Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Essential Oil Extraction, and Mass Spectrometric Analysis
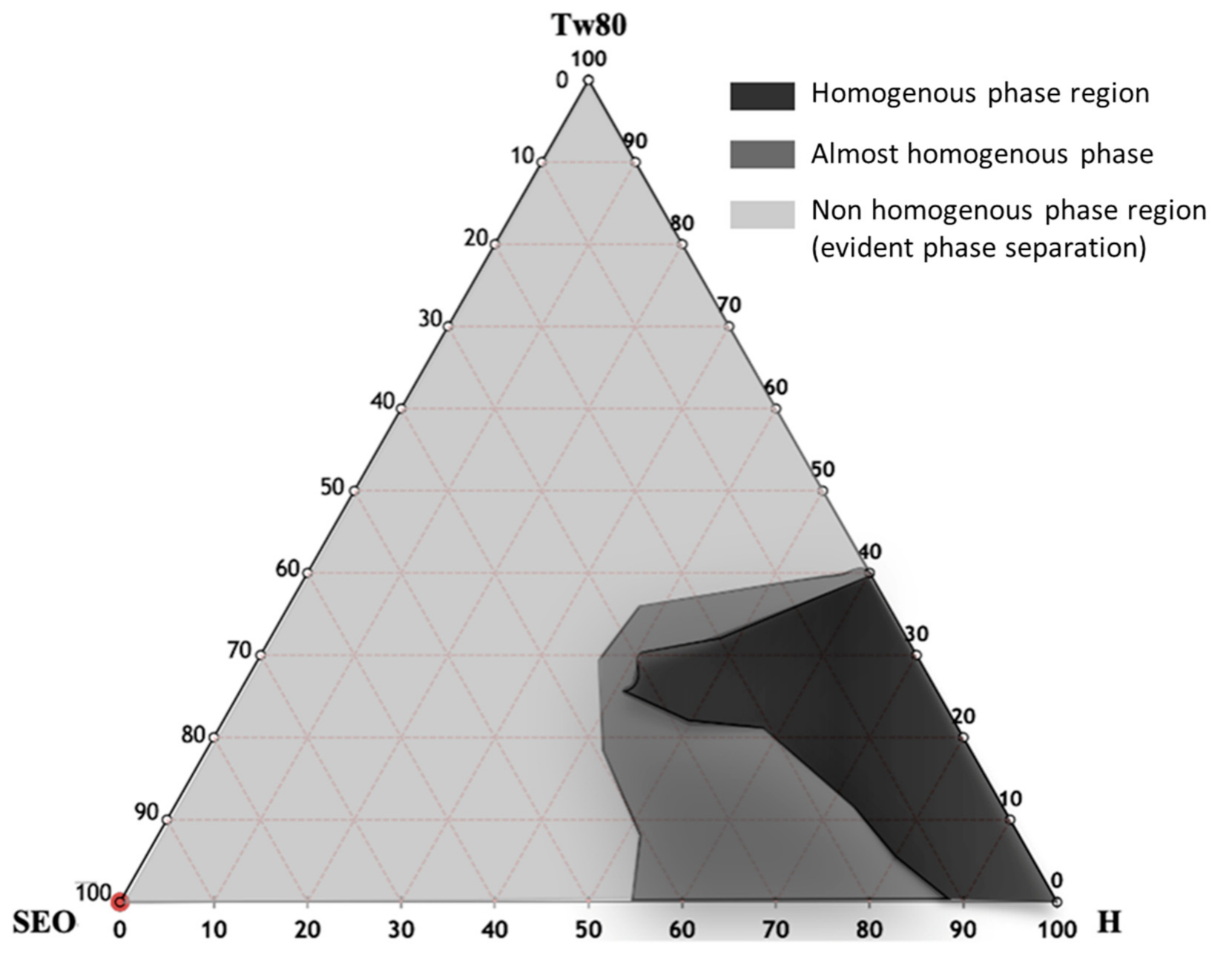
2.2. Pseudoternary Phase Diagram Costruction and Nanoemulsion Preparation
2.3. NE Characterization
2.4. Stability Studies
2.5. Bacterial Strains
2.6. Evaluation of Microbial Biofilm Formation
- OD ≤ ODc = no biofilm producer
- ODc < OD ≤ (2 × ODc) = weak biofilm producer
- (2 × ODc) < OD ≤ (4 × ODc) = moderate biofilm producer
- (4 × ODc) < OD = strong biofilm producer
2.7. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.8. Evaluation of Biofilm Inhibition
2.9. Evaluation of Biofilm Eradication
2.10. Scanning Electron Microscopy (SEM)
2.11. Statistical Analysis
3. Results
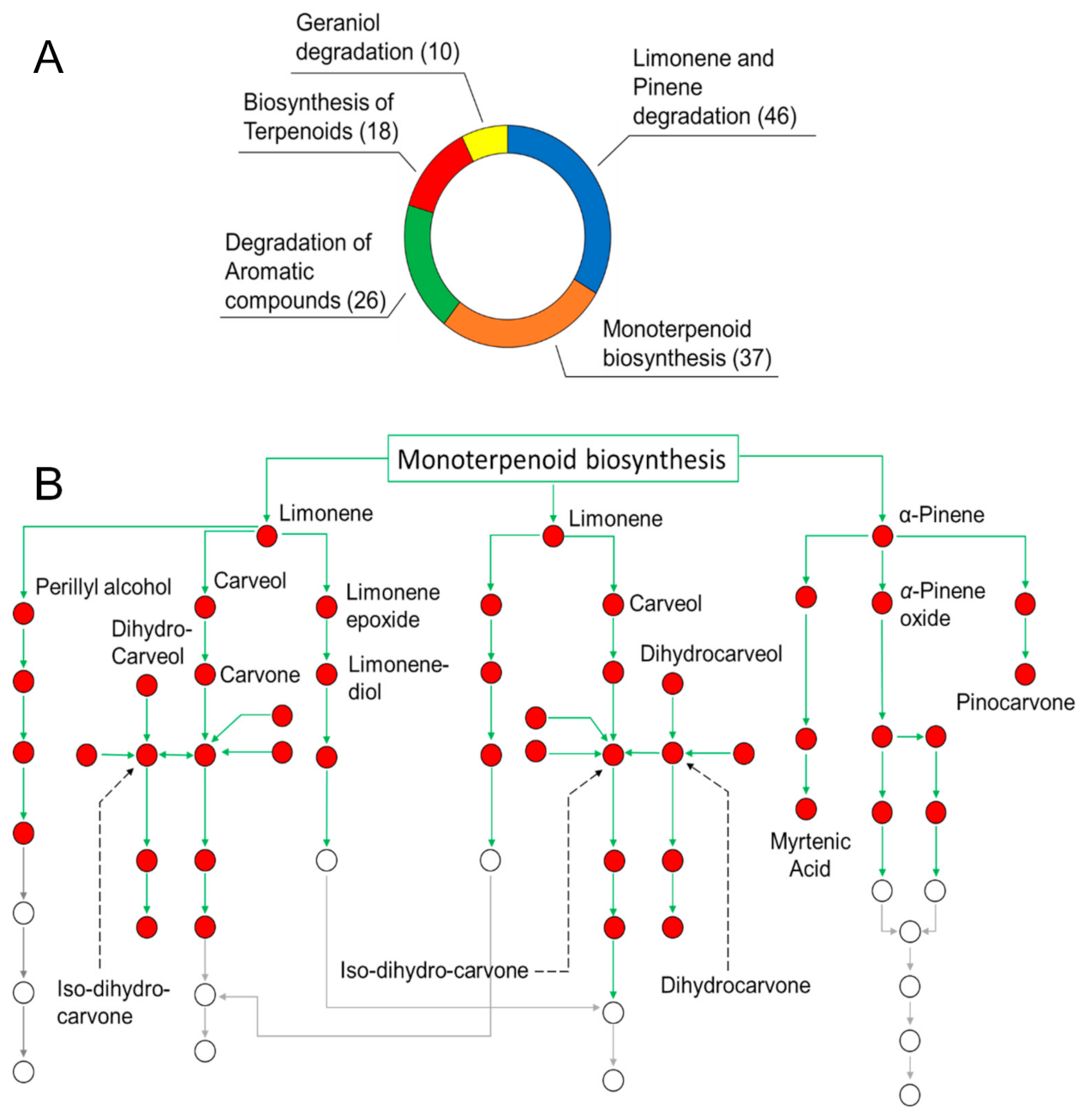
3.1. Oil Composition and Determination of the Role of SEO Components in Biological Pathways
3.2. NE Design and Characterization
3.3. Avian E. coli Strain Characterization for Biofilm Production Ability
3.4. Antibacterial Activity of SEO and NEs against Planktonic E. coli Cells
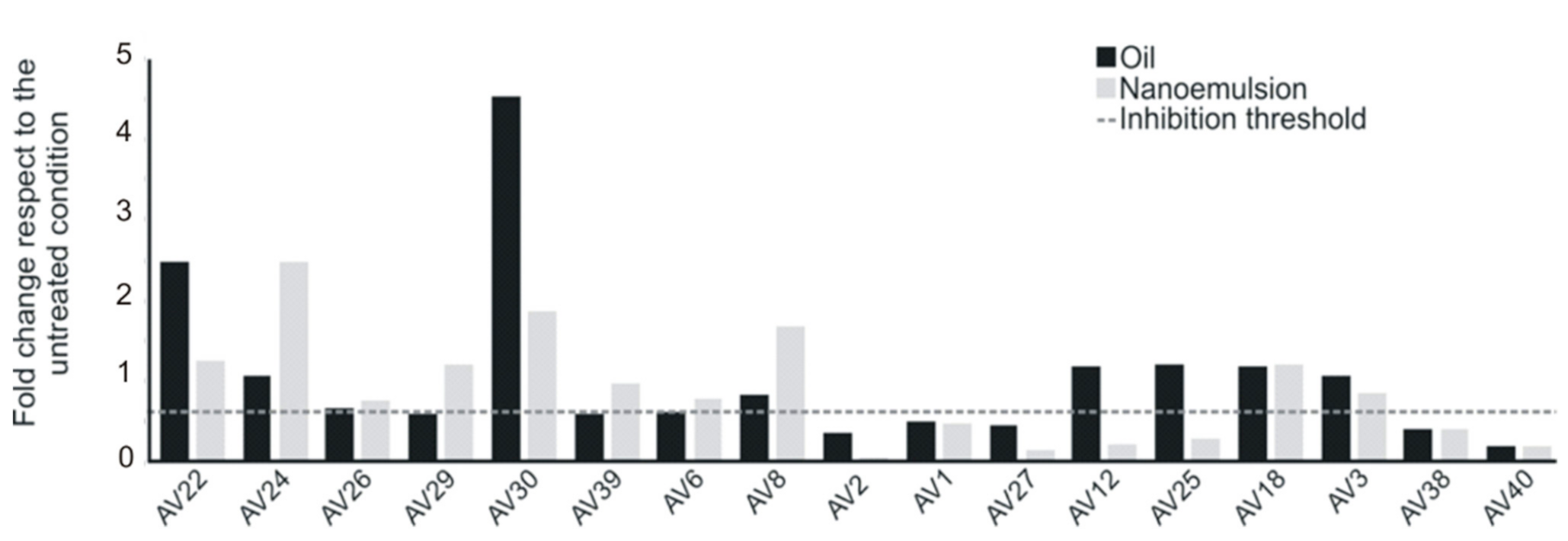
3.5. Antibiofilm Activity of SEO and NEs against Sessile E. coli Cells
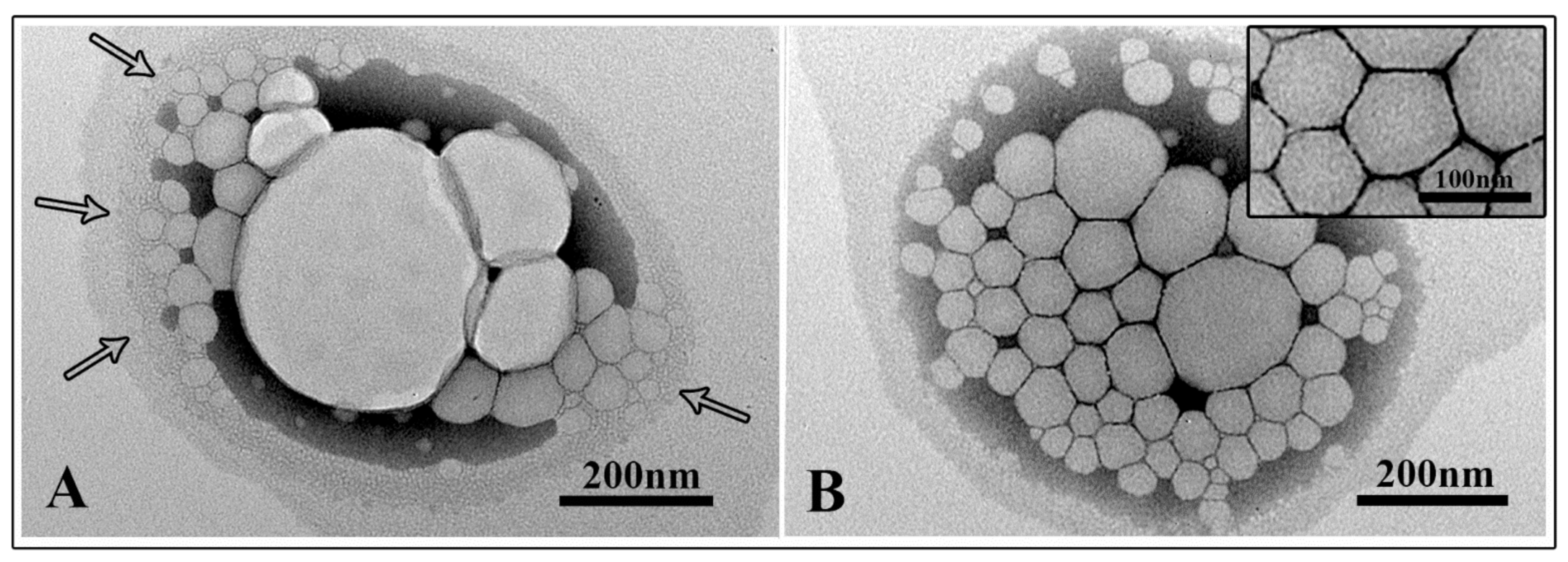
3.6. Scanning Electron Microscopy Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- De Mello Santos, A.C.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of hybrid-and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Riley, L. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Alteri, C.J.; Mobley, H.L. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Curr. Opin. Microbiol. 2012, 15, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, C.-D.; Dobrindt, U. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011, 301, 642–647. [Google Scholar] [CrossRef]
- Manges, A.; Johnson, J. Reservoirs of Extraintestinal Pathogenic Escherichia coli. Microbiol. Spectr. 2015. [CrossRef]
- Manges, A. Escherichia coli and urinary tract infections: The role of poultry-meat. Clin. Microbiol. Infect. 2016, 22, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Stromberg, Z.R.; Johnson, J.R.; Fairbrother, J.M.; Kilbourne, J.; Van Goor, A.; Curtiss, R., 3rd; Mellata, M. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS ONE 2017, 12, e0180599. [Google Scholar] [CrossRef] [Green Version]
- Pitout, J. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Ewers, C.; Antão, E.-M.; Diehl, I.; Philipp, H.-C.; Wieler, L.H. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl. Environ. Microbiol. 2009, 75, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giufrè, M.; Graziani, C.; Accogli, M.; Luzzi, I.; Busani, L.; Cerquetti, M. Escherichia coli of human and avian origin: Detection of clonal groups associated with fluoroquinolone and multidrug resistance in Italy. J. Antimicrob. Chemother. 2012, 67, 860–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fevre, E.M.; Woolhouse, M.E.; Van Bunnik, B.A. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J. 2016, 14, 4380. [Google Scholar]
- Hufnagel, D.A.; Tükel, Ç.; Chapman, M.R. Disease to dirt: The biology of microbial amyloids. PLoS Pathog. 2013, 9, e1003740. [Google Scholar] [CrossRef] [Green Version]
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M. Understanding Antimicrobial Resistance (AMR) Profiles of Salmonella Biofilm and Planktonic Bacteria Challenged with Disinfectants Commonly Used During Poultry Processing. Foods 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Hu, X.; Guo, D.; Shi, C.; Zhang, C.; Peng, X.; Yang, H.; Xia, X. Disinfectant resistance profiles and biofilm formation capacity of Escherichia coli isolated from retail chicken. Microb. Drug Resist. 2019, 25, 703–711. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv. Colloid Interface Sci. 2020, 102318. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent advances in the structural design of photosensitive agent formulations using “soft” colloidal nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B. 2016, 137, 191–202. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Franklyne, J.; Mukherjee, A.; Chandrasekaran, N. Essential oil micro-and nanoemulsions: Promising roles in antimicrobial therapy targeting human pathogens. Lett. Appl. Microbiol. 2016, 63, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S. Satureja montana l. Essential oils: Chemical profiles/phytochemical screening, antimicrobial activity and o/w nanoemulsion formulations. Pharmaceutics 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Witkowska, D.; Sowińska, J. The effectiveness of peppermint and thyme essential oil mist in reducing bacterial contamination in broiler houses. Poult. Sci. 2013, 92, 2834–2843. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef]
- Pope, M.; Cherry, T. An evaluation of the presence of pathogens on broilers raised on poultry litter treatment-treated litter. Poult. Sci. 2000, 79, 1351–1355. [Google Scholar] [CrossRef]
- Suhre, K.; Schmitt-Kopplin, P. MassTRIX: Mass translator into pathways. Nucleic Acids Res. 2008, 36, W481–W484. [Google Scholar] [CrossRef] [Green Version]
- Marazzato, M.; Aleandri, M.; Massaro, M.R.; Vitanza, L.; Conte, A.L.; Conte, M.P.; Nicoletti, M.; Comanducci, A.; Goldoni, P.; Maurizi, L. Escherichia coli strains of chicken and human origin: Characterization of antibiotic and heavy-metal resistance profiles, phylogenetic grouping, and presence of virulence genetic markers. Res. Vet. Sci. 2020, 132, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Svabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.P.; Aleandri, M.; Marazzato, M.; Conte, A.L.; Ambrosi, C.; Nicoletti, M.; Zagaglia, C.; Gambara, G.; Palombi, F.; De Cesaris, P. The adherent/invasive Escherichia coli strain LF82 invades and persists in human prostate cell line RWPE-1, activating a strong inflammatory response. Infect. Immun. 2016, 84, 3105–3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagha, R.; Ben Abdallah, F.; Al-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Maraie, N.K. Overview on Nanoemulsion as a recently developed approach in Drug Nanoformulation. Res. J. Pharm. Technol. 2019, 12, 5554–5560. [Google Scholar] [CrossRef]
- Ishii, S.; Sadowsky, M.J. Escherichia coli in the environment: Implications for water quality and human health. Microbes Environ. 2008, 23, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Idalia, V.-M.N.; Bernardo, F. Escherichia coli as a model organism and its application in biotechnology. Recent Adv. Physiol. Pathog. Biotechnol. Appl. Tech Open Rij. Croat. 2017, 253–274. [Google Scholar]
- Skyberg, J.; Siek, K.; Doetkott, C.; Nolan, L. Biofilm formation by avian Escherichia coli in relation to media, source and phylogeny. J. Appl. Microbiol. 2007, 102, 548–554. [Google Scholar] [CrossRef]
- Pasquali, F.; Lucchi, A.; Braggio, S.; Giovanardi, D.; Franchini, A.; Stonfer, M.; Manfreda, G. Genetic diversity of Escherichia coli isolates of animal and environmental origins from an integrated poultry production chain. Vet. Microbiol. 2015, 178, 230–237. [Google Scholar] [CrossRef]
- Bergholz, P.W.; Noar, J.D.; Buckley, D.H. Environmental patterns are imposed on the population structure of Escherichia coli after fecal deposition. Appl. Environ. Microbiol. 2011, 77, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, F.M.; Dantas, M.C.; E Silva, L.S.; Aona, L.Y.; Tavares, I.F.; De Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crop. Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Pei, R.s.; Zhou, F.; Ji, B.p.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A.C. Essential oils and their components as modulators of antibiotic activity against gram-negative bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.; Mohamed, M.; Khalil, M.; Azmy, M.; Mabrouk, M. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. Toxicity of essential oil of Satureja khuzistanica: In vitro cytotoxicity and anti-microbial activity. J. Immunotoxicol. 2014, 11, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Hoseini-Alfatemi, S.M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, M. Composition, cytotoxic and antimicrobial activities of Satureja intermedia CA Mey essential oil. Int. J. Mol. Sci. 2015, 16, 17812–17825. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periasamy, V.S.; Adisa, A.R.; Al-Shuniaber, M.A.; Gassem, M.A.; Alshatwi, A.A. Antimicrobial activity of nanoemulsion on drug-resistant bacterial pathogens. Microb. Pathog. 2018, 120, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Syed, H.K.; Peh, K.K. Identification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagram. Acta Pol Pharm 2014, 71, 301–309. [Google Scholar] [PubMed]
- Vu, G.T.T.; Phan, N.T.; Nguyen, H.T.; Nguyen, H.C.; Tran, Y.T.H.; Pham, T.B.; Nguyen, L.T.; Nguyen, H.D. Application of the artificial neural network to optimize the formulation of self-nanoemulsifying drug delivery system containing rosuvastatin. J. Appl. Pharm. Sci. 2020, 10, 001–011. [Google Scholar]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Taherian, A.A.; Babae, M.; Vafaei, A.A.; Jarrahi, M.; Jadidi, M.; Sadeghi, H. Antinociceptive effects of hydroalcoholic extract of Thymus vulgaris. Pak. J. Pharm. Sci. 2009, 83–89. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [Green Version]
- Juliani, H.; Koroch, A.; Simon, J. Chemical diversity of essential oils of Ocimum species and their associated antioxidant and antimicrobial activity. Essent. Oils Aromas 2009, 568–571. [Google Scholar]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd.(Lep., Noctuidae) larvae. Ind. Crop. Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Bassler, B.L. Small talk: Cell-to-cell communication in bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Vega, P.; Xu, Y.; Chen, C.Y.; Irudayaraj, J. Exploring the anti-quorum sensing activity of ad-limonene nanoemulsion for Escherichia coli O157: H7. J. Biomed. Mater. Res. Part A 2018, 106, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.; Măruțescu, L.; Oprea, E.; Bleotu, C.; Kamerzan, C.; Chifiriuc, M.C.; Grădișteanu Pircalabioru, G. In Vitro Evaluation of the Antimicrobial and Immunomodulatory Activity of Culinary Herb Essential Oils as Potential Perioceutics. Antibiotics 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Chaabouni, Y.; Fedhila, K.; Bakhrouf, A.; Mahdouani, K.; Chaieb, K. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb. Pathog. 2016, 99, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Furukawa, S.; Ogihara, H.; Morinaga, Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011, 16, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Yuk, H.-G. Effects of sublethal thymol, carvacrol, and trans-cinnamaldehyde adaptation on virulence properties of Escherichia coli O157: H7. Appl. Environ. Microbiol. 2019, 85, e00271–e00219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nostro, A.; Marino, A.; Blanco, A.R.; Cellini, L.; Di Giulio, M.; Pizzimenti, F.; Roccaro, A.S.; Bisignano, G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009, 58, 791–797. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.; Viljoen, A. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Meincken, M.; Holroyd, D.; Rautenbach, M. Atomic force microscopy study of the effect of antimicrobial peptides on the cell envelope of Escherichia coli. Antimicrob. Agents Chemother. 2005, 49, 4085–4092. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Yu, C.; Park, H. Bacteriocidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003, 37, 61–65. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, J.B.; De Carvalho, R.J.; De Souza, N.T.; De Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; De Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control. 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Correa, M.S.; Schwambach, J.; Mann, M.B.; Frazzon, J.; Frazzon, A.P.G. Antimicrobial and antibiofilm activity of the essential oil from dried leaves of Eucalyptus staigeriana. Arq. Do Inst. Biológico 2019, 86, e0202018. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, L.A.; El-Hindawy, M.M.; Alagawany, M.; Salah, A.S.; El-Sayed, S.A. Effect of low-or high-CP diet with cold-pressed oil supplementation on growth, immunity and antioxidant indices of growing quail. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1380–1387. [Google Scholar] [CrossRef]
- Penalver, P.; Huerta, B.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. Apmis 2005, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]


| Sample | SEO (g) | Tw80 (g) | HEPES (g) | SEO% w/w | Tw80% w/w | HEPES% w/w |
|---|---|---|---|---|---|---|
| 1 | 0.180 | 0.020 | 0.600 | 22.00 | 3.00 | 75.00 |
| 2 | 0.180 | 0.020 | 0.800 | 18.00 | 2.00 | 80.00 |
| 3 | 0.193 | 0.086 | 0.300 | 33.00 | 16.00 | 51.00 |
| 4 | 0.193 | 0.086 | 0.450 | 26.00 | 13.00 | 61.00 |
| 5 | 0.500 | 0.083 | 0.500 | 38.00 | 6.00 | 56.00 |
| 6 | 0.180 | 0.020 | 0.400 | 30.00 | 4.00 | 66.00 |
| 7 | 0.050 | 0.450 | 0.500 | 5.00 | 45.00 | 50.00 |
| 8 | 0.193 | 0.086 | 0.150 | 45.00 | 20.00 | 35.00 |
| 9 | 0.500 | 0.083 | 0.250 | 60.00 | 10.00 | 30.00 |
| 10 | 0,500 | 0.083 | 0.250 | 46.00 | 8.00 | 46.00 |
| 11 | 0.200 | 0.266 | 0.200 | 30.00 | 40.00 | 30.00 |
| 12 | 0.180 | 0.020 | 0.200 | 45.00 | 5.00 | 50.00 |
| 13 | 0.072 | 0.142 | 0.500 | 10.00 | 20.00 | 70.00 |
| 14 | 0.083 | 0.250 | 0.500 | 10.00 | 30.00 | 60.00 |
| 15 | 0.050 | 0.450 | 1.000 | 2.50 | 22.50 | 75.00 |
| 16 | 0.225 | 0.225 | 0.300 | 30.00 | 30.00 | 40.00 |
| 17 | 0.225 | 0.225 | 0.600 | 21.50 | 21.50 | 57.00 |
| 18 | 0.200 | 0.266 | 0.400 | 23.00 | 31.00 | 46.00 |
| 19 | 0.050 | 0.450 | 0.500 | 3.00 | 30.00 | 67.00 |
| 20 | 0.200 | 0.266 | 0.200 | 19.00 | 25.00 | 56.00 |
| 21 | 0.098 | 0.098 | 5.000 | 2.00 | 2.00 | 96.00 |
| Sample 21 | Hydrodynamic Diameter (nm) ± SD | ζ-Potential (mV) ± SD | PDI ± SD |
|---|---|---|---|
| Pre–sonication | 816.3 ± 90.0 | −15.9 ± 0.61 | 0.56 ± 0.38 |
| Post–sonication | 112.2 ± 13.1 | −15.9 ± 0.55 | 0.22 ± 0.12 |
| Number (%) | Strains | Antibiotic Resistance | Phylogenetic Group | |
|---|---|---|---|---|
| STRONG BIOFILM PRODUCERS | N = 9/30 (30) | AV1 | MDR | A |
| AV2 | D | |||
| AV3 | D | |||
| AV12 | SU,TET | B1 | ||
| AV18 | SU | A | ||
| AV25 | FULL SENSITIVE | A | ||
| AV27 | B1 | |||
| AV38 | A | |||
| AV40 | A | |||
| MODERATE BIOFILM PRODUCERS | N = 8/30 (27) | AV6 | GM,KM,SM,TB | A |
| AV8 | GM,SM,SU | A | ||
| AV22 | GM | D | ||
| AV24 | FULL SENSITIVE | A | ||
| AV26 | D | |||
| AV29 | A | |||
| AV30 | A | |||
| AV39 | B1 | |||
| WEAK BIOFILM PRODUCERS | N = 7/30 (23) | AV5 | MDR | A |
| AV7 | A | |||
| AV11 | GM,SU | B1 | ||
| AV23 | GM | D | ||
| AV17 | KM | B1 | ||
| AV21 | SM | B1 | ||
| AV34 | FULLSENSITIVE | B1 | ||
| NO BIOFILM PRODUCERS | N = 6/30 (20) | AV4 | MDR | A |
| AV14 | TET | A | ||
| AV15 | TET | A | ||
| AV33 | FULL SENSITIVE | D | ||
| AV35 | A | |||
| AV37 | A |
| STRAINS | MIC SEO 1 | MBC SEO 1 | MIC Nes 1 | MBC Nes 1 | |
|---|---|---|---|---|---|
| STRONG BIOFILM PRODUCERS | AV1 | 3.12 | 3.12 | 0.78 | 1.56 |
| AV2 | 0.78 | 0.78 | 0.78 | 1.56 | |
| AV3 | 1.56 | 1.56 | 1.56 | 1.56 | |
| AV12 | 1.56 | 3.12 | 1.56 | 1.56 | |
| AV18 | 1.56 | 1.56 | 1.56 | 1.56 | |
| AV25 | 0.78 | 1.56 | 0.78 | 1.56 | |
| AV27 | 1.56 | 1.56 | 0.78 | 0.78 | |
| AV38 | 0.78 | 1.56 | 0.78 | 0.78 | |
| AV40 | 0.78 | 1.56 | 0.78 | 0.78 | |
| MODERATE BIOFILM PRODUCERS | AV6 | 3.12 | 3.12 | 0.78 | 0.78 |
| AV8 | 1.56 | 0.78 | 0.78 | 1.56 | |
| AV22 | 3.12 | 3.12 | 0.78 | 1.56 | |
| AV24 | 1.56 | 1.56 | 1.56 | 1.56 | |
| AV26 | 0.78 | 1.56 | 0.78 | 0.78 | |
| AV29 | 1.56 | 1.56 | 0.78 | 0.78 | |
| AV30 | 0.78 | 1.56 | 1.56 | 1.56 | |
| AV39 | 3.12 | 3.12 | 0.78 | 1.56 | |
| WEAK BIOFILM PRODUCERS | AV5 | 3.12 | 3.12 | 1.56 | 1.56 |
| AV7 | 1.56 | 1.56 | 0.78 | 1.56 | |
| AV11 | 3.12 | 3.12 | 0.78 | 1.56 | |
| AV23 | 0.78 | 3.12 | 0.78 | 0.78 | |
| AV17 | 3.12 | 3.12 | 1.56 | 1.56 | |
| AV21 | 0.78 | 3.12 | 1.56 | 0.78 | |
| AV34 | 3.12 | 3.12 | 0.78 | 1.56 | |
| NO BIOFILM PRODUCERS | AV4 | 3.12 | 3.12 | 0.78 | 1.56 |
| AV14 | 1.56 | 3.12 | 0.78 | 0.78 | |
| AV15 | 3.12 | 3.12 | 1.56 | 1.56 | |
| AV33 | 3.12 | 3.12 | 0.78 | 1.56 | |
| AV35 | 3.12 | 3.12 | 0.78 | 1.56 | |
| AV37 | 3.12 | 3.12 | 0.78 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, F.; Maurizi, L.; Conte, A.L.; Marazzato, M.; Maccelli, A.; Crestoni, M.E.; Hanieh, P.N.; Forte, J.; Conte, M.P.; Zagaglia, C.; et al. Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains. Pharmaceutics 2021, 13, 134. https://doi.org/10.3390/pharmaceutics13020134
Rinaldi F, Maurizi L, Conte AL, Marazzato M, Maccelli A, Crestoni ME, Hanieh PN, Forte J, Conte MP, Zagaglia C, et al. Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains. Pharmaceutics. 2021; 13(2):134. https://doi.org/10.3390/pharmaceutics13020134
Chicago/Turabian StyleRinaldi, Federica, Linda Maurizi, Antonietta Lucia Conte, Massimiliano Marazzato, Alessandro Maccelli, Maria Elisa Crestoni, Patrizia Nadia Hanieh, Jacopo Forte, Maria Pia Conte, Carlo Zagaglia, and et al. 2021. "Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains" Pharmaceutics 13, no. 2: 134. https://doi.org/10.3390/pharmaceutics13020134
APA StyleRinaldi, F., Maurizi, L., Conte, A. L., Marazzato, M., Maccelli, A., Crestoni, M. E., Hanieh, P. N., Forte, J., Conte, M. P., Zagaglia, C., Longhi, C., Marianecci, C., Ammendolia, M. G., & Carafa, M. (2021). Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains. Pharmaceutics, 13(2), 134. https://doi.org/10.3390/pharmaceutics13020134