MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
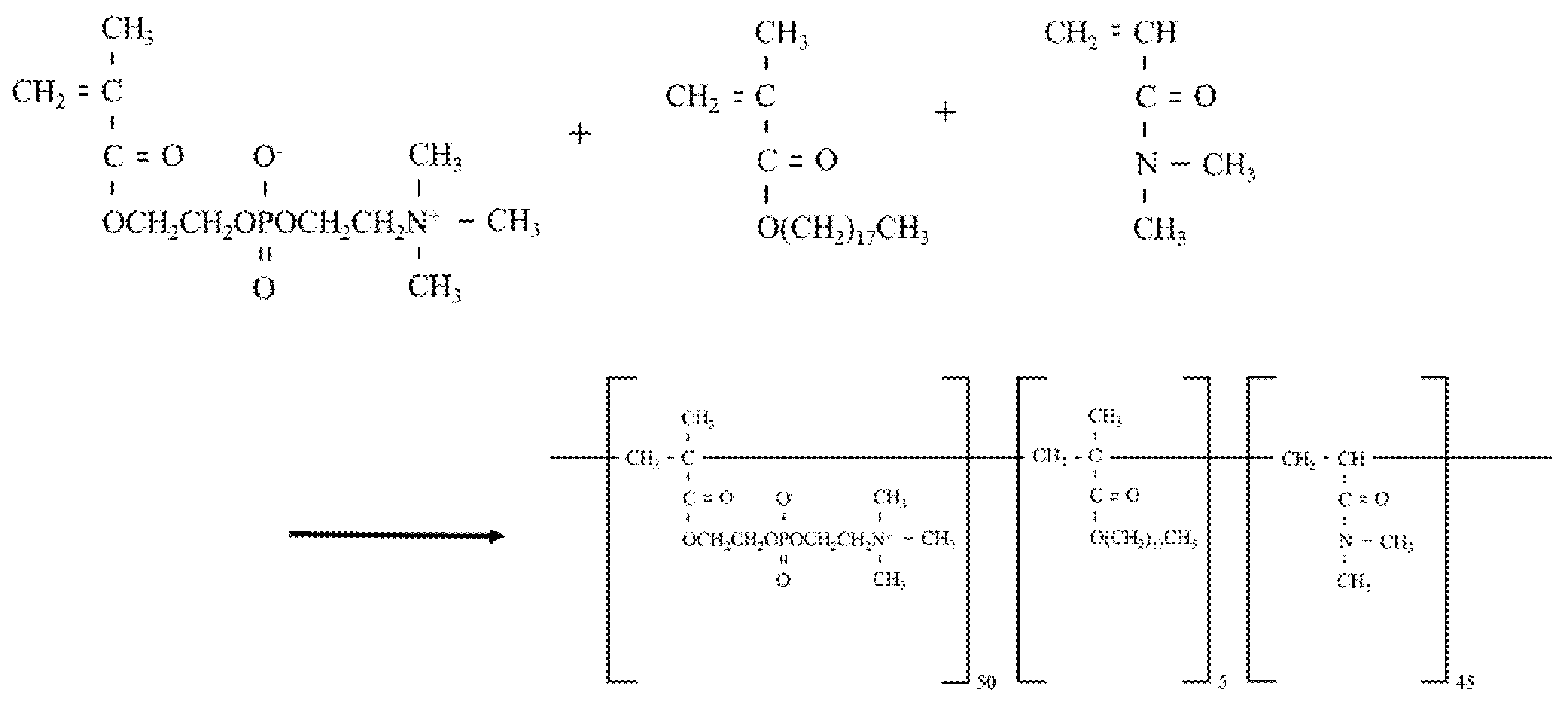
2.3. Preparation of MPCP
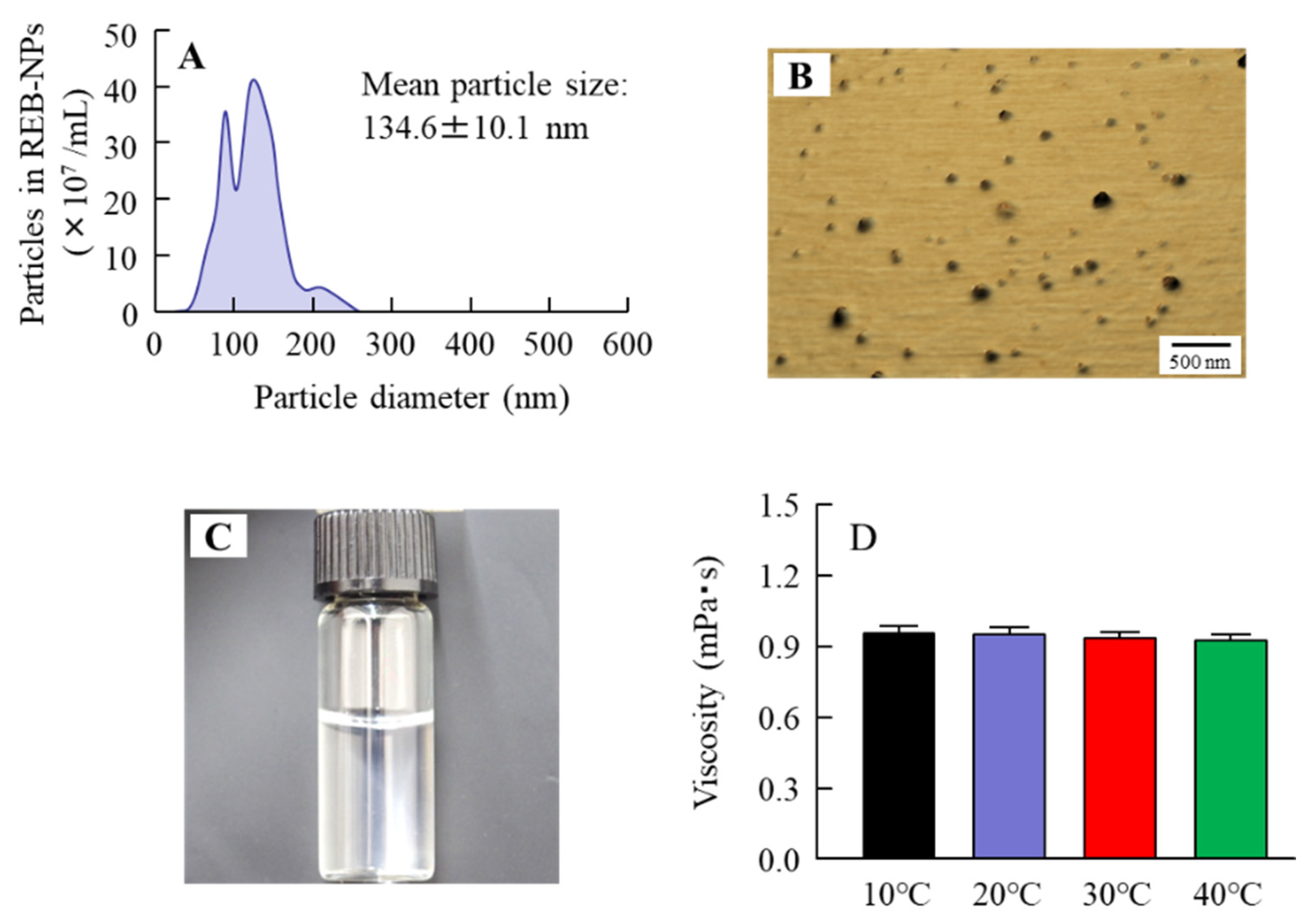
2.4. Measurement of Characteristics in MPCP
2.5. Cell Culture and Treatment
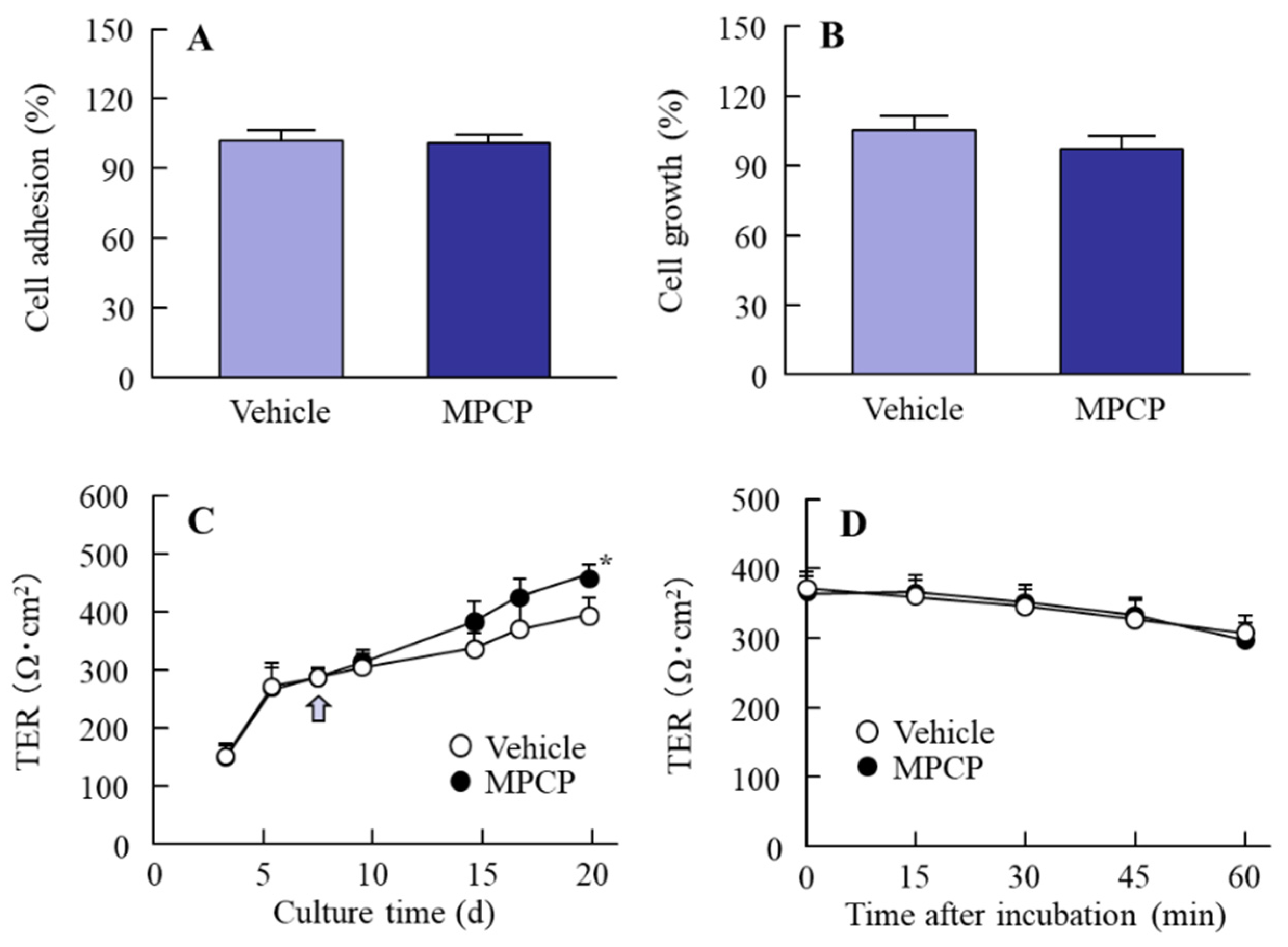
2.6. Measurement of Cell Adhesion
2.7. Measurement of Cell Proliferation
2.8. Preparation of HCE-T Cell Layer Model
2.9. Cell Toxicity of MPCP
2.10. Measurement of Water Retention in the Cornea
2.11. Monitoring the Ocular Surface of Rabbits Instilled with MPCP
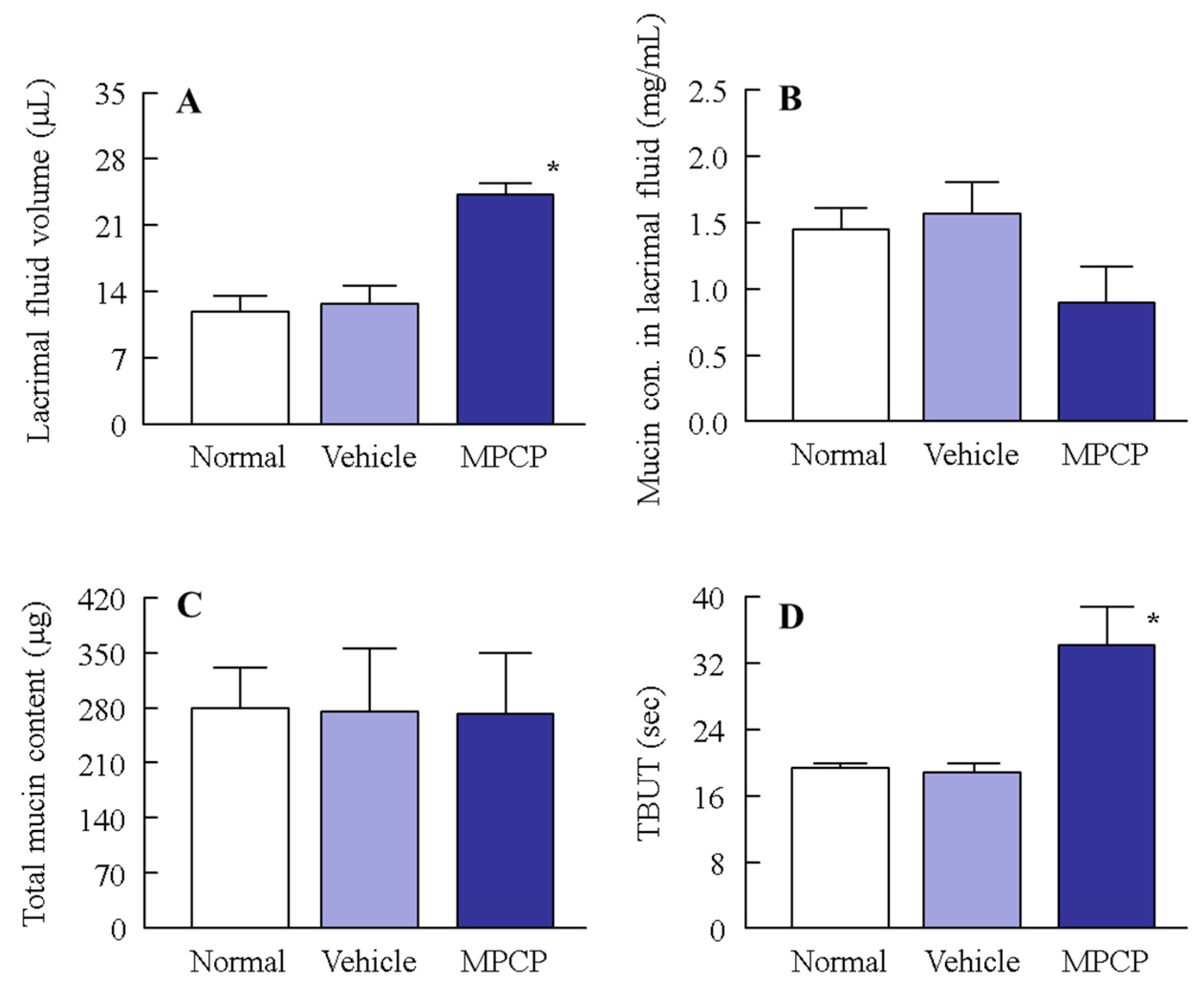
2.12. Lacrimal Fluid and Mucin Levels in Rabbits Instilled with MPCP
2.13. Statistical Analysis
3. Results
3.1. Design of the MPCP
3.2. Changes in Cell Conditions in the Immortalized Human Corneal Epithelial Cell Line (HCE-T Cell) Treated with MPCP
3.3. Effect of MPCP on the Ocular Surface Stability in the Normal Model
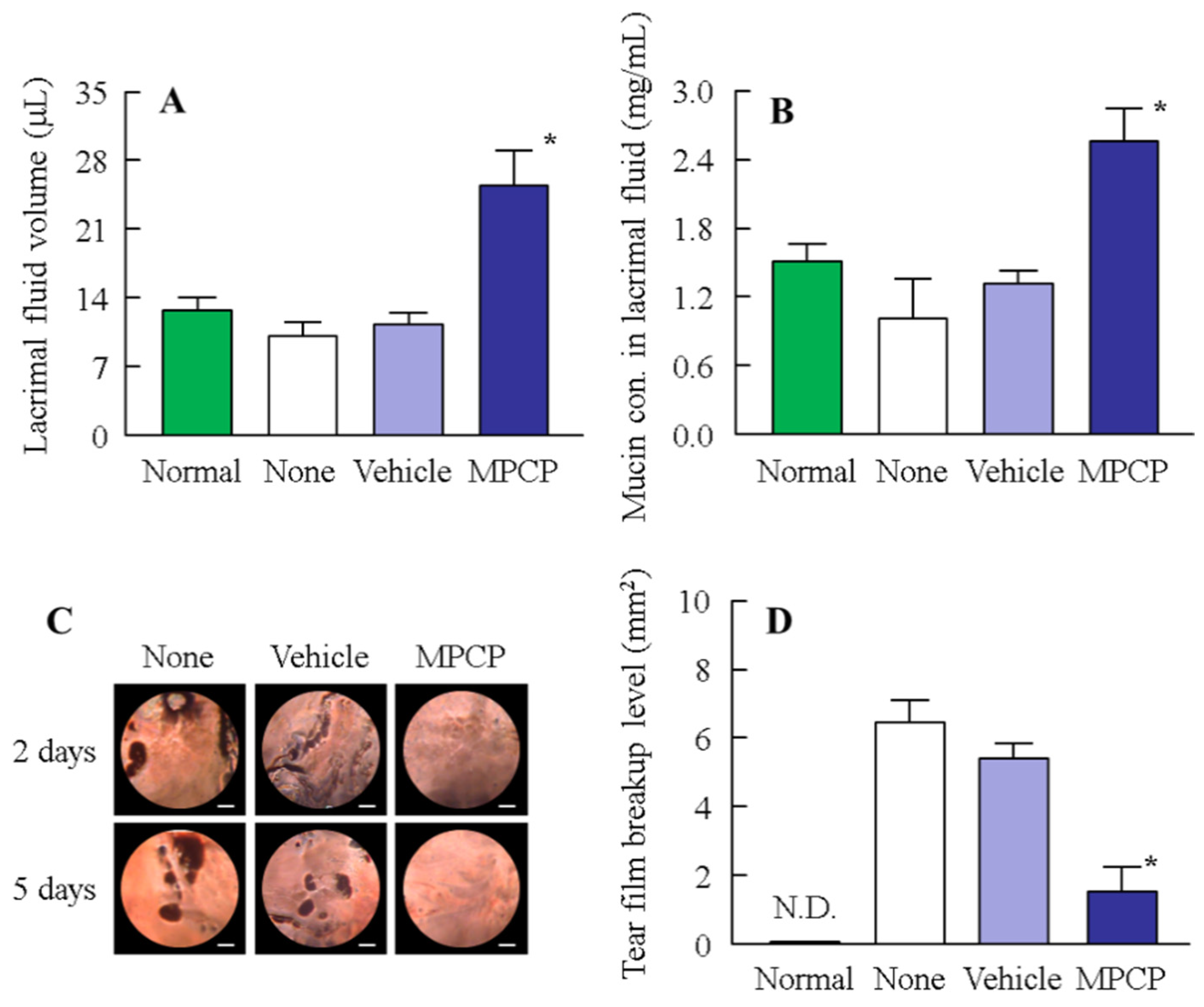
3.4. Therapeutic Potential of the MPCP for Dry Eye Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.L.; Roach, J.M. Treatment of dry eye disease. Consult. Pharm. 2016, 31, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye Work Shop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Ogenesis of dry eye. Inflamm. Regen. 2007, 27, 559–564.
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Garrett, Q.; Chan, C.; Craig, J.P. The epidemiology of dry eye diseas. In Dry Eye: A Practical Approach, Essentials in Ophthalmology; Chan, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Nebbioso, M.; Fameli, V.; Gharbiya, M.; Sacchetti, M.; Zicari, A.M.; Lambiase, A. Investigational drugs in dry eye disease. Expert Opin. Investig. Drugs 2016, 25, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Takai, M. Bioinspired interface for nanobiodevices based on phospholipid polymer chemistry. J. R. Soc. Interface 2009, 6, S279–S291. [Google Scholar] [CrossRef]
- Ishihara, K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci. Technol. Adv. Mater. 2000, 1, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K. New polymeric biomaterials–phospholipid polymers with a biocompatible surface. Front. Med. Biol. Eng. 2000, 10, 83–95. [Google Scholar] [CrossRef]
- Hall, B.; Janes, S.; Young, G.; Coleman, S. The on-eye dehydration of proclear compatibles lenses. CLAO J. 1999, 25, 233–237. [Google Scholar]
- Ayaki, M.; Iwasawa, A.; Niwano, Y. Cytotoxicity assays of new artificial tears containing 2-methacryloyloxyehtyl phospholylcholine polymer for ocular surface cells. Jpn. J. Ophthalmol. 2011, 55, 541–546. [Google Scholar] [CrossRef]
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel sustained-release drug delivery system for dry eye therapy by rebamipide nanoparticles. Pharmaceutics 2020, 12, E155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Isaka, T.; Deguchi, S.; Minami, M.; Yamaguchi, M.; Otake, H.; Okamoto, N.; Nakazawa, Y. In situ gelling systems using pluronic F127 enhance corneal permeability of indomethacin nanocrystals. Int. J. Mol. Sci. 2020, 21, 7083. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Nagai, N.; Fukuoka, Y.; Ishii, M.; Otake, H.; Yamamoto, T.; Taga, A.; Okamoto, N.; Shimomura, Y. Instillation of sericin enhances corneal wound healing through the erk pathway in rat debrided corneal epithelium. Int. J. Mol. Sci. 2018, 19, 1123. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [Green Version]
- Toropainen, E.; Ranta, V.P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar]
- Minami, M.; Yamaguchi, M.; Yamasaki, Y.; Otake, H.; Sakurai, S.; Harata, E.; Nagai, N. Effect of MPC polymer on corneal toxicity and corneal drug permeation of benzalkonium chloride in corneal epithelial cells. J. Eye 2020, 37, 1309–1314. [Google Scholar]
- Gipson, I.K.; Hori, Y.; Argueso, P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul. Surf. 2004, 2, 131–148. [Google Scholar] [CrossRef]
- Gipson, I.K.; Argueso, P. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar]
- Rose, M.C. Mucins:structure, function, and role in pulmonary diseases. Am. J. Physiol. 1992, 263, L413–L492. [Google Scholar] [PubMed]
- Sheffner, A.L.; Medler, E.M.; Jacobs, L.W.; Sarett, H.P. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am. Rev. Respir. Dis. 1964, 90, 721–729. [Google Scholar] [PubMed]
- Thermes, F.; Molon-Noblot, S.; Grove, J. Effects of acetylcysteine on rabbit conjunctival and corneal surfaces. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2958–2963. [Google Scholar] [PubMed]
- Anderton, P.; Tragoulias, S. Mucous contribution to rat tear-film thickness measured with a microelectrode technique. In Lacrimal Gland, Tear Film, and Dry Eye Syndrome 2; Plenum Press: New York, NY, USA, 1998; pp. 247–252. [Google Scholar]
- Urashima, H.; Okamoto, T.; Takeji, Y.; Shinohara, H.; Fujisawa, S. Rebamipide increases the amount of mucin-like substances on the conjunctiva and cornea in the N-acetylcysteine-treated in vivo model. Cornea 2004, 23, 613–619. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Sakurai, S.; Seiriki, R.; Minami, M.; Yamaguchi, M.; Deguchi, S.; Harata, E. MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface. Pharmaceutics 2021, 13, 168. https://doi.org/10.3390/pharmaceutics13020168
Nagai N, Sakurai S, Seiriki R, Minami M, Yamaguchi M, Deguchi S, Harata E. MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface. Pharmaceutics. 2021; 13(2):168. https://doi.org/10.3390/pharmaceutics13020168
Chicago/Turabian StyleNagai, Noriaki, Shunsuke Sakurai, Ryotaro Seiriki, Misa Minami, Mizuki Yamaguchi, Saori Deguchi, and Eiji Harata. 2021. "MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface" Pharmaceutics 13, no. 2: 168. https://doi.org/10.3390/pharmaceutics13020168
APA StyleNagai, N., Sakurai, S., Seiriki, R., Minami, M., Yamaguchi, M., Deguchi, S., & Harata, E. (2021). MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface. Pharmaceutics, 13(2), 168. https://doi.org/10.3390/pharmaceutics13020168





