Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Donor and Acceptor Lipid Nanodispersions
2.3. Preparation of Trimyristin-Containing Alginate Beads
2.4. Particle Size Analysis
2.5. pH Measurements
2.6. Microscopy
2.7. Differential Scanning Calorimetry
2.8. Lipid Quantification Via High Performance Liquid Chromatography
2.9. Investigation of Drug Transfer
3. Results
3.1. Characteristics of Donor and Acceptor Particles
3.1.1. Particle Sizes
3.1.2. Drug Load of Donor Emulsions
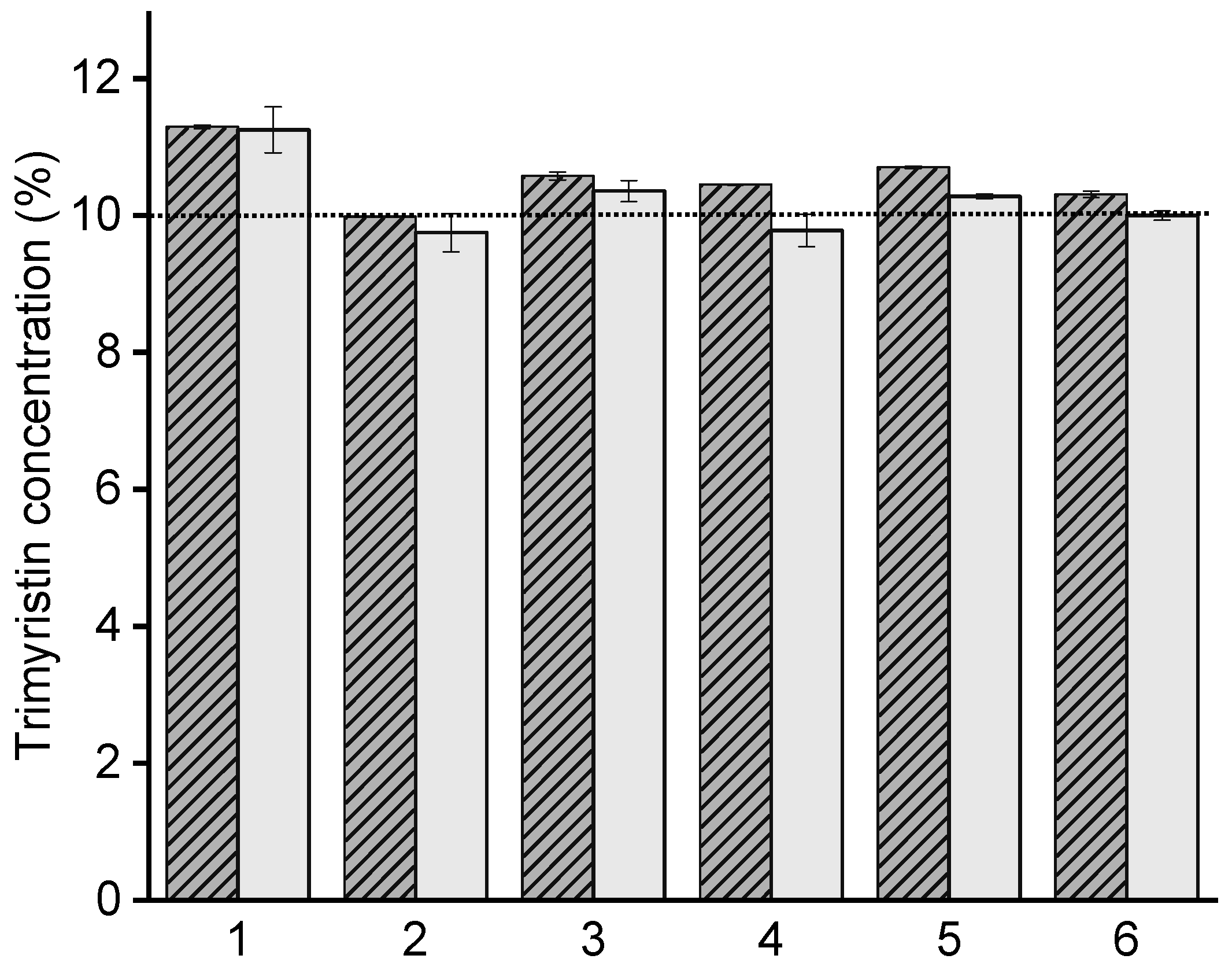
3.1.3. Lipid Concentration and Nanoemulsion Integrity in Hydrogel Particles
3.2. Investigation of Drug Transfer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunjes, H. Lipid nanoparticles for the delivery of poorly water-soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Boyd, B.J. Drug release from nanomedicines: Selection of appropriate encapsulation and release methodology. Drug. Deliv. Transl. Res. 2012, 2, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Heeremans, J.L.M.; Gerritsen, H.R.; Meusen, S.P.; Mijnheer, F.W.; Panday, R.S.G.; Prevost, R.; Kluft, C.; Crommelin, D.J.A. The preparation of tissue-type plasminogen activator (t-PA) containing liposomes: Entrapment efficiency and ultracentrifugation damage. J. Drug. Target. 1995, 3, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Washington, C.; Koosha, F. Drug release from microparticulates; deconvolution of measurement errors. Int. J. Pharm. 1990, 59, 79–82. [Google Scholar] [CrossRef]
- Magenheim, B.; Levy, M.Y.; Benita, S. A new in vitro technique for the evaluation of drug release profile from colloidal carriers- ultrafiltration technique at low pressure. Int. J. Pharm. 1993, 94, 115–123. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef]
- Levy, M.Y.; Benita, S. Drug release from submicronized o/w emulsion: A new in vitro kinetic evaluation model. Int. J. Pharm. 1990, 66, 29–37. [Google Scholar] [CrossRef]
- Washington, C. Drug release from microdisperse systems: A critical review. Int. J. Pharm. 1990, 58, 1–12. [Google Scholar] [CrossRef]
- Xie, L.; Beyer, S.; Vogel, V.; Wacker, M.G.; Mäntele, W. Assessing the drug release from nanoparticles: Overcoming the shortcomings of dialysis by using novel optical techniques and a mathematical model. Int. J. Pharm. 2015, 488, 108–119. [Google Scholar] [CrossRef]
- Landry, F.B.; Bazile, D.V.; Spenlehauer, G.; Veillard, M.; Kreuter, J. Release of the fluorescent marker Prodan® from poly(D,L-lactic acid) nanoparticles coated with albumin or polyvinyl alcohol in model digestive fluids (USP XXII). J. Control. Release 1997, 44, 227–236. [Google Scholar] [CrossRef]
- Washington, C.; Evans, K. Release reate measurements of model hydrophobic solutes hydrophobic solutes from submicron triglyceride emulsions. J. Control Release 1995, 33, 383–390. [Google Scholar] [CrossRef]
- Rosenblatt, K.M.; Douroumis, D.; Bunjes, H. Drug release from differently structured monoolein/poloxamer nanodispersions studied with differential pulse polarography and ultrafiltration at low pressure. J. Pharm. Sci. 2007, 96, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Gil, D.; Frank-Kamenetskii, A.; Barry, J.; Reukov, V.; Xiang, Y.; Das, A.; Varma, A.K.; Kindy, M.S.; Banik, N.L.; Vertegel, A. Albumin-assisted method allows assessment of release of hydrophobic drugs from nanocarriers. Biotechnol. J. 2018, 13, 1700337. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Fahr, A.; Bunjes, H. Flow cytometry as a new approach to investigate drug transfer between lipid particles. Mol. Pharm. 2010, 7, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Hinna, A.; Steiniger, F.; Hupfeld, S.; Brandl, M.; Kuntsche, J. Asymmetrical flow field-flow fractionation with on-line detection for drug transfer studies: A feasibility study. Anal. Bioanal. Chem. 2014, 406, 7827–7839. [Google Scholar] [CrossRef]
- Decker, C.; Steiniger, F.; Fahr, A. Transfer of a lipophilic drug (temoporfin) between small unilamellar liposomes and human plasma proteins: Influence of membrane composition on vesicle integrity and release characteristics. J. Liposome Res. 2013, 23, 154–165. [Google Scholar] [CrossRef]
- Holzschuh, S.; Kaeß, K.; Bossa, G.V.; Decker, C.; Fahr, A.; May, S. Investigations of the influence of liposome composition on vesicle stability and drug transfer in human plasma: A transfer study. J. Liposome Res. 2018, 28, 22–34. [Google Scholar] [CrossRef]
- Roese, E.; Bunjes, H. Drug release studies from lipid nanoparticles in physiological media by a new DSC method. J. Control Release 2017, 256, 92–100. [Google Scholar] [CrossRef]
- Strasdat, B.; Bunjes, H. Development of a new approach to investigating the drug transfer from colloidal carrier systems applying lipid nanosuspension-containing alginate microbeads as acceptor. Int. J. Pharm. 2015, 489, 203–209. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Rosenblatt, K.M.; Bunjes, H. Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading. Eur. J. Pharm. Biopharm. 2017, 117, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Strasdat, B.; Bunjes, H. Incorporation of lipid nanoparticles into calcium alginate beads and characterization of the encapsulated particles by differential scanning calorimetry. Food Hydrocoll. 2013, 30, 567–575. [Google Scholar] [CrossRef]
- Tall, A.R.; Deckelbaum, R.J.; Small, D.M.; Shipley, G.G. Thermal behavior of human plasma high density lipoprotein. Biochim. Biophys. Acta 1977, 487, 145–153. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Shipley, G.G.; Small, D.M. Structure and interactions of lipids in human plasma low density lipoproteins. J. Biol. Chem. 1977, 252, 744–754. [Google Scholar] [CrossRef]
- Westesen, K.; Siekmann, B. Investigation of the gel formation of phospholipid-stabilized solid lipid nanoparticles. Int. J. Pharm. 1997, 151, 35–45. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Influence of polymorphic transformations on gelation of tripalmitin solid lipid nanoparticle suspensions. J. Am. Oil Chem. Soc. 2008, 85, 501–511. [Google Scholar] [CrossRef]
- Shabbits, J.A.; Chiu, G.N.C.; Mayer, L.D. Development of an in vitro drug release assay that accurately predicts in vivo drug retention for liposome-based delivery systems. J. Control. Release 2002, 84, 161–170. [Google Scholar] [CrossRef]
- Hefesha, H.; Loew, S.; Liu, X.; May, S.; Fahr, A. Transfer mechanism of temoporfin between liposomal membranes. J. Control. Release 2011, 150, 279–286. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Sasaki, H.; Takakura, Y.; Hashida, M.; Kimura, T.; Sezaki, H. Antitumor activity of lipophilic prodrugs of mitomycin C entrapped in liposome or O/W emulsion. J. Pharmacobio-Dyn. 1984, 7, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Washington, C. Evaluation of non-sink dialysis methods for the measurement of drug release from colloids: Effects of drug partition. Int. J. Pharm. 1989, 56, 71–74. [Google Scholar] [CrossRef]
- Takino, T.; Konishi, K.; Takakura, Y.; Hashida, M. Long circulating emulsion carrier systems for highly lipophilic drugs. Biol. Pharm. Bull. 1994, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Zorin, V.; Siupa, A.; D’Hallewin, M.-A.; Guillemin, F.; Bezdetnaya, L. Interaction of liposomal formulations of meta-tetra(hydroxyphenyl)chlorin (temoporfin) with serum proteins: Protein binding and liposome destruction. Photochem. Photobiol. 2012, 88, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef]






| Drug | Abbreviation | LogP (Drugbank; ALOGPS) | LogD (SciFinder; ACD/Labs) |
|---|---|---|---|
| Fenofibrate | FFB | 4.86 | - |
| Cannabidiol | CBD | 6.1 | - |
| Retinyl acetate | RA | 6.56 | - |
| Orlistat | ORL | 7.61 | |
| Lumefantrine | LU | 8.34 | 6.17 (pH 6) 7.04 (pH 7) |
| Investigated Drug | Mean Diameter (µm) ± SD | D10 (µm) ± SD | D90 (µm) ± SD | Lipid Content (mg/mL) ± SD as Determined Via DSC | Transferred Amount of Drug after 1 h (%) ± SD |
|---|---|---|---|---|---|
| Fenofibrate | 40 ± 3 | 10 ± 0.6 | 88 ± 8 | 45.3 ± 2 | 90 ± 0.8 |
| Cannabidiol | 40 ± 1 | 9 ± 0.2 | 90 ± 6 | 46.0 ± 4 | 88 ± 2 |
| Retinyl acetate | 39 ± 2 | 10 ± 0.4 | 79 ± 3 | 43.4 ± 3 | 71 ± 4 |
| Orlistat | 36 ± 1 * | 9 ± 0.1 * | 81 ± 3 * | 42.5 ± 0.4 * | 36 ± 13 |
| Lumefantrine | 42 ± 3 | 10 ± 0.8 | 90 ± 7 | 45.1 ± 4 | 47 ± 6 |
| Lumefantrine pH = 10.8 | 39 ± 1 * | 9 * | 87 ± 2 * | 44.1 ± 2 * | 11 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoke, S.; Bunjes, H. Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres. Pharmaceutics 2021, 13, 173. https://doi.org/10.3390/pharmaceutics13020173
Knoke S, Bunjes H. Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres. Pharmaceutics. 2021; 13(2):173. https://doi.org/10.3390/pharmaceutics13020173
Chicago/Turabian StyleKnoke, Sabrina, and Heike Bunjes. 2021. "Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres" Pharmaceutics 13, no. 2: 173. https://doi.org/10.3390/pharmaceutics13020173
APA StyleKnoke, S., & Bunjes, H. (2021). Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres. Pharmaceutics, 13(2), 173. https://doi.org/10.3390/pharmaceutics13020173







