Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Co-Processing by Fluid-Bed Melt Granulation
2.2.2. Particle Size Distribution Determination
2.2.3. Flow through an Orifice
2.2.4. Determination of Bulk and Tapped Density, Compressibility Index and Hausner Ratio
2.2.5. Evaluation of Tableting Behaviour
2.2.6. Tablet Disintegration
3. Results and Discussion
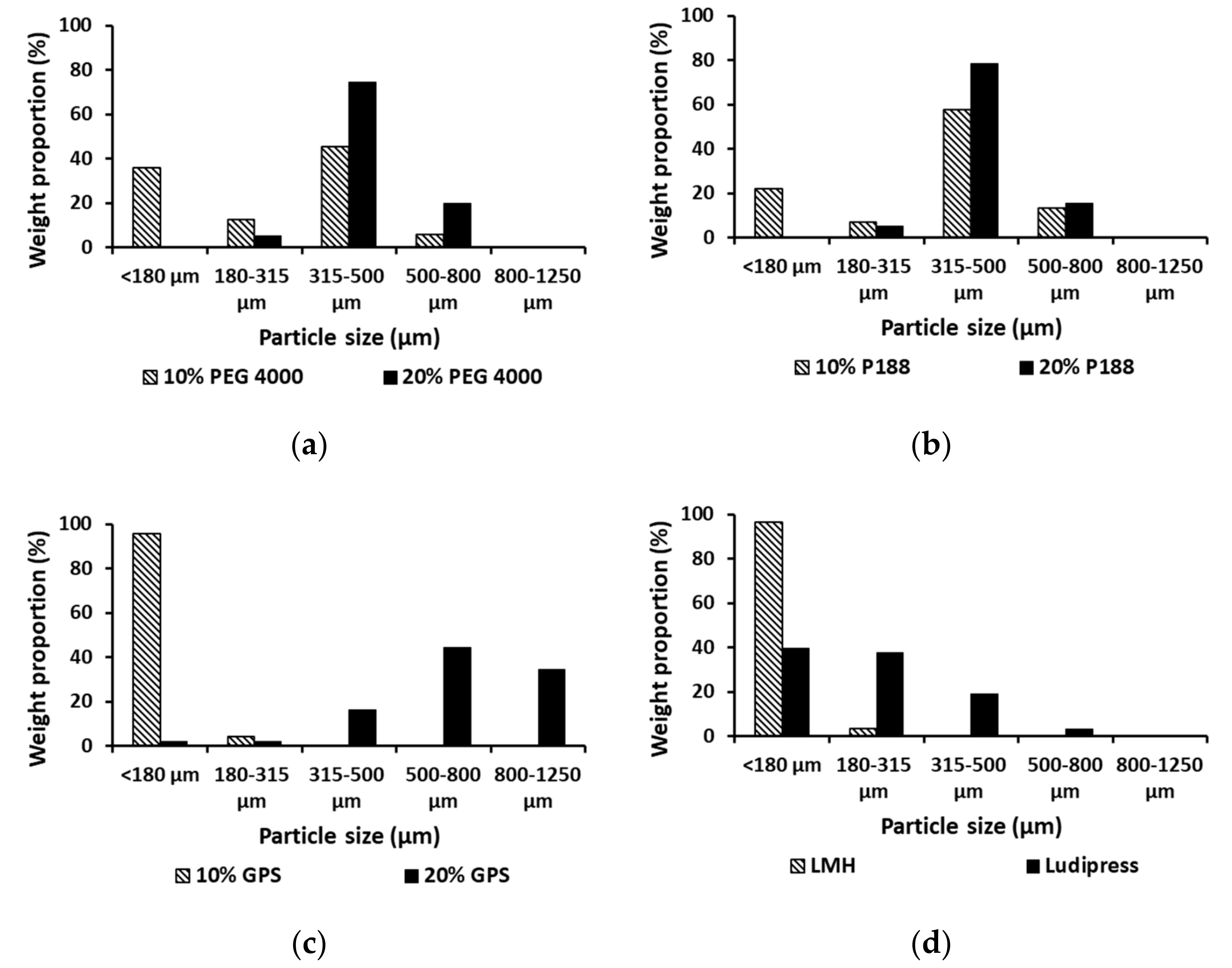
3.1. Particle Size Determination
3.2. Flowability Testing
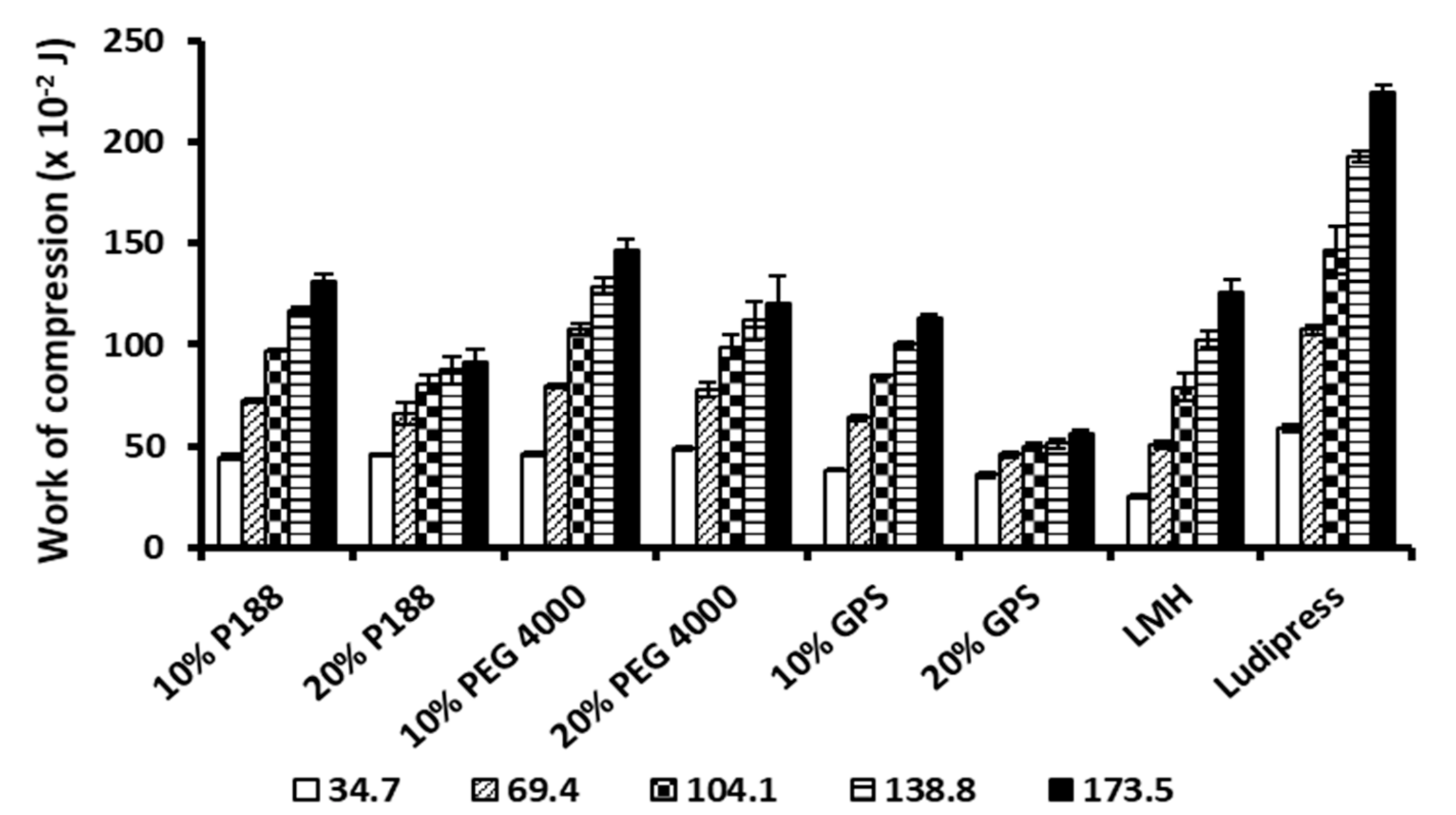
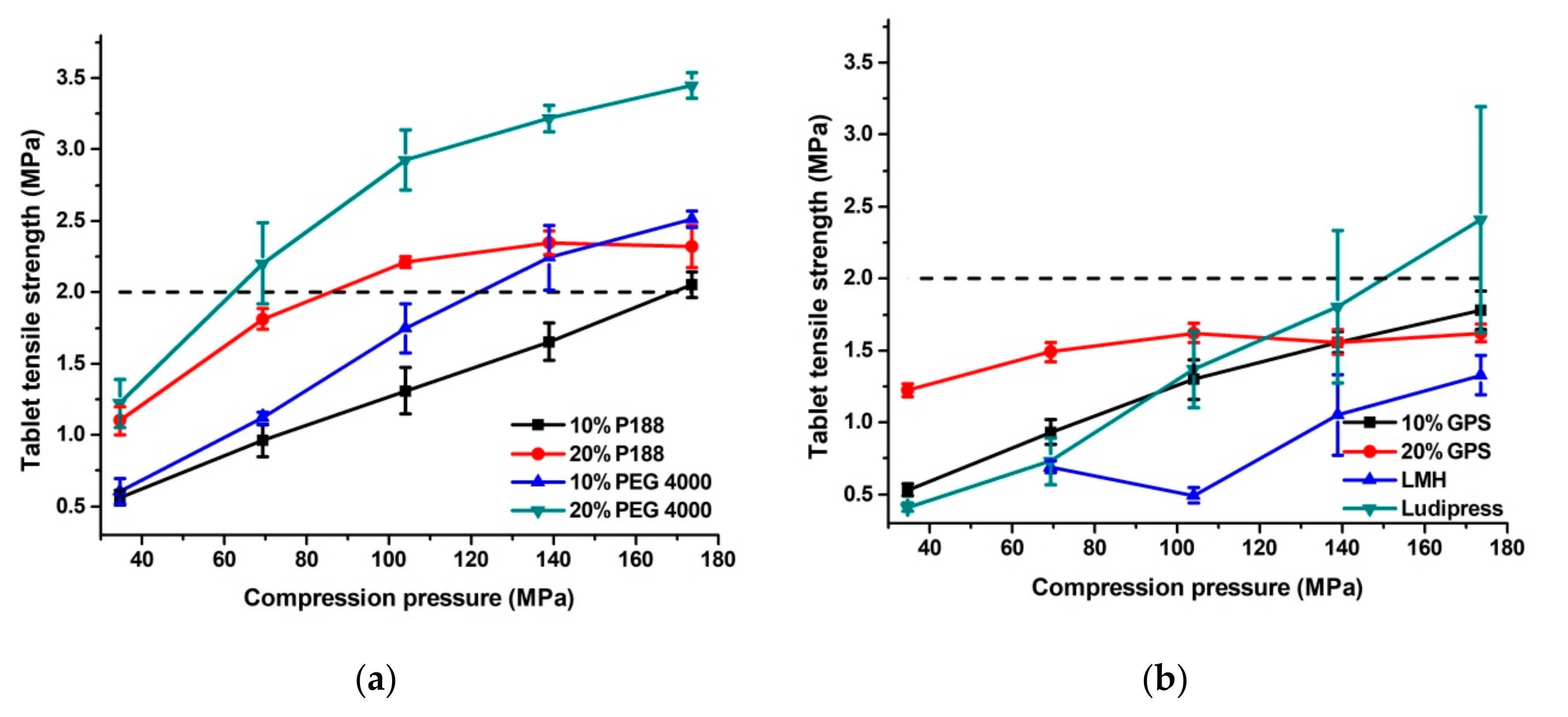
3.3. Evaluation of Tableting Behaviour
3.4. Tablet Disintegration Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar] [PubMed]
- Ilić, I.; Kása, P., Jr.; Dreu, R.; Pintye-Hódi, K.; Srcic, S. The compressibility and compactibility of different types of lactose. Drug. Dev. Ind. Pharm. 2009, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.A. Tablet Manufacture by Direct Compression. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare Inc.: New York, NY, USA, 2007; pp. 3673–3683. [Google Scholar]
- Rojas, J. Excipient design by co-processing for direct compression applications. In Excipient Applications in Formulation Design and Drug Delivery; Narang, A., Boddu, S., Eds.; Springer: Cham, Switzerland, 2015; pp. 589–612. [Google Scholar]
- Saha, S.; Shahiwala, A.F. Multifunctional coprocessed excipients for improved tableting performance. Expert. Opin. Drug. Deliv. 2009, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Buckner, I.; Kumar, V. Co-proccessed excipients with enhanced direct compression functionality for improved tableting performance. Drug. Dev. Ind. Pharm. 2012, 38, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Abu Fara, D.; Rashid, I.; Alkhamis, K.; Al-Omari, M.; Chowdhry, B.Z.; Badwan, A. Modification of α-lactose monohydrate as a direct compression excipient using roller compaction. Drug. Dev. Ind. Pharm. 2018, 44, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Ruangchayajatuporn, J.; Amornsakchai, T.; Sinchaipanid, N.; Mitrevej, A. Compaction behavior and optimization of spray-dried lactose with various amorphous content. J. Drug. Deliv. Sci. Technol. 2011, 21, 175–181. [Google Scholar] [CrossRef]
- Paul, S.; Sun, C.C. Systematic evaluation of common lubricants for optimal use in tablet formulation. Eur. J. Pharm. Sci. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.L.; Tian, C.; Ke, X. Comparative evaluation of a co-processed self-lubricating excipient LubriTose SD as a direct compression vehicle. J. Drug. Deliv. Sci. Technol. 2012, 22, 562–567. [Google Scholar] [CrossRef]
- Madhvi, K.; Mehta, K.; Vadalia, K.R.; Jay, C.; Sandip, K. Design and development of co-processed excipients for fast dissolving tablets of irbesartan by melt agglomeration technique. J. Pharma. Investig. 2015, 45, 163–186. [Google Scholar] [CrossRef]
- Gohel, M.C.; Jogani, P.D. Exploration of melt granulation technique for the development of coprocessed directly compressible adjuvant containing lactose and microcrystalline cellulose. Pharm. Dev. Technol. 2003, 8, 175–185. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019.
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Osamura, T.; Takeuchi, Y.; Onodera, R.; Kitamura, M.; Takahashi, Y.; Tahara, K.; Takeuchi, H. Characterization of tableting properties measured with a multi-functional compaction instrument for several pharmaceutical excipients and actual tablet formulations. Int. J. Pharm. 2016, 510, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Benabbas, R.; Sanchez-Ballester, N.M.; Aubert, A.; Sharkawi, T.; Bataille, B.; Soulairol, I. Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release. Polymers 2021, 13, 988. [Google Scholar] [CrossRef] [PubMed]
- Šantl, M.; Ilić, I.; Vrečer, F.; Baumgartner, S. A compressibility and compactibility study of real tableting mixtures: The impact of wet and dry granulation versus a direct tableting mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhou, M.; Lin, X.; Shen, L.; Feng, Y. Differences in fundamental and functional properties of HPMC co-processed fillers prepared by fluid-bed coating and spray drying. Eur. J. Pharm. Sci. 2018, 119, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic development of a high dosage formulation to enable direct compression of a poorly flowing API: A case study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Uzondu, B.; Leung, L.Y.; Mao, C.; Yang, C.Y. A mechanistic study on tablet ejection force and its sensitivity to lubrication for pharmaceutical powders. Int. J. Pharm. 2018, 543, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Djuris, J.; Cirin-Varadjan, S.; Aleksic, I.; Djuris, M.; Cvijic, S.; Ibric, S. Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients. Pharmaceutics 2021, 13, 663. [Google Scholar] [CrossRef] [PubMed]

| Sample | LMH (%) | Calcium Dihydrogen Phosphate Dihydrate (%) | Croscarmellose Sodium (%) | PEG 4000 (%) | P188 (%) | GPS (%) |
|---|---|---|---|---|---|---|
| 10% PEG 4000 | 70 | 15 | 5 | 10 | x | x |
| 20% PEG 4000 | 60 | 15 | 5 | 20 | x | x |
| 10% P188 | 70 | 15 | 5 | x | 10 | x |
| 20% P188 | 60 | 15 | 5 | x | 20 | x |
| 10% GPS | 70 | 15 | 5 | x | x | 10 |
| 20% GPS | 60 | 15 | 5 | x | x | 20 |
| Sample | Compressibility Index | Hausner Ratio (%) | Flowability | |
|---|---|---|---|---|
| According to Scale of Ph. Eur. 10.0 (Table 2.9.36.-2.) | Flow Rate Measured by Flow through an Orifice (g/s) | |||
| 10% PEG 4000 | 10.76 ± 0.70 | 1.12 ± 0.01 | good | 10.87 ± 0.10 |
| 20% PEG 4000 | 8.25 ± 0.95 | 1.09 ± 0.01 | excellent | 9.59 ± 0.25 |
| 10% P188 | 9.14 ± 0.35 | 1.10 ± 0.01 | excellent | 10.66 ± 0.20 |
| 20% P188 | 6.69 ± 0.90 | 1.07 ± 0.01 | excellent | 9.40 ± 0.32 |
| 10% GPS | 13.72 ± 2.24 | 1.16 ± 0.03 | good | 7.41 ± 0.09 |
| 20% GPS | 8.22 ± 0.59 | 1.09 ± 0.01 | excellent | 8.82 ± 0.13 |
| LMH | 35.70 ± 1.12 | 1.55 ± 0.03 | very poor | 1.24 ± 0.13 |
| Ludipress® | 15.52 ± 2.66 | 1.18 ± 0.04 | fair | 7.46 ± 0.09 |
| Sample | Detachment Parameters | Compression Pressure (MPa) | ||||
|---|---|---|---|---|---|---|
| 34.7 | 69.4 | 104.1 | 138.8 | 173.5 | ||
| 10% PEG 4000 | DW (×10−2 J) | 7.90 ± 4.43 | 16.06 ± 5.86 b | 21.58 ± 7.36 a,b | 36.64 ± 11.00 a,b | 44.13 ± 12.62 a,b |
| DS (MPa) | 1.51 ± 0.27 | 2.79 ± 0.09 b | 3.75 ± 0.32 | 4.35 ± 0.76 | 4.68 ± 0.59 | |
| 20% PEG 4000 | DW (×10−2 J) | 4.00 ± 2.05 | 11.52 ± 2.87 b | 22.09 ± 5.85 a,b | 25.55 ± 5.63 a,b | 32.13 ± 2.39 a,b |
| DS (MPa) | 1.53 ± 0.42 | 3.27 ± 0.34 | 4.20 ± 0.39 | 4.08 ± 1.36 | 4.91 ± 0.38 | |
| 10% P188 | DW (×10−2 J) | 2.23 ± 1.58 b | 6.12 ± 4.21 a,b | 13.29 ± 9.15 a,b | 13.73 ± 5.35 a,b | 15.98 ± 5.88 a,b |
| DS (MPa) | 0.77 ± 0.12 a,b | 1.59 ± 0.19b | 2.45 ± 0.22 a,b | 2.66 ± 0.63 a,b | 2.89 ± 0.53 a,b | |
| 20% P188 | DW (×10−2 J) | 2.60 ± 0.95 b | 3.84 ± 1.22 a,b | 5.67 ± 2.56 a,b | 9.77 ± 7.25 a,b | 10.25 ± 6.45 a,b |
| DS (MPa) | 1.27 ± 0.33 | 1.87 ± 0.50 b | 2.24 ± 0.70 a,b | 2.88 ± 0.10 a,b | 2.59 ± 0.67 a,b | |
| 10% GPS | DW (×10−2 J) | 1.03 ± 0.57 a,b | 1.72 ± 0.77 a,b | 2.83 ± 1.20 a,b | 3.23 ± 1.13 a,b | 2.96 ± 1.15 a,b |
| DS (MPa) | 0.71 ± 0.10 a,b | 1.08 ± 0.16 b | 1.14 ± 0.26 a,b | 1.31 ± 0.46 a,b | 1.25 ± 0.47 a,b | |
| 20% GPS | DW (×10−2 J) | 1.06 ± 0.48 a,b | 0.86 ± 0.17 a,b | 1.06 ± 0.50 a,b | 1.02 ± 0.41 a,b | 1.21 ± 0.29 a,b |
| DS (MPa) | 0.68 ± 0.11 a,b | 0.76 ± 0.06b | 0.72 ± 0.22 a,b | 0.82 ± 0.22 a,b | 1.00 ± 0.14 a,b | |
| LMH | DW (×10−2 J) | 4.77 ± 2.35 | 15.17 ± 7.76 b | 97.57 ± 47.92 | 144.55 ± 59.15 | 139.61 ± 84.42 |
| DS (MPa) | 1.40 ± 0.47 | 1.13 ± 0.54 b | 3.95 ± 1.22 | 5.79 ± 1.65 | 5.16 ± 1.87 | |
| Ludipress® | DW (×10−2 J) | 10.43 ± 6.85 | 47.74 ± 13.78 | 53.42 ± 14.16 | 77.92 ± 39.95 a | 111.64 ± 36.83 |
| DS (MPa) | 1.71 ± 0.42 | 4.18 ± 1.05 | 4.57 ± 1.09 | 5.56 ± 2.03 | 8.02 ± 3.49 | |
| Sample | Ejection Parameters | Compression Pressure (MPa) | ||||
|---|---|---|---|---|---|---|
| 34.7 | 69.4 | 104.1 | 138.8 | 173.5 | ||
| 10% PEG 4000 | EW (×10−2 J) | 4.21 ± 0.69 a,b | 7.16 ± 1.43 a,b | 7.15 ± 0.56 a,b | 8.46 ± 0.68 a,b | 9.22 ± 0.69 a,b |
| ES (MPa) | 0.94 ± 0.06 a,b | 1.68 ± 0.16 a,b | 1.78 ± 0.16 a,b | 3.18 ± 0.08 a,b | 4.29 ± 0.47 a,b | |
| 20% PEG 4000 | EW (×10−2 J) | 2.96 ± 0.42 a,b | 4.35 ± 0.93 a,b | 5.59 ± 1.06 a,b | 5.97 ± 1.76 a,b | 6.39 ± 2.13 a,b |
| ES (MPa) | 0.89 ± 0.18 a,b | 1.70 ± 0.15 a,b | 3.02 ± 0.64 a,b | 3.10 ± 0.61 a,b | 3.58 ± 0.94 a,b | |
| 10% P188 | EW (×10−2 J) | 3.14 ± 0.84 a,b | 4.31 ± 0.24 a,b | 6.02 ± 0.77 a,b | 6.87 ± 0.72 a,b | 6.91 ± 0.45 a,b |
| ES (MPa) | 0.52 ± 0.11 a,b | 1.14 ± 0.19 a,b | 1.76 ± 0.25 a,b | 1.92 ± 0.27 a,b | 2.06 ± 0.19 a,b | |
| 20% P188 | EW (×10−2 J) | 3.09 ± 0.52 a,b | 3.66 ± 0.21 a,b | 3.61 ± 0.22 a,b | 3.88 ± 0.17 a,b | 3.96 ± 0.53 a,b |
| ES (MPa) | 0.70 ± 0.15 a,b | 0.89 ± 0.17 a,b | 1.07 ± 0.19 a,b | 1.51 ± 0.36 a,b | 1.66 ± 0.55 a,b | |
| 10% GPS | EW (×10−2 J) | 1.48 ± 0.28 a,b | 2.40 ± 0.54 a,b | 2.50 ± 0.23 a,b | 2.81 ± 0.26 a,b | 2.93 ± 0.24 a,b |
| ES (MPa) | 0.31 ± 0.08 a,b | 0.46 ± 0.02 a,b | 0.55 ± 0.08 a,b | 0.68 ± 0.14 a,b | 0.73 ± 0.10 a,b | |
| 20% GPS | EW (×10−2 J) | 0.79 ± 0.19 a,b | 1.00 ± 0.12 a,b | 1.22 ± 0.25 a,b | 1.28 ± 0.21 a,b | 1.34 ± 0.20 a,b |
| ES (MPa) | 0.17±0.02ab | 0.20 ± 0.01 a,b | 0.24 ± 0.03 a,b | 0.26 ± 0.04 a,b | 0.29 ± 0.04 a,b | |
| LMH | EW (×10−2 J) | 15.10 ± 5.54 | 30.67 ± 4.18 b | 131.22 ± 23.20 | 142.80 ± 16.73 | 156.06 ± 8.13 |
| ES (MPa) | 1.30 ± 0.31 | 2.66 ± 0.23 b | 11.84 ± 3.56 | 15.91 ± 4.15 | 18.90 ± 4.02 | |
| Ludipress® | EW (×10−2 J) | 20.38 ± 11.67 | 120.21 ± 41.44 | 139.90 ± 37.30 | 137.85 ± 38.29 | 165.53 ± 77.82 |
| ES (MPa) | 1.33 ± 0.59 | 7.68 ± 2.71 | 9.57 ± 3.56 | 13.82 ± 6.40 | 13.35 ± 4.98 | |
| Sample | Disintegration Time (min) |
|---|---|
| 10% PEG 4000 | 1.88 |
| 20% PEG 4000 | 4.07 |
| 10% P188 | 2.33 |
| 20% P188 | 5.45 |
| 10% GPS | 0.57 |
| 20% GPS | 10.97 |
| LMH | 1.77 |
| Ludipress® | 5.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medarević, D.; Djuriš, J.; Krkobabić, M.; Ibrić, S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics 2021, 13, 2165. https://doi.org/10.3390/pharmaceutics13122165
Medarević D, Djuriš J, Krkobabić M, Ibrić S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics. 2021; 13(12):2165. https://doi.org/10.3390/pharmaceutics13122165
Chicago/Turabian StyleMedarević, Djordje, Jelena Djuriš, Mirjana Krkobabić, and Svetlana Ibrić. 2021. "Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing" Pharmaceutics 13, no. 12: 2165. https://doi.org/10.3390/pharmaceutics13122165
APA StyleMedarević, D., Djuriš, J., Krkobabić, M., & Ibrić, S. (2021). Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics, 13(12), 2165. https://doi.org/10.3390/pharmaceutics13122165









