters of bioflavonoid quercetin by cocrystallization. CrystEngComm 2016, 18, 1403–1415. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C. Overcoming poor tabletability of pharmaceutical crystals by surface modification. Pharm. Res. 2011, 28, 3248–3255. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Serajuddin, A. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J.; Nair, R.-H. Solubility advantage of pharmaceutical cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]



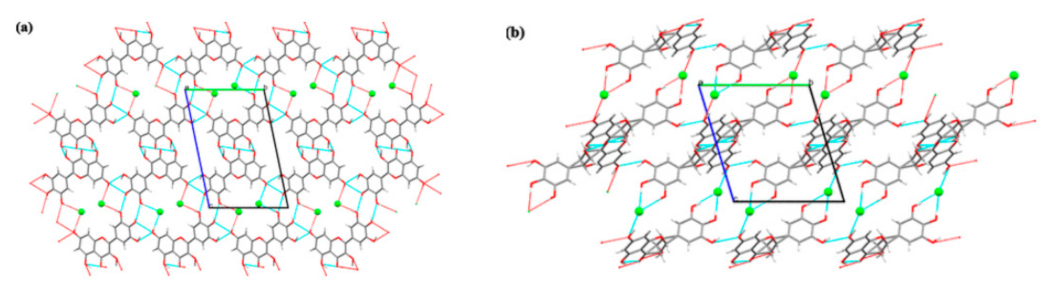






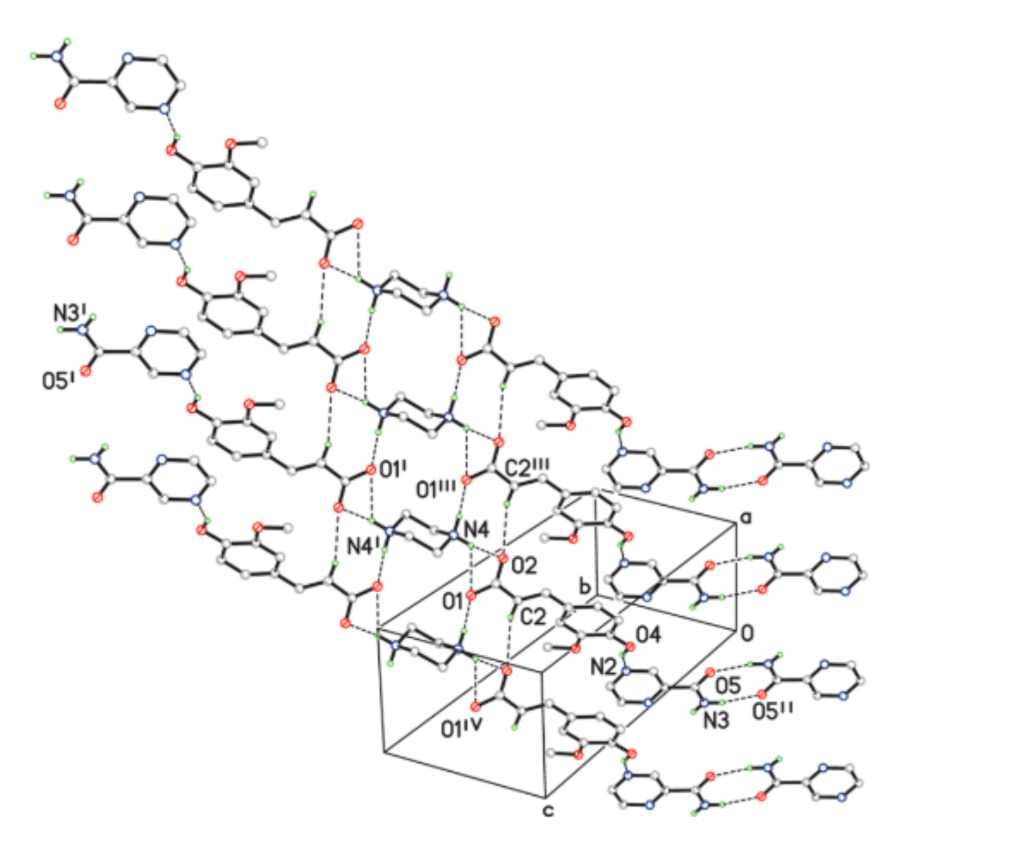
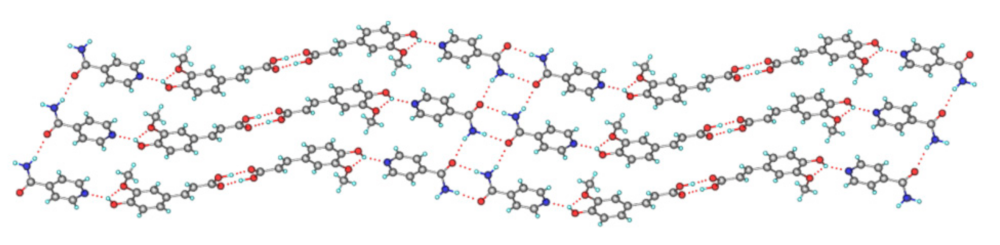

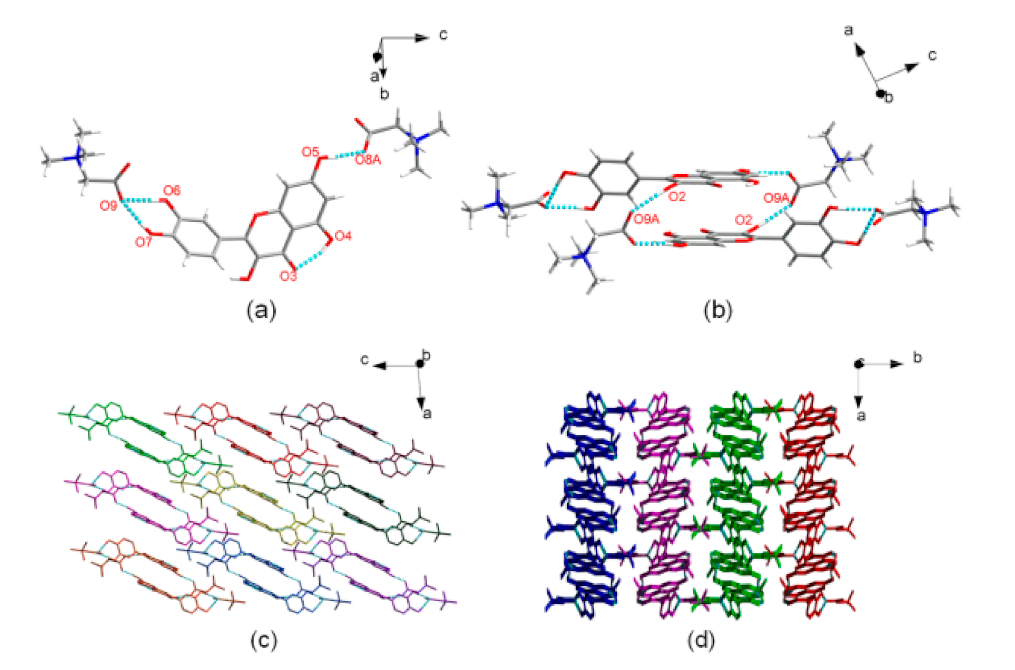
| Name of T-APIs | Main Sources of Plants | Problematic Physicochemical Properties | Major Indications |
|---|---|---|---|
| Alkaloids | |||
| Dauricine | Menispermum dauricum DC. | Slightly soluble | Tachyarrhythmia |
| Lycorine | Lycoris radiata (L’Her.) Herb., Narcissus tazetta Linn. var. chinensis M.Roener, Zephyranthes candida Herb., Crinum asiaticum Linn. var. sinicum (Roxb.ex Herb.) Baker, Galanthus woronawii Losink., Clivia miniata Regel. | Practically insoluble | Intestinal and external amoeba |
| Matrine | Sophora flavescens Alt., Euchresta japonica Hook. f. ex Regel. | - | Chronic cervicitis, dysentery, enteritis, skin disease |
| Sophocarpine | Sophora alopecuroides Linn., Sophora flavescens Alt., Sophora japonica L., Sophora davidii (Franch.) Skeels. | Slightly soluble | Cancer, chronic bronchial asthma, malignant mole |
| Toddaline | Chelidonium majus Linn., Toddalia asiatica (L.) Lam. | Slightly soluble | Rheumatic pain |
| Flavonoids | |||
| Apigenin | Apium graveolens Linn., Veronica linariifolia Pall.ex Link subsp.dilatata (NakaietKitag.) Hong., Reynoutria japonica Houtt., Veratrum grandiflorum Loes. | Practically insoluble | HIV and other viral infections, inflammation |
| Baicalein | Scutellaria baicalensis Georgi, Oroxylum indicum (Linn.) Bentham ex Kurz, Plantago major Linn. | Practically insoluble, unstable, prone to oxidation | Fever, sore throat, and upper respiratory tract infection |
| Chrysin | Oroxylum indicum (Linn.) Bentham ex Kurz, Pinus mon-ticola Dougl., P.aristata Engelm. | Practically insoluble | Cardiovascular and cerebrovascular diseases, inflammation |
| Dihydromyricetin | Ampelopsis grossedentata (Hand-Mazz)WT Wan | Hygroscopic, prone to oxidation and decomposition | Alcoholism, alcoholic liver, fatty liver |
| Genistein | Euchresta japonica Hook. f. ex Regel, Genista tinctoria Linn. | Practically insoluble | Cancer |
| Hesperidin | Citrus sinensis (Linn.) Osbeck, Citrus limon (L.) Burm. F. | Practically insoluble | Various diseases related to venous and lymphatic insufficiency, hypertension, and myocardial infarction |
| Kaempferol | Kaempferia galanga Linn. | Slightly soluble | Osteoclast bone resorption |
| Luteolin | Reseda odorata Linn., Lonicera japonica Thunb., Dendranthema morifolium (Ramat.) Tzvel., Nepeta cataria Linn., Ajuga nipponensis Makino. | Slightly soluble, low bioavailability | Cardiovascular disease, amyotrophic lateral sclerosis |
| Myricetin | Myricaceae, Vitaceae, Leguminosae, Ericaceae, and Euphorbiaceae. | Slightly soluble, low bioavailability | Cardiovascular disease |
| Naringenin | Amacardi-um occidentale L., Prunus yedoensis Mats., Armeniaca mume Sieb. | Practically insoluble, low bioavailability | Bacterial infection, cough, cancer |
| Quercetin | Sophora japonica Linn., Platycladus orientalis (Linn.) Franco, Alpinia officinarum Hance, Tussilago farfara Linn., Taxillus sutchuenensis (Lecomte) Danser, Ginkgo biloba Linn., Sambucus williamsii Hance, etc. | Practically insoluble, low bioavailability | Bacterial infection, viral infection, tumor, diabetes, hyperlipidemia, and immune system diseases |
| Phenolic acids | |||
| Curcumin | Curcuma aromatica Salisb., Curcuma longa Linn., Curcuma zedoaria (Christm.) Rosc., Acorus calamus Linn. | Practically insoluble, poor stability, prone to degradation, poor solubility in acidic condition, low dissolution rate and bioavailability | Inflammatory bowel disease, pancreatitis, arthritis |
| Ferulic acid | Ferula assafoetida L., Angelica sinensis (Oliv.) Diels, Ligusticum chuanxiong Hort., Cimicifuga foetida L., Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H.F.Chow. | Practically insoluble | Cardiovascular diseases, cerebrovascular diseases, leukopenia, and other diseases |
| Gallic acid monohydrate | Rhus chinensis Mill. | Slightly soluble | High blood pressure, hyperlipidemia, colon cancer, skin tumors |
| Oleanolic acid | Olea europaea Linn., Swertia mileensis T. N. Ho et W. L. Shi, Fructus Ligustri Lucidi, Hemsleya macrosperma C. Y. Wu ex C. Y. Wu et C. L. Chen. | Practically insoluble | Bronchitis, pneumonia, acute tonsillitis, periodontitis, bacillary dysentery, acute gastroenteritis, urinary infection, acute hepatitis |
| Pterostilbene | Pterocarpus indicus willd., Vitis vinifera Linn., and Ormosia henryi Prain. | Practically insoluble | High blood pressure, hyperlipidemia, colon cancer, skin tumors |
| Resveratrol | Reynoutria japonica Houtt., Cassia tora Linn., Morus alba L. | Practically insoluble, photosensitive, thermally unstable | Cancer, high blood cholesterol |
| Rhein | Rheum officinale Baill. | Practically insoluble | Hyperlipidemia, constipation |
| Ursolic acid | Prunella vulgaris Linn., Ilex rotunda Thunb. | Practically insoluble | Viral hepatitis, depression, primary hyperlipidemia |
| Salvianolic acid B | Salvia miltiorrhiza Bge. | Photosensitive, thermally unstable, prone to degradation | Ischemic stroke |
| Terpenoids | |||
| Loganin | Strychnos nux-vomica Linn., Cornus officinalis Sieb. Et Zucc. | Practically insoluble | Cancer |
| Triptolide | Tripterygium wilfordii Hook. F. | Practically insoluble | Rheumatoid arthritis |
| Name of T-APIs | Main Sources of Plants | Problematic Physicochemical Properties | Major Indications |
|---|---|---|---|
| Alkaloids | |||
| Berberine chloride | Coptis chinensis Franch., Phellodendron amurense Rupr. | Slightly soluble, low bioavailability, bitter taste | Diabetes, high blood pressure |
| Bulleyaconitine A | The subgenus Subgen. Aconitum. | Practically insoluble | Rheumatoid arthritis |
| Homoharringtonine | The genus Cephalotaxus. | Slightly soluble | Acute myelogenous leukemia, acute monocytic leukemia, malignant lymphoma |
| Huperzine-A | The genus Huperzia, i.e., Huperzia serrata (Thunb. ex Murray) Trev. | Practically insoluble, hygroscopic | Senile dementia, myasthenia gravis |
| Pseudoephedrine hydrochloride | Ephedra sinica Stapf. | - | Nasal congestion caused by cold, allergic rhinitis, rhinitis, sinusitis |
| Raceanisodamine | Atropa belladonna Linn., Datura metel L. | - | Choline drug resistance, smooth muscle spasm, gastrointestinal colic, biliary tract spasm, organophosphorus poisoning |
| Reserpine | The genus Rauvolfia. | Slightly soluble, photosensitive | Mild and moderate hypertension |
| Tetrahydropalmatine sulfate | Corydalis yanhusuo W. T. Wang. | Slightly soluble, prone to oxidation, photosensitive | medical ailments, prenatal labor pains, postpartum contractions, menstrual pain, headache, insomnia |
| Ligustrazine hydrochloride | Ligusticum chuanxiong Hort., Curcuma aromatica Salisb. | Prone to sublimation, hygroscopic | Vasodilator, occlusive cerebrovascul- ar disease, ischemic vascular disease |
| Vinblastine sulfate | Catharanthus roseus (Linn.) G. Don. | Hygroscopic, photosensitive, prone to thermal degradation | Hodgkin’s disease, chorioepithelial carcinoma, lymphoid sarcoma, acute leukemia, breast cancer, testicular tumor, choriocarcinoma |
| Vincristine sulfate | Catharanthus roseus (Linn.) G. Don. | Hygroscopic, photosensitive, prone to thermal degradation | Acute leukemia |
| Flavonoids | |||
| Baicalin | Scutellaria baicalensis Georgi. | Slightly soluble, low bioavailability, poor tabletability | Acute and chronic hepatitis, persistent hepatitis, nephritis, pyelonephritis, and allergic diseases |
| Rutinum | Sophora japonica Linn., Platycladus orientalis (Linn.) Franco, Alpinia officinarum Hance, Tussilago farfara Linn., Taxillus sutchuenensis (Lecomte) Danser, Ginkgo biloba Linn., Sambucus williamsii Hance, etc. | Practically insoluble, hygroscopic | Hypertensive encephalopathy, cerebral hemorrhage, retinal hemorrhage |
| Phenolic acids | |||
| 7-Hydroxy-4-methylcoumarin | - | Practically insoluble | Cholecystitis, cholelithiasis, biliary tract infection, cholecystectomy syndrome |
| Propyl gallate | Rhus chinensis Mill. | Slightly soluble | Cerebral thrombosis, coronary heart disease, thrombophlebitis |
| Terpenoids | |||
| Andrographolide | Andrographis paniculata (Burm. f.) Nees. | Practically insoluble | Upper respiratory tract infection, bacterial dysentery, bacterial and viral upper respiratory tract infections, dysentery |
| Artemether | Artemisia annua Linn. | Practically insoluble | Plasmodium falciparum, dangerous malaria resistant to chloroquine |
| Artemisinin | Artemisia annua Linn. | Practically insoluble, low bioavailability, short biological half-life, frequent drug administration is needed | Malaria, pulmonary hypertension |
| Artesunate | Artemisia annua Linn. | Slightly soluble | Cerebral malaria and various critical malaria |
| Dihydroartemisinin | - | Slightly soluble | Malaria Plasmodium falciparum, dangerous malaria resistant to chloroquine and piperaquine |
| (R)-Camphor | Cinnamomum camphora (Linn.) Presl. | Low aqueous soluable, volatile | Skin irritant |
| Taxol | Some various plants of the genus Taxus Linn. | Practically insoluble | Ovarian cancer, breast cancer, non-small cell lung cancer |
| Drug | Coformer | Single Crystal Structure | Supramolecular Interactions |
|---|---|---|---|
| Alkaloids | |||
| Berberine chloride | Citric acid [60] Ibuprofen [61] Fumaric acid [62] Lactic acid * [63] Myricetin [64] Dihydromyricetin [64] | Reported - Reported - Reported Reported | −COOH···Cl - O−H···Cl, π–π interaction - O−H···Cl O−H···Cl |
| Berberine | Phthalic acid * [65] Chrysin [66] | - Reported | - O−H···O, interaction |
| Ligustrazine | Bexarotene [67] | Reported | O−H···N |
| Flavonoids | |||
| Apigenin | 4,4′-Bipyridine * [68] | Reported | O−H···N, π–π interaction |
| Baicalein | Nicotinamide [69] Caffeine [69] Isoniazid [69] Isonicotinamide [70] Theophylline [70] Betaine [71] | Reported Reported - Reported Reported Reported | O−H···N, O−H···O, N−H···O O−H···N, O−H···O, π–π interaction - O−H···Narom O−H···Narom O−H···O |
| Chrysin | Cytosine [72] Thiamine hydrochloride * [72] | Reported Reported | N−H···N N−H···O |
| Dihydromyricetin | Pentoxifylline [73] Caffeine [74] Urea* [74] | Reported - - | O−H···O - - |
| Fisetin | Nicotinamide [75] Isonicotinamide [75] | Reported Reported | O−H···Narom O−H···Narom |
| Genistein | Isonicotinamide [76] 4,4′-Bipyridine * [77] Caffeine [78] Nicotinamide [75] | Reported Reported Reported Reported | O−H···N O−H···N O−H···N, O−H···O O−H···Narom |
| Hesperidin | Caffeine [79] Nicotinamide [79] Picolinic acid * [79] Temozolomide [80] | Reported Reported Reported Reported | O−H···N N−H···Narom, C=O···OH− C=O···OH− O−H···N |
| Kaempferol | 5-Fluorouracil [81] 4,4′-Bipyridine * [81] Propylthiouracil [82] | Reported Reported Reported | O−H···N, O−H···O, N−H···O, C−H···O O−H···N, π–π interaction C−H···O |
| Luteolin | Isonicotinamide [75] | Reported | O−H···Narom |
| Myricetin | 4,4′-Bipyridine * [81] | Reported | O−H···N, π–π interaction |
| Naringenin | 4-Hydroxypyridine [83] Anthranilamide [83] Flavone [83] 4,4′-Bipyridine * [83] Isonicotinamide [84] Picolinic acid * [84] Betaine [84] Carbamazepine [85] | Reported Reported Reported Reported Reported - Reported Reported | O−H···C=O O−H···C=O O−H···C=O O−H···C=O O−H···N, N−H···O - O−H···O O−H···O, N−H···O |
| Puerarin | Lornoxicam [86] | - | - |
| Quercetin | 4,4′-Bipyridine * [81] Caffeine [7] Isonicotinamide [7] Theobromine dihydrate [7] Isoniazid [87] Nicotinamide [88] Pyrazole * [89] Imidazolidinone * [89] Baclofen [89] | Reported Reported Reported - Reported - - - - | O−H···N, π–π interaction O−H···O, O−H···Narom O−H···C=O, O−H···Narom - N−H···O - - - - |
| Phenolic acids | |||
| Curcumin | Isoniazid [90] Hydroquinone * [91] Phloroglucinol [92] Resorcinol [93] Pyrogallol [93] Ascorbic acid [94] Trimesic acid [95] | - - - Reported Reported - - | - - - O−H···O, C−H···O, C−H···π O−H···O, C−H···O, C−H···π - - |
| Gallic acid | Metronidazole [96] Lenalidomide [97] Isoniazid [98] | Reported Reported Reported | O−H···O O−H···O, N−H···O O−H···O, O−H···N, π–π interaction |
| Ferulic acid | Pyrazinamide [99] Urea [100] Nicotinamide [100] Isonicotinamide [100] | Reported Reported Reported Reported | C=O···N−H N−H···N O−H···NO−H···N |
| Oleanolic acid | Isoniazid [101] | - | - |
| Pterostilbene | Ethylenediamine * [102] Caffeine [103] Carbamazepine [103] | Reported Reported Reported | C−H···π O−H···N, O−H···O O−H···N, O−H···O |
| Resveratrol | Curcumin [104] Isoniazid [105] 4-Aminobenzamide * [106] | - Reported - | - N−H···O - |
| Ursolic acid | Ethylenediamine * [107] Piperazine [108] Metformin [109] Arginine [109] Lysine [109] N-Methylglucamine [109] | - - - - - - | - - - - - - |
| Terpenoids | |||
| Andrographolide | Salicylic acid [110] Vanillin [110] Vanillic acid [110] Guaiacol [110] Resorcinol [110] | Reported Reported Reported Reported Reported | O−H···O, C−H···O O−H···O, C−H···O O−H···O, C−H···O O−H···O O−H···O |
| Artemisinin | Orcinol [18] Resorcinol [18] | Reported - | C−H···O, O−H···π C−H···O, O−H···O |
| Artesunate | Nicotinamide [111] | - | - |
| 11-Aza-artemisinin | Benzoic acid [54] Salicylic acid [54] Succinic acid [54] Heptanedioic acid [54] | - - - - | - - - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, D.; Xuan, B.; Wang, C.; Long, R.; Jiang, Y.; Mao, L.; Kang, J.; Wang, Z.; Chow, S.F.; Zhou, Q. Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering. Pharmaceutics 2021, 13, 2160. https://doi.org/10.3390/pharmaceutics13122160
Guan D, Xuan B, Wang C, Long R, Jiang Y, Mao L, Kang J, Wang Z, Chow SF, Zhou Q. Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering. Pharmaceutics. 2021; 13(12):2160. https://doi.org/10.3390/pharmaceutics13122160
Chicago/Turabian StyleGuan, Danyingzi, Bianfei Xuan, Chengguang Wang, Ruitao Long, Yaqin Jiang, Lina Mao, Jinbing Kang, Ziwen Wang, Shing Fung Chow, and Qun Zhou. 2021. "Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering" Pharmaceutics 13, no. 12: 2160. https://doi.org/10.3390/pharmaceutics13122160
APA StyleGuan, D., Xuan, B., Wang, C., Long, R., Jiang, Y., Mao, L., Kang, J., Wang, Z., Chow, S. F., & Zhou, Q. (2021). Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering. Pharmaceutics, 13(12), 2160. https://doi.org/10.3390/pharmaceutics13122160