Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bead Preparation
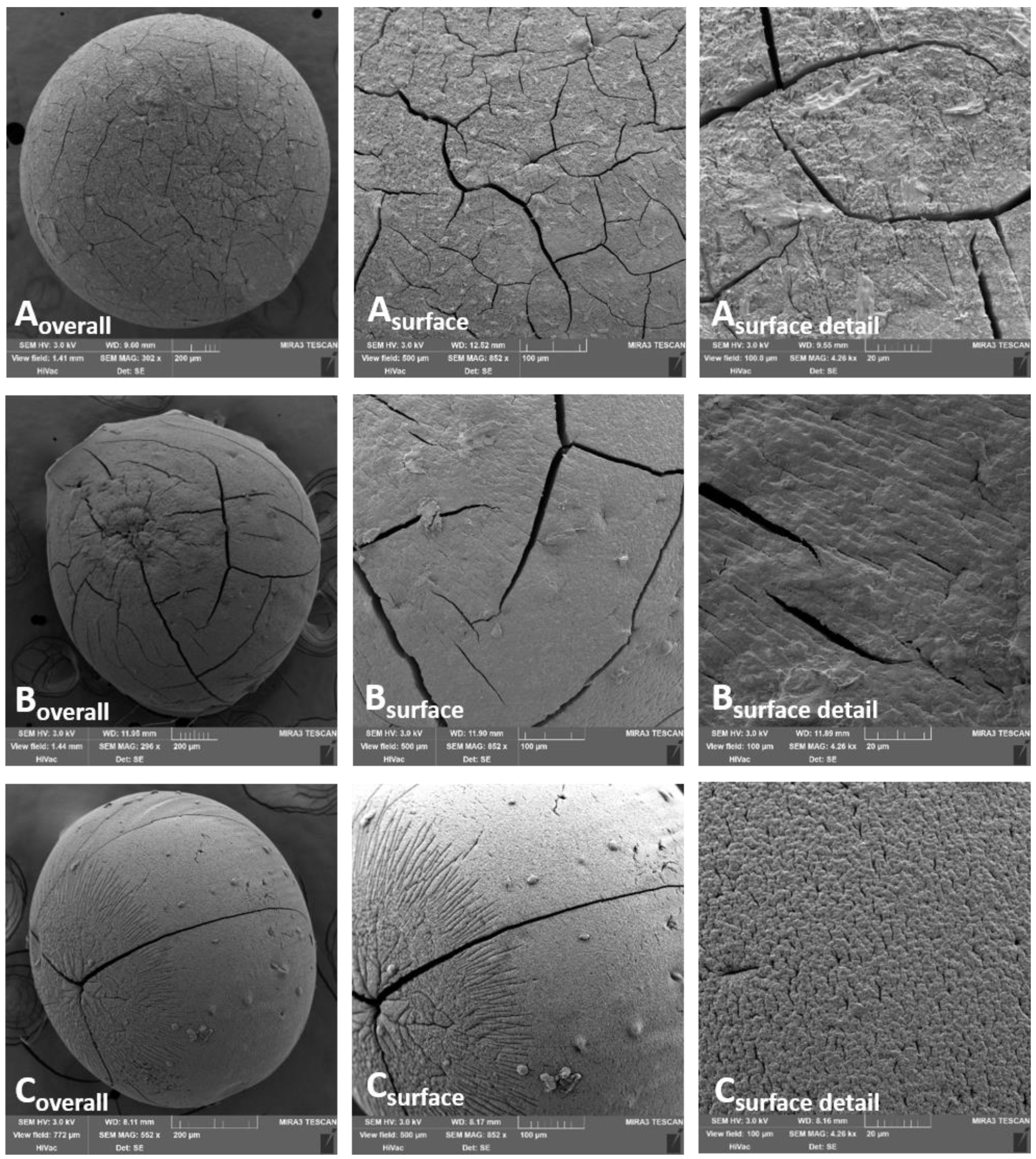
2.3. Evaluation of Morphological Parameters
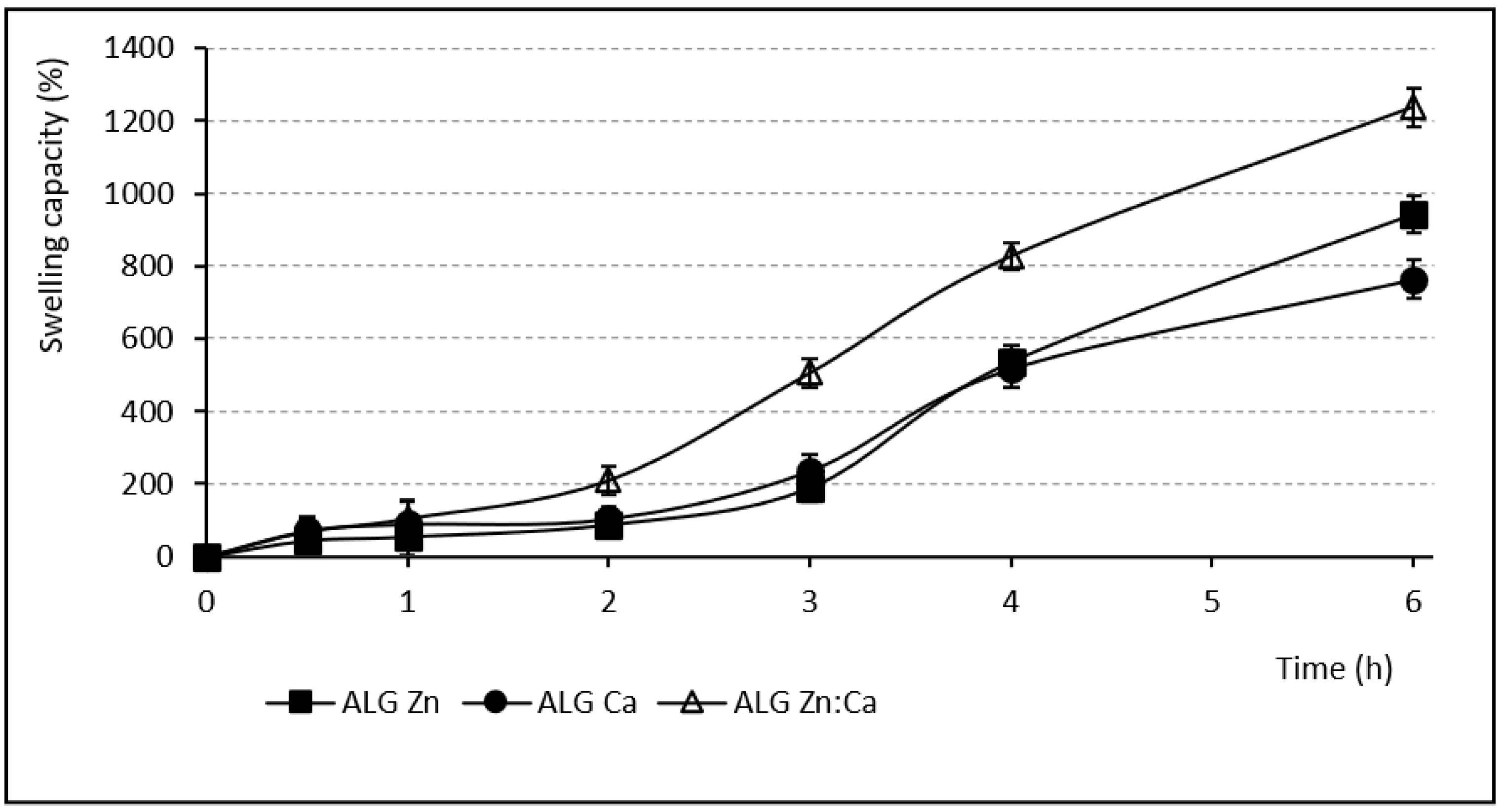
2.4. Swelling Capacity
2.5. Ion Content
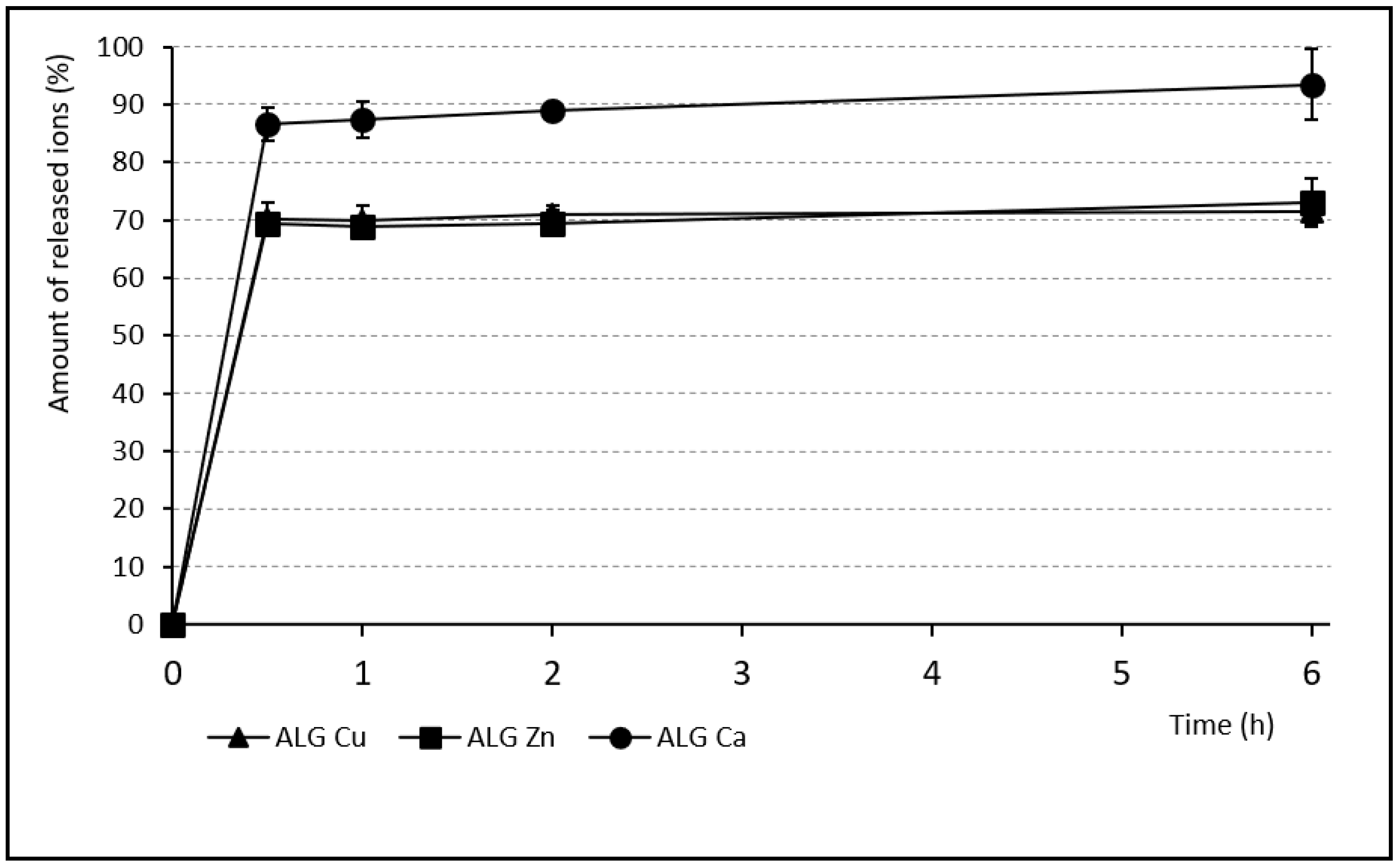
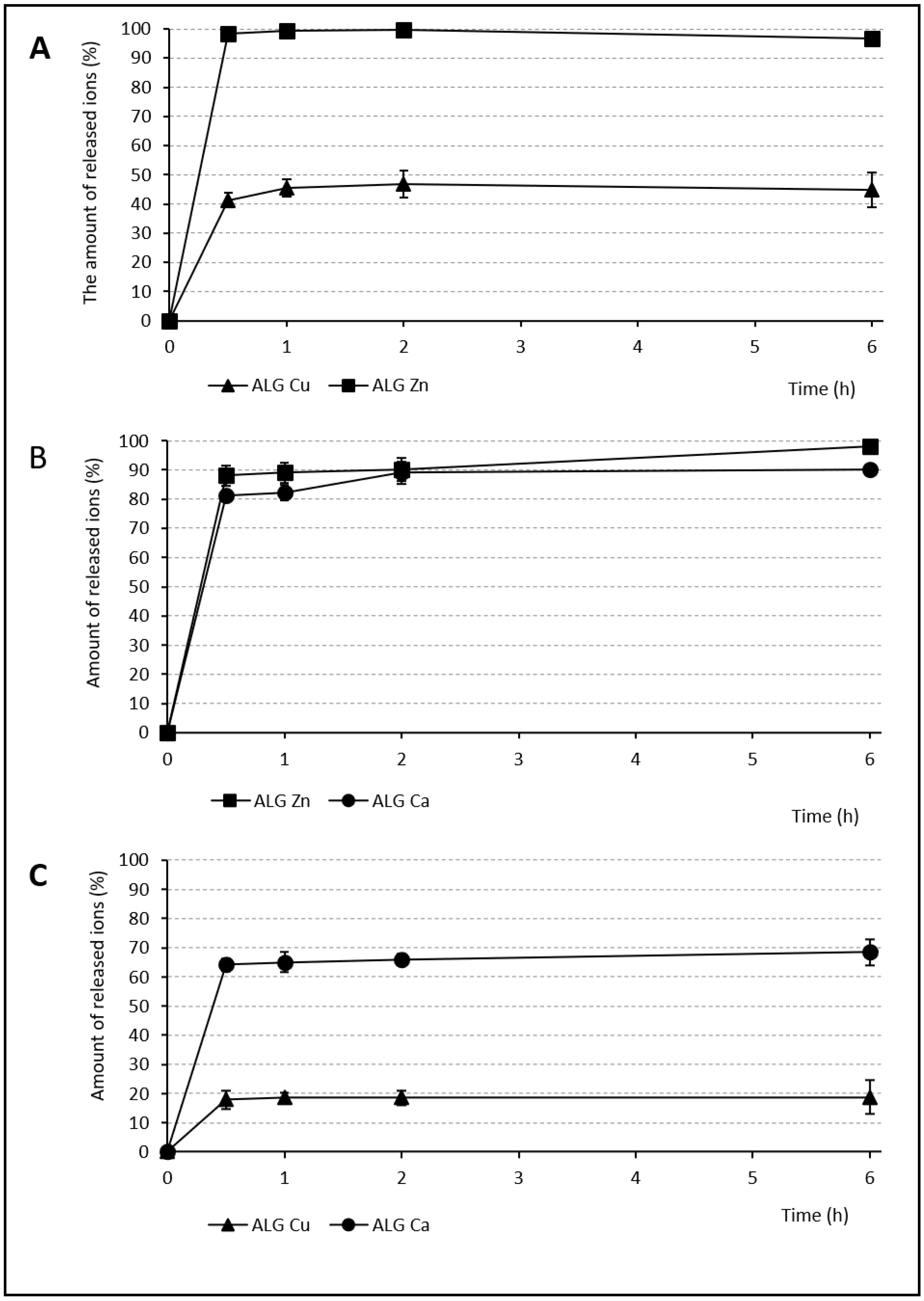
2.6. Release Profiles of Individual Ions from Beads
2.7. Biological Effects
3. Results and Discussion
3.1. The Evaluation of Morphological Parameters
3.2. Ion Content
3.3. Swelling Capacity
3.4. Release of Individual Ions
3.5. Cytotoxicity Evaluation
3.6. Antimicrobial Activity
3.7. Antiviral Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mühlen, S.; Dersch, P. Anti-virulence Strategies to Target Bacterial Infections. In How to Overcome the Antibiotic Crisis; Stadler, M., Dersch, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 147–183. [Google Scholar] [CrossRef]
- Frieri, M.; Krishan, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginjupalli, K.; Alla, R.; Shaw, T.; Tellapragada, C.; Gupta, L.K.; Upadhya, P.N. Comparative evaluation of efficacy of Zinc oxide and Copper oxide nanoparticles as antimicrobial additives in alginate impression materials. Mater. Today Proc. 2018, 5, 16258–16266. [Google Scholar] [CrossRef]
- Frígols, B.; Martí, M.; Salesa, B.; Hernandéz-Oliver, C.; Aartsad, O.; Ulset, A.-S.T.; Saetrom, G.; Aachmann, F.; Serrano-Aroca, A. Graphene oxide in zinc alginate films: Antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mary, G.; Bajpai, S.K.; Chand, N. Copper (II) ions and copper nanoparticles-loaded chemically modified cotton cellulose fibers with fair antibacterial properties. J. Appl. Polym. Sci. 2009, 113, 757–766. [Google Scholar] [CrossRef]
- Pavelková, M.; Kubová, K.; Vysloužil, J.; Kejdušová, M.; Vetchý, D.; Celer, V.; Molinková, D.; Lobová, D.; Pechová, A.; Vysloužil, J.; et al. Biological effects of drug-free alginate beads cross-linked by copper ions prepared using external ionotropic gelation. AAPS PharmSciTech 2017, 18, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Zarubica, A.; Stojković, N.; Vasić, M.; Cakić, M.; Nikolić, G. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Adv. Technol. 2016, 5, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Alginate-Based Hydrogel Systems for Drug Releasing in Wound Healing. In Alginates in Drug Delivery, 1st ed.; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 323–358. [Google Scholar]
- Schmid, W.; Picker-Fryer, K.M. Tableting and tablet properties of alginates: Characterisation and potential for soft tableting. Eur. J. Pharm. Biopharm. 2009, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sathyabama, S.; Ranjith Kumar, M.; Brunthadevi, P.; Vijayabharathi, R.; Brindha, V. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- De Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliver. Rev. 2014, 67, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Sahiwala, A. Multiparticulate formulation approach to pulsatile drug delivery: Current perspective. J. Control. Rel. 2009, 134, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Dulieu, C.; Poncelet, D.; Neufeld, R.J. Encapsulation and Immobilization Techniques. In Cell Encapsulation Technology and Therapeutics; Kühtreiber, W.M., Lanza, R.P., Chick, W.L., Eds.; Birkhäuser: Boston, MA, USA, 1999; pp. 9–11. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. The effect of divalent metals on the properties of alginate solutions II. comparison of different metal ions. Acta Chem. Scand. 1965, 19, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Qi, M.; Gundersen, P.O.M.; Formo, K.; Lacik, I.; Skjåk-Bræk, G.; Oberholzer, J.; Strand, B.L. Binding and leakage of barium in alginate microbeads. J. Biomed. Mater. Res. Part A 2012, 100, 2939–2947. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Mazukhina, S.; Tereshchenko, T.; Drogobuzhskaya, S.; Pozhilenko, V. The Speciation of Chemical Elements in Water and Their Possible Impact on Human Health. In Proceedings of the 16th International Symposium on Water-Rock Interaction (WRI-16) and 13th International Symposium on Applied Isotope Geochemistry (1st IAGC International Conference), Tomsk, Russia, 21–26 July 2019. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Cerciello, A.; Del Gaudio, P.; Granata, V.; Sala, M.; Aquino, R.P.; Russo, P. Synergistic effect of divalent cations in improving technological properties of cross-linked alginate beads. Int. J. Biol. Macromol. 2017, 101, 100–106. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [Green Version]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloid. Surf. A Physicochem. Eng. Aspects 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Atmaca, S.; Gül, K.; Ҫíҫek, R. The effect of zinc on microbial growth. Turk. J. Med. Sci. 1998, 28, 595–597. [Google Scholar]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Laurienzo, P. Novel zinc alginate hydrogels prepared by internal setting method with intrinsic antibacterial activity. Carbohydr. Polym. 2015, 125, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, N.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Han, G.; Zhang, Y.; Pan, Y.; Li, X.; Xia, Y.; Wu, Y. Antifungal activity and cytotoxicity of zinc, calcium, or copper alginate fibers. Biol. Trace Elem. Res. 2012, 148, 415–419. [Google Scholar] [CrossRef]
- Miller, L.P.; McCallan, S.E.A. Fungicides, toxic action of metal ions to fungus spores. J. Agric. Food Chem. 1957, 5, 116–122. [Google Scholar] [CrossRef]
- Wei, Z.; Burwinkel, M.; Pallissa, C.; Ephraim, E.; Schmidt, M.F. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet. Microbiol. 2012, 160, 468–472. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horsky, J.; Vetchy, D.; et al. Structure and dynamics of alginate gels cross-linked by polyvalent ions probed via solid state NMR spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [Green Version]
- Smýkalová, I.; Horáček, J.; Hýbl, M.; Bjelková, M.; Pavelek, M.; Krulikovská, T.; Hampel, D. Seed type identification by image analysis—Correlation of nutrients with size, shape and colour characteristics of seeds. Chem. Listy. 2011, 105, 138–145. [Google Scholar]
- Rabišková, M.; Häring, A.; Minczingerová, K.; Havlásek, M.; Musilová, P. Microcrystalline cellulose in oral dosage forms. Chem. Listy 2007, 101, 70–77. [Google Scholar]
- Younis, M.K.; Tareq, A.Z.; Kamal, I.M. Optimization of Swelling, Drug Loading and Release from Natural Polymer Hydrogels. In Proceedings of the IOP Conference Series: Materials Science and Engineering, International Conference on Materials Engineering and Science 8, Istanbul, Turkey, 8–9 August 2018; IOP Science: Bristol, UK, 2018. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commision. Buffer Solutions. In European Pharmacopoeia, 10th ed.; Supplement 8.0; Council of Europe: Strasbourg, France, 2020; Chapter 4.1.3. [Google Scholar]
- Caillouette, J.C.; Sharp, C.F., Jr.; Zimmerman, G.J.; Roy, S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am. J. Obstet. Gynecol. 1997, 176, 1270–1277. [Google Scholar] [CrossRef]
- Latronico, T.; Pati, I.; Ciavarella, R.; Fasano, A.; Mengoni, F.; Lichtner, M.; Vullo, V.; Mastroianni, C.M.; Liuzzi, G.M. In vitro effect of antiretroviral drugs on cultured primary astrocytes: Analysis of neurotoxicity and matrix metalloproteinase inhibition. J. Neurochem. 2018, 144, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliver. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural regime identification in ionotropic alginate gels: Influence of the cation nature and alginate structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Aslani, P.; Kennedy, R.A. Effect of gelation conditions and dissollution media on the release of paracetamol from alginate gel beads. J. Microencapsul. 1996, 13, 601–614. [Google Scholar] [CrossRef]
- Yokoyama, F.; Achife, C.E.; Takahira, K.; Yamashita, Y.; Monobe, K.; Kusano, F.; Nishi, K. Morphologies of oriented alginate gels crosslinked with various divalent metal ions. J. Macromol. Sci. Part B 1992, 31, 463–483. [Google Scholar] [CrossRef]
- Deasy, P.B.; Law, M.F.L. Use of extrusion-spheronization to develop an improved oral dosage form of indomethacin. Int. J. Pharm. 1997, 148, 201–209. [Google Scholar] [CrossRef]
- Lyn, M.E.; Ying, D.Y. Drying model for calcium alginate beads. Ind. Eng. Chem. Res. 2010, 49, 1986–1990. [Google Scholar] [CrossRef]
- Rodrigues, J.R.; Lagoa, R. Copper Ions Binding in Cu-Alginate Gelation. J. Carbohydr. Chem. 2006, 25, 219. [Google Scholar] [CrossRef]
- Velings, N.M.; Mestdagh, M.M. Physico-chemical properties of alginate gel beads. Polym. Gels Netw. 1995, 3, 311–330. [Google Scholar] [CrossRef]
- Chowdary, K.P.R.; Rao, Y.S. Mucoadhesive microspheres for controlled drug delivery. Biol. Pharm. Bull. 2004, 27, 1717–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanova, M.; Pavelkova, M.; Czernek, J.; Kubova, K.; Vyslouzil, J.; Pechova, A.; Molinkova, D.; Vyslouzil, J.; Vetchy, D.; Brus, J. Interaction pathways and structure−chemical transformations of alginate gels in physiological environments. Biomacromolecules 2019, 20, 4158–4170. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Eiselt, P.; Rowley, J.A.; Mooney, D.J. PEG Cross-Linked Alginate Hydrogels with Controlled Mechanical Properties. In MRS Proceedings; Cambridge University Press: Cambridge, UK, 1998; pp. 37–42. [Google Scholar]
- Baloglu, E.; Bernkop-Schnürch, A.; Karavana, S.Y.; Senyigit, Z.A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336. [Google Scholar] [CrossRef]
- Esmaeili, L.; Perez, M.G.; Jafari, M.; Paquin, J.; Ispas-Szabo, P.; Pop, V.; Andruh, M.; Byers, J.; Mateescu, M.A. Copper complexes for biomedical applications: Structural in-sights, antioxidant activity and neuron compatibility. J. Inorg. Biochem. 2019, 192, 87–97. [Google Scholar] [CrossRef]
- Wataha, J.C.; Hanks, C.T.; Craig, R.G. In vitro effect of metal ions on cellular metabolism and the correlation between these effects and the uptake of the ions. J. Biomed. Mater. Res. 1994, 28, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Kass, G.E.; Orrenius, S. Calcium signaling and cytotoxicity. Environ. Health Perspect. 1999, 107, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen. Med. 2018, 3, 1–20. [Google Scholar] [CrossRef]
- Xing, J.Z.; Zhu, L.; Jackson, J.A.; Gabos, S.; Sun, X.J.; Wang, X.; Xu, X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; He, J.; Zhu, C. In vitro cytotoxicity of Cu2+, Zn2+, Ag+ and their mixtures on primary human endometrial epithelial cells. Contraception 2012, 85, 509–518. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release. 2005, 103, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.A.; Olarte, K.T.; Michalek, J.L.; Jandu, G.S.; Michel, S.L.J.; Bruno, V.M. Regulation of copper toxicity by Candida albicans GPA2. Eukaryot. Cell 2013, 12, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Zhu, C.; Chen, J.; Liang, D.; Wo, G. Absorption and release of zinc and copper ions by chitosan fibers. J. Appl. Polym. Sci. 2007, 105, 527–532. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Routson, L.B.; Bonifacino, A.C.; Lytle, C.D. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997, 41, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Kümel, G.; Schrader, S.; Zentgraf, H.; Daus, H.; Brendel, M. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 1990, 71, 2989–2997. [Google Scholar] [CrossRef]
- Arens, M.; Travis, S. Zinc salts inactivate clinical isolates of Herpes simplex virus In vitro. J. Clin. Microbiol. 2000, 38, 1758–1762. [Google Scholar] [CrossRef] [Green Version]
- Panteva, M.; Varadinova, T.; Turel, I. Effect of copper acyclovir complexes on Herpes simplex virus type 1 and type 2 (HHV-1, HSV-2) infection in cultured cells. Met. Based Drugs 1998, 5, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Kydd, J.H.; Townsend, H.G.G.; Hannant, D. The equine immune response to equine herpesvirus-1: The virus and its vaccines. Vet. Immunol. Immunopathol. 2006, 111, 15–30. [Google Scholar] [CrossRef]
- Lodmell, D.L.; Niwa, A.; Hayashi, K.; Notkins, A.L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J. Exp. Med. 1973, 137, 706–720. [Google Scholar] [CrossRef] [PubMed]


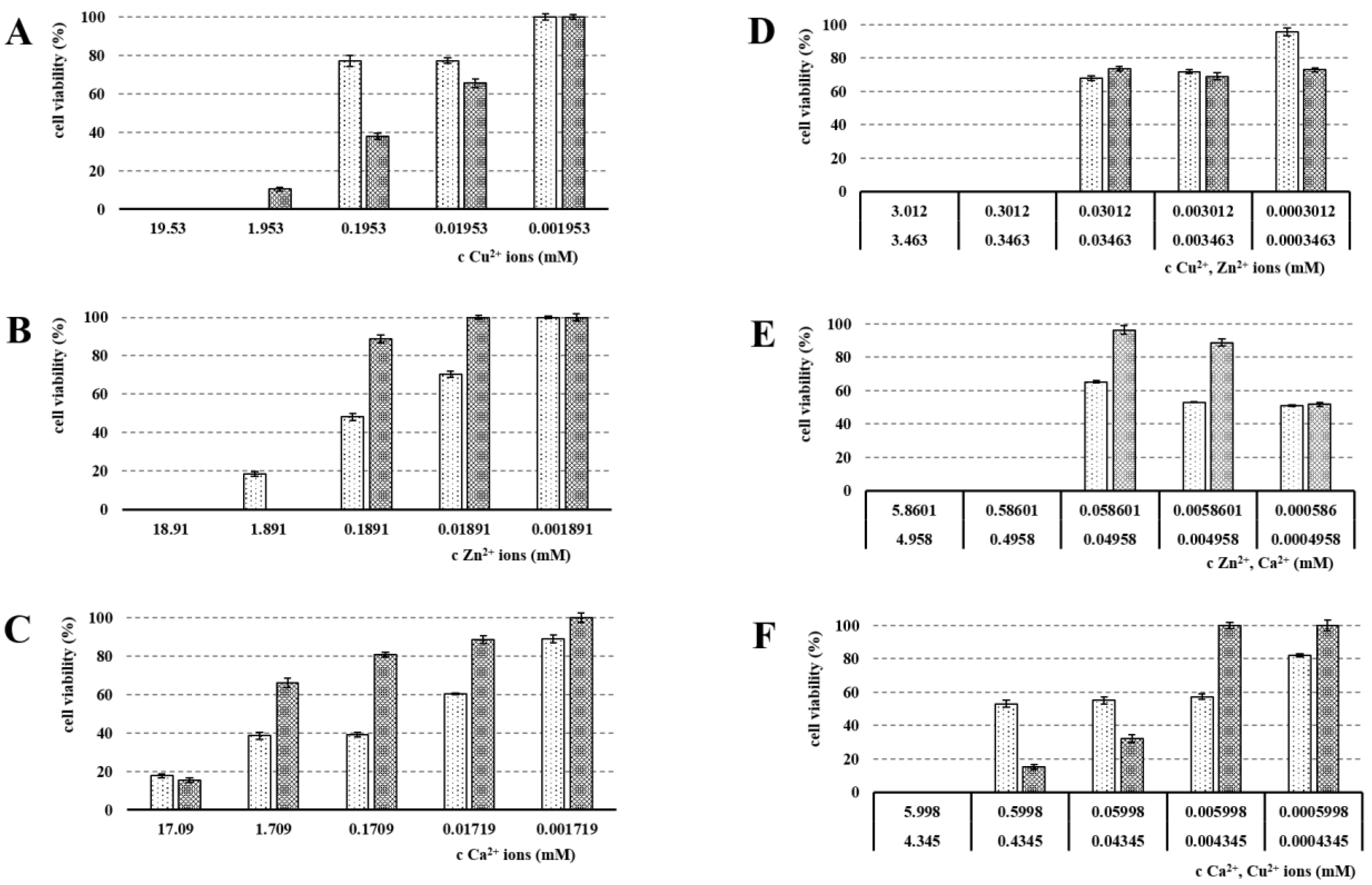

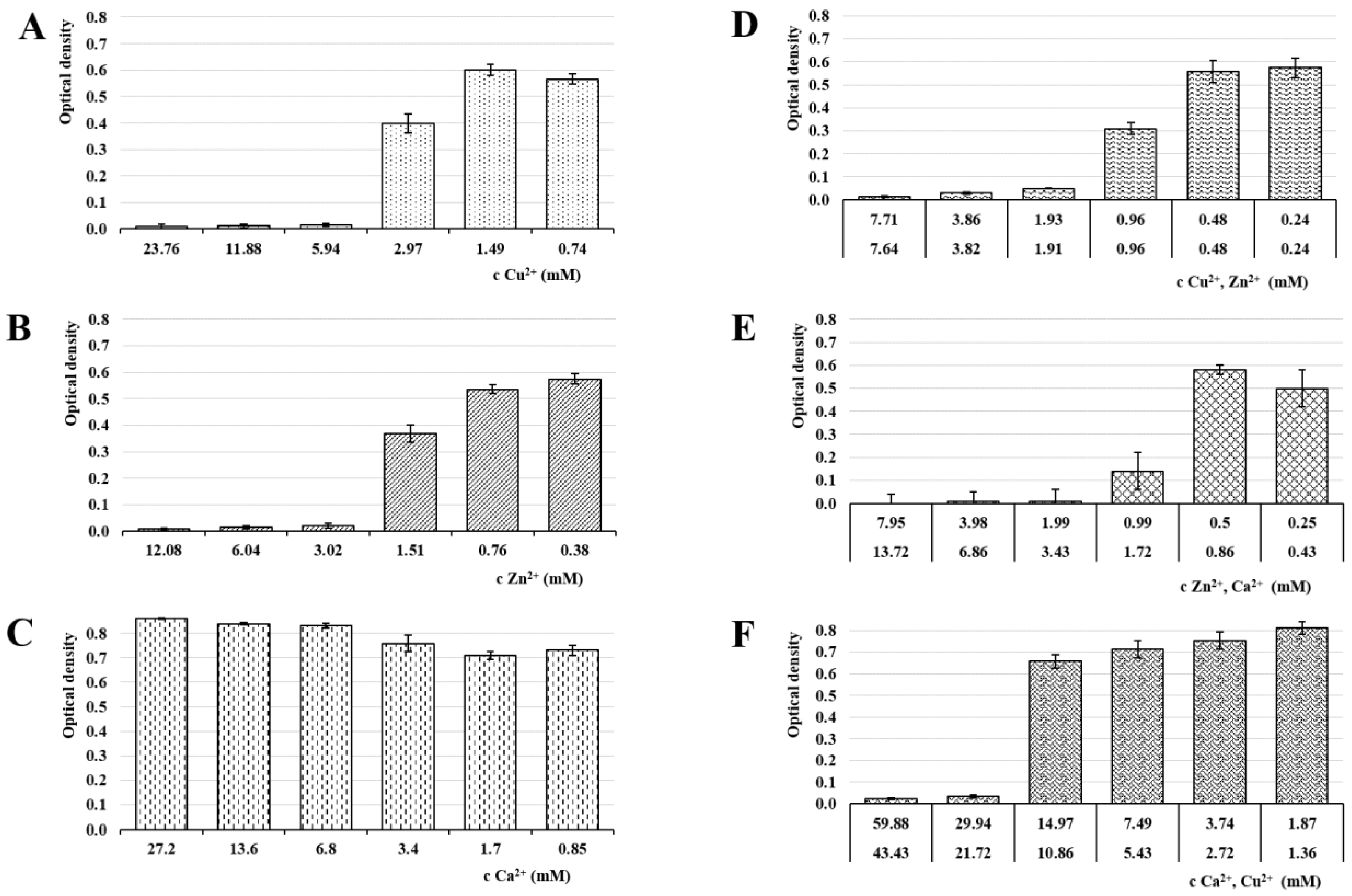
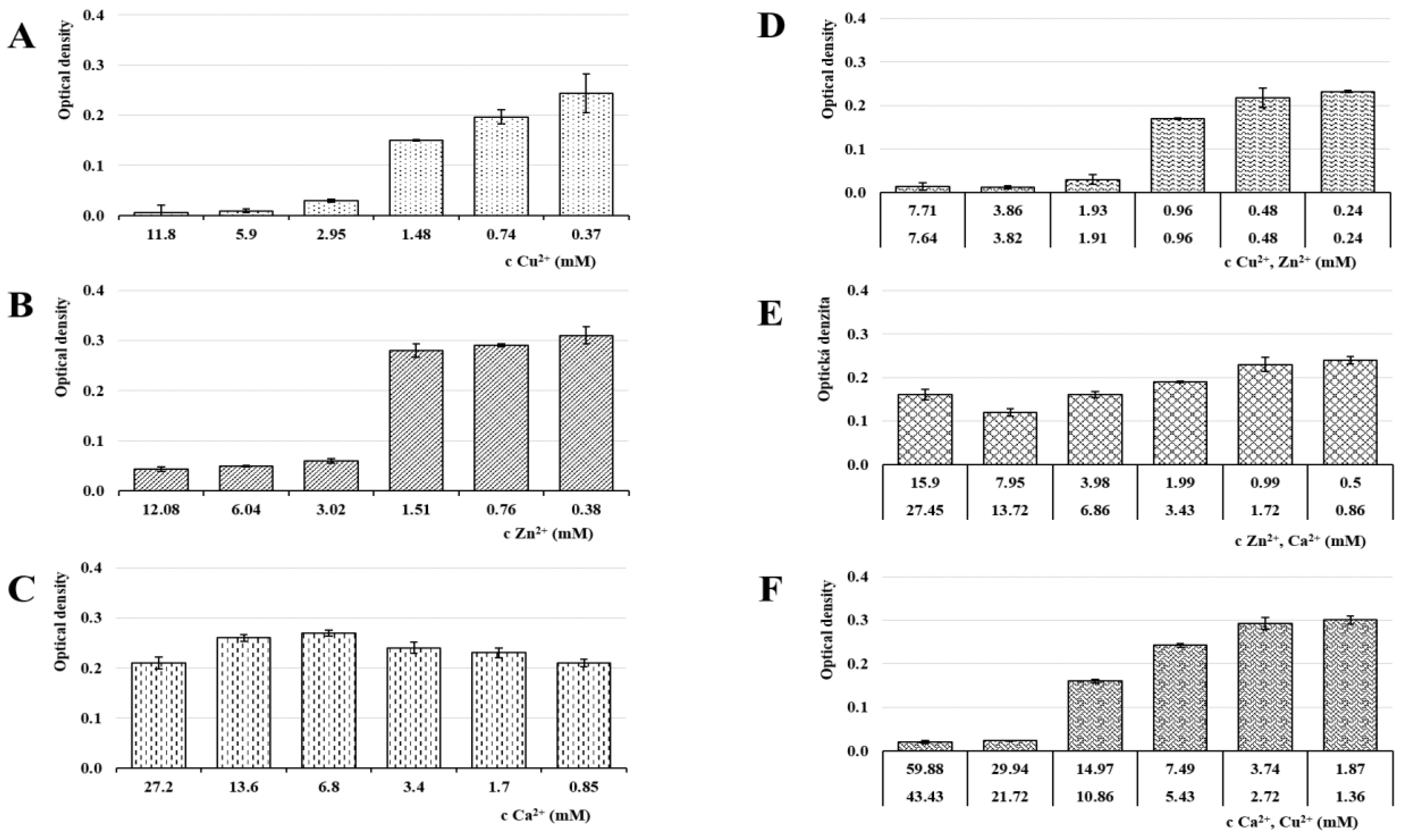

| Sample | NaALG (%) | CuCl2 (mol/dm3) | ZnCl2 (mol/dm3) | CaCl2 (mol/dm3) |
|---|---|---|---|---|
| ALG_Cu | 6 | 1 | - | - |
| ALG_Zn | 6 | - | 1 | - |
| ALG_Ca | 6 | - | - | 1 |
| ALG_Cu:Zn | 6 | 0.5 | 0.5 | - |
| ALG_Zn:Ca | 6 | - | 0.5 | 0.5 |
| ALG_Ca:Cu | 6 | 0.5 | - | 0.5 |
| Sample | Weigh of Dry Samples (g) | Equivalent Diameter (µm) | Sphericity |
|---|---|---|---|
| ALG_Cu | 13.99 | 1455.68 ± 18.71 | 0.97 ± 0.06 |
| ALG_Zn | 14.22 | 1688.44 ± 12.45 | 0.90 ± 0.08 |
| ALG_Ca | 12.95 | 1501.50 ± 15.61 | 0.94 ± 0.07 |
| ALG_Cu:Zn | 17.24 | 1691.77 ± 15.92 | 0.91 ± 0.07 |
| ALG_Zn:Ca | 15.29 | 1756.31 ± 16.58 | 0.86 ± 0.04 |
| ALG_Ca:Cu | 15.11 | 1593.31 ± 11.39 | 0.95 ± 0.07 |
| Sample | Cu2+ (g/kg) | Zn2+ (g/kg) | Ca2+ (g/kg) |
|---|---|---|---|
| ALG_Cu | 205.3 ± 25.1 | - | - |
| ALG_Zn | - | 223.8 ± 21.3 | - |
| ALG_Ca | - | - | 117.4 ± 15.9 |
| ALG_Cu:Zn | 158.7 ± 11.3 | 87.4 ± 4.4 | - |
| ALG_Zn:Ca | - | 123.4 ± 13.7 | 71.8 ± 5.1 |
| ALG_Ca:Cu | 118.9 ± 4.1 | - | 36.5 ± 2.7 |
| Samples | Ion Concentration (mM) | Average Amounts of CPEs | ||
|---|---|---|---|---|
| Preventive Efficiency | Therapeutic Efficiency | |||
| ALG_Cu | Cu2+ | 0.060 | 0.8 | 3.1 |
| ALG_Zn | Zn2+ | 0.600 | 1.8 | 5.2 |
| ALG_Ca | Ca2+ | 3.410 | 8.0 | 9.2 |
| ALG_Cu:Zn | Cu2+ | 0.035 | 2.0 | 7.6 |
| Zn2+ | 0.034 | |||
| ALG_Zn:Ca | Zn2+ | 0.040 | 3.0 | 4.8 |
| Ca2+ | 0.037 | |||
| ALG_Ca:Cu | Ca2+ | 0.065 | 1.0 | 2.8 |
| Cu2+ | 0.048 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavelková, M.; Vysloužil, J.; Kubová, K.; Pavloková, S.; Molinková, D.; Celer, V.; Pechová, A.; Mašek, J.; Vetchý, D. Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration. Pharmaceutics 2021, 13, 165. https://doi.org/10.3390/pharmaceutics13020165
Pavelková M, Vysloužil J, Kubová K, Pavloková S, Molinková D, Celer V, Pechová A, Mašek J, Vetchý D. Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration. Pharmaceutics. 2021; 13(2):165. https://doi.org/10.3390/pharmaceutics13020165
Chicago/Turabian StylePavelková, Miroslava, Jakub Vysloužil, Kateřina Kubová, Sylvie Pavloková, Dobromila Molinková, Vladimír Celer, Alena Pechová, Josef Mašek, and David Vetchý. 2021. "Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration" Pharmaceutics 13, no. 2: 165. https://doi.org/10.3390/pharmaceutics13020165
APA StylePavelková, M., Vysloužil, J., Kubová, K., Pavloková, S., Molinková, D., Celer, V., Pechová, A., Mašek, J., & Vetchý, D. (2021). Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration. Pharmaceutics, 13(2), 165. https://doi.org/10.3390/pharmaceutics13020165








