Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects
Abstract
:1. Introduction
2. Inhaled Medicines for Obstructive Airway Diseases
3. The Fate of Aerosol Drugs after Deposition in the Lungs
4. Novel Inhaled Medicines for Obstructive Airway Diseases
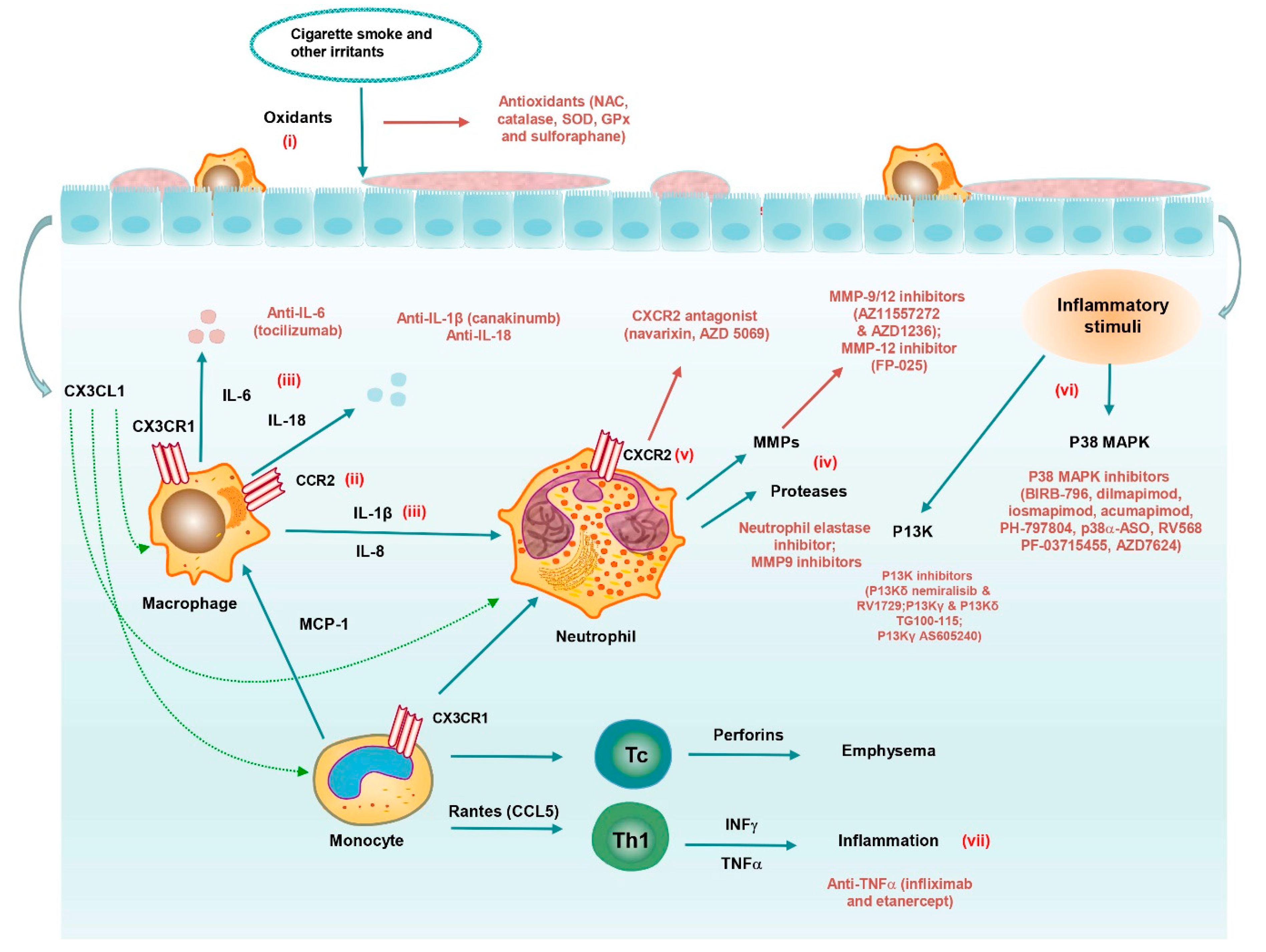
4.1. Antioxidants
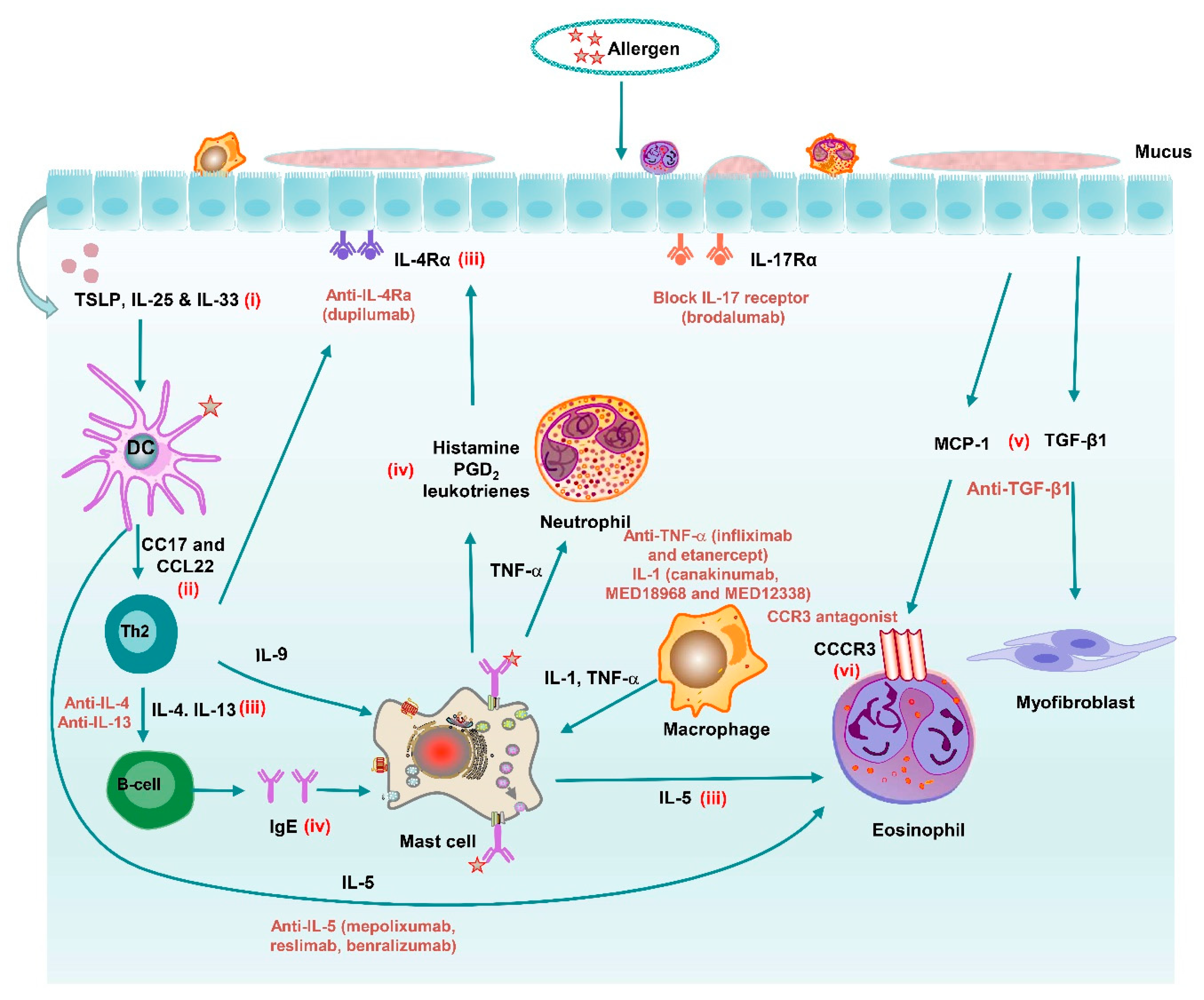
4.2. Mediator Antagonists
4.2.1. Cytokine/Chemokine Inhibitors
4.2.2. Inflammasome Inhibitors
4.2.3. Protease Inhibitors
4.3. Kinase Inhibitors
4.3.1. p38 MAPK Inhibitors
4.3.2. PI3K Inhibitors
4.4. RNA Therapeutics
5. Dry Powder-Based Inhaled Medicines
5.1. Milling
5.2. Spray Drying
5.3. Spray Freeze Drying
5.4. Supercritical Fluid Technology
5.5. Non-Wetting Templates (PRINT)
6. Critical Quality Attributes of Inhalable Dry Powders
7. Delivery Systems for Inhaled RNA Therapeutics
7.1. Microparticles
7.2. Nanoembedded Microparticles
7.2.1. Lipid-Based Delivery Systems
7.2.2. Polymer-Based Delivery Systems
7.2.3. Lipid-Polymer Hybrid Delivery Systems
7.2.4. Peptide-Based Delivery Systems
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami-Afshar, S.; FitzGerald, J.M.; Doyle-Waters, M.M.; Sadatsafavi, M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2016, 20, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol.-Mech. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [Green Version]
- Postma, D.S.; Rabe, K.F. The asthma–COPD overlap syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis, clinical features, and therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef]
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 1 December 2017).
- Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 20 May 2020).
- Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults--United States, 2011. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 938–943. [Google Scholar]
- Tan, M.J.; Tan, J.S.; File, T.M., Jr.; Hamor, R.H.; Breiman, R.F. The radiologic manifestations of Legionnaire‘s disease. Chest 2000, 117, 398–403. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar]
- Harris, D.M.; Martin, L.E.; Harrison, C.; Jack, D. The effect of oral and inhaled beclomethasone dipropionate on adrenal function. Clin. Allergy 1973, 3, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inhaled corticosteroids in COPD: A controversy. Respiration 2010, 80, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Garcha, D.S.; Thurston, S.J.; Patel, A.R.; Mackay, A.J.; Goldring, J.J.; Donaldson, G.C.; McHugh, T.D.; Wedzicha, J.A. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012, 67, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Gotfried, M.H. Macrolides for the Treatment of Chronic Sinusitis, Asthma, and COPD. Chest 2004, 125, 52S–61S. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.; Welte, T.; Polverino, E.; De Soyza, A.; Greville, H.; O’Donnell, A.; Alder, J.; Reimnitz, P.; Hampel, B. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: A phase II randomised study. Eur. Respir. J. 2013, 41, 1107–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.; Penn, R.; Hanania, N.; Dickey, B.F.; Bond, R. New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma. Br. J. Pharmacol. 2011, 163, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Billington, C.K.; Penn, R.B.; Hall, I.P. β 2 Agonists. Handb. Exp. Pharmacol. 2017, 237, 23–40. [Google Scholar]
- Cazzola, M.; Rogliani, P.; Segreti, A.; Matera, M.G. An update on bronchodilators in Phase I and II clinical trials. Expert Opin. Investig. Drugs 2012, 21, 1489–1501. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R., Jr. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wada, H.; Rossios, C.; Takagi, D.; Higaki, M.; Mikura, S.i.; Goto, H.; Barnes, P.J.; Ito, K. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J. Pharmacol. Exp. Ther. 2013, 345, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.; Wilkinson, T.M.; Hurst, J.R.; Perera, W.R.; Sapsford, R.J.; Wedzicha, J.A. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am. J. Respir. Crit. Care Med. 2008, 178, 1139–1147. [Google Scholar] [CrossRef]
- Korhonen, K.; Dunder, T.; Klaukka, T.; Reijonen, T.; Issakoff, K.; Kiviharju, M.; Linna, O.; Remes, K.; Korppi, M. Do inhaled steroids differ from cromones in terms of hospital admission rates for asthma in children? Acta Paediatr. 2004, 93, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, C.; Zhang, Z.; Kim, C.S. Combined inertial and gravitational deposition of microparticles in small model airways of a human respiratory system. J. Aerosol Sci. 2007, 38, 1047–1061. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Patton, J. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996, 19, 3–36. [Google Scholar] [CrossRef]
- Zanen, P.; Go, L.T.; Lammers, J.W. Optimal particle size for beta 2 agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax 1996, 51, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Kleinstreuer, C.; Zhang, Z. Particle deposition in the human tracheobronchial airways due to transient inspiratory flow patterns. J. Aerosol Sci. 2007, 38, 625–644. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Laminar-to-turbulent fluid-particle flows in a human airway model. Int. J. Multiph. Flow 2003, 29, 271–289. [Google Scholar] [CrossRef]
- Patton, J.S.; Brain, J.D.; Davies, L.A.; Fiegel, J.; Gumbleton, M.; Kim, K.J.; Sakagami, M.; Vanbever, R.; Ehrhardt, C. The particle has landed—characterizing the fate of inhaled pharmaceuticals. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S-71–S-87. [Google Scholar] [CrossRef] [Green Version]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Voynow, J.A.; Rubin, B.K. Mucins, mucus, and sputum. Chest 2009, 135, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.B.; Gudis, D.A.; Cohen, N.A. Epithelium, cilia, and mucus: Their importance in chronic rhinosinusitis. Immunol. Allergy. Clin. 2009, 29, 631–643. [Google Scholar] [CrossRef]
- Schürch, S.; Gehr, P.; Im Hof, V.; Geiser, M.; Green, F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir. Physiol. 1990, 80, 17–32. [Google Scholar] [CrossRef]
- Smith, D.J.; Gaffney, E.A.; Blake, J.R. Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 2008, 163, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Boger, E.; Evans, N.; Chappell, M.; Lundqvist, A.; Ewing, P.; Wigenborg, A.; Friden, M. Systems Pharmacology Approach for Prediction of Pulmonary and Systemic Pharmacokinetics and Receptor Occupancy of Inhaled Drugs. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 201–210. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Weaver, T.E. Pulmonary surfactant pathophysiology: Current models and open questions. Physiology 2010, 25, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gil, J. Structure of pulmonary surfactant membranes and films: The role of proteins and lipid–protein interactions. Biochim. Biophys. Acta 2008, 1778, 1676–1695. [Google Scholar] [CrossRef] [Green Version]
- Harishchandra, R.K.; Saleem, M.; Galla, H.J. Nanoparticle interaction with model lung surfactant monolayers. J. R. Soc. Interface 2010, 7 (Suppl. 1), S15–S26. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of Deposition, Lung Surface Area and Lung Fluid for Simulation of Inhaled Compounds. Front. Pharmacol. 2016, 7, 181. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, L.A.; Jekel, N.; Fink, L.; Gessler, T.; Schmehl, T.; Wittmar, M.; Kissel, T.; Seeger, W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol. Appl. Pharmacol. 2006, 215, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Edsbäcker, S.; Johansson, C.J. Airway selectivity: An update of pharmacokinetic factors affecting local and systemic disposition of inhaled steroids. Basic Clin. Pharmacol. 2006, 98, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Agu, R.U.; Ugwoke, M.I.; Armand, M.; Kinget, R.; Verbeke, N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir. Res. 2001, 2, 198. [Google Scholar]
- Tronde, A.; Nordén, B.; Marchner, H.; Wendel, A.K.; Lennernäs, H.; Bengtsson, U.H. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: Structure–absorption relationships and physicochemical profiling of inhaled drugs. J. Pharm. Sci. 2003, 92, 1216–1233. [Google Scholar] [CrossRef]
- Arora, D.; Shah, K.A.; Halquist, M.S.; Sakagami, M. In vitro aqueous fluid-capacity-limited dissolution testing of respirable aerosol drug particles generated from inhaler products. Pharm. Res. 2010, 27, 786–795. [Google Scholar] [CrossRef]
- Yang, W.; Johnston, K.P.; Williams, R.O. Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur. J. Pharm. Biopharm. 2010, 75, 33–41. [Google Scholar] [CrossRef]
- Morales, J.O.; Peters, J.I.; Williams, R.O. Surfactants: Their critical role in enhancing drug delivery to the lungs. Ther. Deliv. 2011, 2, 623–641. [Google Scholar] [CrossRef]
- Zheng, J.P.; Wen, F.Q.; Bai, C.X.; Wan, H.Y.; Kang, J.; Chen, P.; Yao, W.Z.; Ma, L.J.; Li, X.; Raiteri, L. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014, 2, 187–194. [Google Scholar] [CrossRef]
- Tse, H.N.; Raiteri, L.; Wong, K.Y.; Ng, L.Y.; Yee, K.S.; Tseng, C.Z.S. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest 2014, 146, 611–623. [Google Scholar] [CrossRef]
- Stav, D.; Raz, M. Effect of N-acetylcysteine on air trapping in COPD: A randomized placebo-controlled study. Chest 2009, 136, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.N.; Raiteri, L.; Wong, K.Y.; Yee, K.S.; Ng, L.Y.; Wai, K.Y.; Loo, C.K.; Chan, M.H. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013, 144, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Khabour, O.F.; Alzoubi, K.H.; Al-Sawalha, N.; Ahmad, M.B.; Shihadeh, A.; Eissenberg, T. The effect of chronic exposure to waterpipe tobacco smoke on airway inflammation in mice. Life Sci. 2018, 200, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A.; Holbrook, J.T.; Criner, G.; Sethi, S.; Rayapudi, S.; Sudini, K.R.; Sugar, E.A.; Burke, A.; Thimmulappa, R.; Singh, A.; et al. Lack of Effect of Oral Sulforaphane Administration on Nrf2 Expression in COPD: A Randomized, Double-Blind, Placebo Controlled Trial. PLoS ONE 2016, 11, e0163716. [Google Scholar] [CrossRef] [Green Version]
- Churg, A.; Wang, R.; Wang, X.; Onnervik, P.O.; Thim, K.; Wright, J.L. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax 2007, 62, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Ostridge, K.; Williams, N.; Kim, V.; Bennett, M.; Harden, S.; Welch, L.; Bourne, S.; Coombs, N.A.; Elkington, P.T.; Staples, K.J. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax 2016, 71, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Dahl, R.; Titlestad, I.; Lindqvist, A.; Wielders, P.; Wray, H.; Wang, M.; Samuelsson, V.; Mo, J.; Holt, A. Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: A randomised controlled trial. Pulm. Pharmacol. Ther. 2012, 25, 169–177. [Google Scholar] [CrossRef]
- Kuna, P.; Jenkins, M.; O’Brien, C.D.; Fahy, W.A. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir. Med. 2012, 106, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Andreadou, I.; Triantafyllidi, H.; Tsoumani, M.; Varoudi, M.; Vlastos, D.; Makavos, G.; Kostelli, G.; et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin. Res. Cardiol. 2019, 108, 1093–1101. [Google Scholar] [CrossRef]
- Rennard, S.I.; Fogarty, C.; Kelsen, S.; Long, W.; Ramsdell, J.; Allison, J.; Mahler, D.; Saadeh, C.; Siler, T.; Snell, P.; et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Nair, P.; Bardin, P.; Humbert, M.; Murphy, K.R.; Hickey, L.; Garin, M.; Vanlandingham, R.; Chanez, P. Efficacy of Intravenous Reslizumab in Oral Corticosteroid–Dependent Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 555–564. [Google Scholar] [CrossRef]
- Castro, M.; Wenzel, S.E.; Bleecker, E.R.; Pizzichini, E.; Kuna, P.; Busse, W.W.; Gossage, D.L.; Ward, C.K.; Wu, Y.; Wang, B.; et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: A phase 2b randomised dose-ranging study. Lancet Respir. Med. 2014, 2, 879–890. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Tohda, Y.; Nakamura, Y.; Fujisawa, T.; Ebisawa, M.; Arima, K.; Miyata, M.; Takahashi, Y.; Rice, M.S.; Deniz, Y.; Rowe, P.; et al. Dupilumab efficacy and safety in Japanese patients with uncontrolled, moderate-to-severe asthma in the phase 3 LIBERTY ASTHMA QUEST study. Allergol. Int. 2020, 69, 578–587. [Google Scholar] [CrossRef]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy. Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Krug, N.; Hohlfeld, J.M.; Kirsten, A.M.; Kornmann, O.; Beeh, K.M.; Kappeler, D.; Korn, S.; Ignatenko, S.; Timmer, W.; Rogon, C.; et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N. Engl. J. Med. 2015, 372, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti–IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens, H.A.; Bjermer, L.; Eriksson, L.; Dahlstrom, K.; Vestbo, J. Tolerability and efficacy of inhaled AZD4818, a CCR1 antagonist, in moderate to severe COPD patients. Respir. Med. 2010, 104, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, A.M.; Forster, K.; Radeczky, E.; Linnhoff, A.; Balint, B.; Watz, H.; Wray, H.; Salkeld, L.; Cullberg, M.; Larsson, B. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm. Pharmacol. Ther. 2015, 31, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Holz, O.; Khalilieh, S.; Ludwig-Sengpiel, A.; Watz, H.; Stryszak, P.; Soni, P.; Tsai, M.; Sadeh, J.; Magnussen, H. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur. Respir. J. 2010, 35, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, P.G.; Albert, R.K.; Bailey, W.C.; Casaburi, R.; Connett, J.E.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; et al. Randomized trial of zileuton for treatment of COPD exacerbations requiring hospitalization. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 21–29. [Google Scholar] [CrossRef]
- Calverley, P.M.; Sethi, S.; Dawson, M.; Ward, C.K.; Finch, D.K.; Penney, M.; Newbold, P.; van der Merwe, R. A randomised, placebo-controlled trial of anti–interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir. Res. 2017, 18, 153. [Google Scholar] [CrossRef]
- Armstrong, J.; Harbron, C.; Lea, S.; Booth, G.; Cadden, P.; Wreggett, K.A.; Singh, D. Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2011, 338, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, S.; Costa, M.; Marks-Konczalik, J.; McKie, E.; Yang, S.; Scherbovsky, P.S. Biological effects of p38 MAPK inhibitor losmapimod does not translate to clinical benefits in COPD. Respir. Med. 2017, 130, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Fisk, M.; Cheriyan, J.; Mohan, D.; Forman, J.; Maki-Petaja, K.M.; McEniery, C.M.; Fuld, J.; Rudd, J.H.F.; Hopkinson, N.S.; Lomas, D.A.; et al. The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: A randomised double-blind placebo-controlled trial. PLoS ONE 2018, 13, e0194197. [Google Scholar] [CrossRef]
- Lomas, D.A.; Lipson, D.A.; Miller, B.E.; Willits, L.; Keene, O.; Barnacle, H.; Barnes, N.C.; Tal-Singer, R.; Losmapimod Study, I. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J. Clin. Pharmacol. 2012, 52, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Strâmbu, I.R.; Kobalava, Z.D.; Magnusson, B.P.; MacKinnon, A.; Parkin, J.M. Phase II study of single/repeated doses of acumapimod (BCT197) to treat acute exacerbations of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Allan, R.J.; Jones, I.; De Salvo, M.C.; Tan, L.F. Efficacy and safety of the oral p38 inhibitor PH-797804 in chronic obstructive pulmonary disease: A randomised clinical trial. Thorax 2013, 68, 738–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Chan, J.H.; McKay, K.; Crosby, J.R.; Choo, H.H.; Leung, B.P.; Karras, J.G.; Wong, W.F. Inhaled p38α mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am. J. Respir. Crit. Care Med. 2005, 171, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Cunoosamy, D.M.; Fageras, M.; Taib, Z.; Asimus, S.; Hegelund-Myrback, T.; Lundin, S.; Pardali, K.; Kurian, N.; Ersdal, E.; et al. The development of AZD7624 for prevention of exacerbations in COPD: A randomized controlled trial. COPD J. Chronic Obstr. Pulm. Dis. 2018, 13, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahn, A.; Hamblin, J.; Begg, M.; Wilson, R.; Dunsire, L.; Sriskantharajah, S.; Montembault, M.; Leemereise, C.; Galinanes-Garcia, L.; Watz, H. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kδ inhibitor under development for the treatment of COPD. Pulm. Pharmacol. Ther. 2017, 46, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Templeton, A.; Leemereise, C.; Eames, R.; Banham-Hall, E.; Hessel, E.M.; Cahn, A. Safety, Tolerability, and Pharmacokinetics of a New Formulation of Nemiralisib Administered via a Dry Powder Inhaler to Healthy Individuals. Clin. Ther. 2019, 41, 1214–1220. [Google Scholar] [CrossRef]
- Doukas, J.; Eide, L.; Stebbins, K.; Racanelli-Layton, A.; Dellamary, L.; Martin, M.; Dneprovskaia, E.; Noronha, G.; Soll, R.; Wrasidlo, W. Aerosolized phosphoinositide 3-kinase γ/δ inhibitor TG100–115 [3-[2, 4-diamino-6-(3-hydroxyphenyl) pteridin-7-yl] phenol] as a therapeutic candidate for asthma and chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2009, 328, 758–765. [Google Scholar] [CrossRef]
- Wei, X.; Han, J.; Chen, Z.Z.; Qi, B.W.; Wang, G.C.; Ma, Y.H.; Zheng, H.; Luo, Y.F.; Wei, Y.Q.; Chen, L.J. A phosphoinositide 3-kinase-gamma inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem. Biophys. Res. Commun. 2010, 397, 311–317. [Google Scholar] [CrossRef]
- DeVincenzo, J.; Cehelsky, J.E.; Alvarez, R.; Elbashir, S.; Harborth, J.; Toudjarska, I.; Nechev, L.; Murugaiah, V.; Van Vliet, A.; Vaishnaw, A.K.; et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral. Res. 2008, 77, 225–231. [Google Scholar] [CrossRef]
- DeVincenzo, J.; Lambkin-Williams, R.; Wilkinson, T.; Cehelsky, J.; Nochur, S.; Walsh, E.; Meyers, R.; Gollob, J.; Vaishnaw, A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 2010, 107, 8800–8805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miah, K.M.; Hyde, S.C.; Gill, D.R. Emerging gene therapies for cystic fibrosis. Expert. Rev. Respir. Med. 2019, 13, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Song, L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: A review. J. Nanobiotechnology 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Houston, M.; Anderson, J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am. Rev. Respir. Dis. 1993, 147, 1461–1464. [Google Scholar] [CrossRef]
- Van Der Vliet, A.; O’Neill, C.A.; Cross, C.E.; Koostra, J.M.; Volz, W.G.; Halliwell, B.; Louie, S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. Lung Cell Mol. Physiol. 1999, 276, L289–L296. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.; Cotgrove, M.; Mudway, I. Respiratory tract lining fluid antioxidants: The first line of defence against gaseous pollutants. Cent. Eur. J. Public Health 1996, 4, 11–14. [Google Scholar]
- Rustow, B.; Haupt, R.; Stevens, P.; Kunze, D. Type II pneumocytes secrete vitamin E together with surfactant lipids. Am. J. Physiol. Lung Cell Mol. Physiol. 1993, 265, L133–L139. [Google Scholar] [CrossRef]
- Moitra, S. N-acetylcysteine (NAC) in COPD: Benefits often lost in trials. QJM-Int. J. Med. 2019, 112, 387–388. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van Molken, M.; Dekhuijzen, P.N.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Cantin, A.M.; Fells, G.A.; Hubbard, R.C.; Crystal, R.G. Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. J. Clin. Investig. 1990, 86, 962–971. [Google Scholar] [CrossRef] [Green Version]
- Pietarinen-Runtti, P.; Raivio, K.O.; Saksela, M.; Asikainen, T.M.; Kinnula, V.L. Antioxidant enzyme regulation and resistance to oxidants of human bronchial epithelial cells cultured under hyperoxic conditions. Am. J. Respir. Cell Mol. Biol. 1998, 19, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Unlü, M.; Ozgüner, F.; Sütcü, R.; Akkaya, A.; Delibas, N. Lipid peroxidation and glutathione peroxidase activity in chronic obstructive pulmonary disease exacerbation: Prognostic value of malondialdehyde. J. Basic Clin. Physiol. Pharmacol. 2001, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rangasamy, T.; Thimmulappa, R.K.; Lee, H.; Osburn, W.O.; Brigelius-Flohé, R.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Glutathione peroxidase 2, the major cigarette smoke–inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006, 35, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Thimmulappa, R.K.; Sethi, S.; Kong, X.; Yarmus, L.; Brown, R.H.; Feller-Kopman, D.; Wise, R.; Biswal, S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011, 3, 78ra32. [Google Scholar] [CrossRef] [Green Version]
- Adcock, I.M.; Caramori, G.; Barnes, P.J. Chronic obstructive pulmonary disease and lung cancer: New molecular insights. Respiration 2011, 81, 265–284. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Ito, I.; Niimi, A.; Izuhara, K.; Ohta, S.; Ono, J.; Iwata, T.; Matsumoto, H.; Mishima, M. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 472–474. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC chemokines in angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar]
- Allen, S.J.; Crown, S.E.; Handel, T.M. Chemokine: Receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007, 25, 787–820. [Google Scholar] [CrossRef]
- Lazarus, S.C.; Chinchilli, V.M.; Rollings, N.J.; Boushey, H.A.; Cherniack, R.; Craig, T.J.; Deykin, A.; DiMango, E.; Fish, J.E.; Ford, J.G.; et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 783–790. [Google Scholar] [CrossRef]
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.; O‘byrne, P.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy. 2012, 42, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Phillips, J.E.; Hipkin, R.W.; Curran, A.K.; Lundell, D.; Fine, J.S. CXCR2 antagonists for the treatment of pulmonary disease. Pharmacol. Ther. 2009, 121, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Pinto, A.; Sorrentino, R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget 2017, 8, 81813–81824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nardo, D.; De Nardo, C.M.; Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 2014, 184, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Suh, G.Y.; Ryter, S.W.; Choi, A.M. Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkington, P.T.G.; Friedland, J.S. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006, 61, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churg, A.; Zhou, S.; Wright, J.L. Matrix metalloproteinases in COPD. Eur. Respir. J. 2012, 39, 197–209. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, B.; Moreau, T.; Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie 2008, 90, 227–242. [Google Scholar] [CrossRef]
- Groutas, W.C.; Dou, D.; Alliston, K.R. Neutrophil elastase inhibitors. Expert Opin. Ther. Pat. 2011, 21, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Renda, T.; Baraldo, S.; Pelaia, G.; Bazzan, E.; Turato, G.; Papi, A.; Maestrelli, P.; Maselli, R.; Vatrella, A.; Fabbri, L.M.; et al. Increased activation of p38 MAPK in COPD. Eur. Respir. J. 2008, 31, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammaker, D.; Firestein, G. “Go upstream, young man”: Lessons learned from the p38 saga. Ann. Rheum. Dis. 2010, 69, i77–i82. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xie, M.; He, X.; Gao, H. Activity of sputum p38 MAPK is correlated with airway inflammation and reduced FEV1 in COPD patients. Med. Sci. Monit. 2013, 19, 1229. [Google Scholar] [PubMed] [Green Version]
- Enslen, H.; Brancho, D.M.; Davis, R.J. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000, 19, 1301–1311. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Chuang, C.Y.; Lee, P.K.; Lee, J.S.; Harper, R.W.; Buckpitt, A.B.; Wu, R.; Oslund, K. TRX-ASK1-JNK signaling regulation of cell density-dependent cytotoxicity in cigarette smoke-exposed human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L921–L931. [Google Scholar] [CrossRef] [Green Version]
- Marwick, J.A.; Chung, K.F.; Adcock, I.M. Phosphatidylinositol 3-kinase isoforms as targets in respiratory disease. Ther. Adv. Respir. Dis. 2010, 4, 19–34. [Google Scholar] [CrossRef] [Green Version]
- To, Y.; Ito, K.; Kizawa, Y.; Failla, M.; Ito, M.; Kusama, T.; Elliott, W.M.; Hogg, J.C.; Adcock, I.M.; Barnes, P.J. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Dowd, G.C.; Bhalla, M.; Kean, B.; Thomas, R.; Ireton, K. Role of Host Type IA Phosphoinositide 3-Kinase Pathway Components in Invasin-Mediated Internalization of Yersinia enterocolitica. Infect Immun. 2016, 84, 1826–1841. [Google Scholar] [CrossRef] [Green Version]
- Bewley, M.A.; Belchamber, K.B.; Chana, K.K.; Budd, R.C.; Donaldson, G.; Wedzicha, J.A.; Brightling, C.E.; Kilty, I.; Donnelly, L.E.; Barnes, P.J.; et al. Differential Effects of p38, MAPK, PI3K or Rho Kinase Inhibitors on Bacterial Phagocytosis and Efferocytosis by Macrophages in COPD. PLoS ONE 2016, 11, e0163139. [Google Scholar] [CrossRef] [Green Version]
- Fung-Leung, W.P. Phosphoinositide 3-kinase delta (PI3Kδ) in leukocyte signaling and function. Cell. Signal. 2011, 23, 603–608. [Google Scholar] [CrossRef]
- Heit, B.; Liu, L.; Colarusso, P.; Puri, K.D.; Kubes, P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell. Sci. 2008, 121, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhai, X.; Ma, C.; Sun, P.; Fu, Z.; Liu, W.; Xu, J. An inhalable β2-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of siRNA to lung. J. Control. Release 2012, 162, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, M.; Bohr, A.; Thanki, K.; Wan, F.; Zeng, X.; Boetker, J.P.; Yang, M.; Foged, C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 2017, 120, 9–21. [Google Scholar] [CrossRef]
- Choi, M.; Gu, J.; Lee, M.; Rhim, T. A new combination therapy for asthma using dual-function dexamethasone-conjugated polyethylenimine and vitamin D binding protein siRNA. Gene Ther. 2017, 24, 727–734. [Google Scholar] [CrossRef]
- Thanki, K.; Zeng, X.; Justesen, S.; Tejlmann, S.; Falkenberg, E.; Van Driessche, E.; Morck Nielsen, H.; Franzyk, H.; Foged, C. Engineering of small interfering RNA-loaded lipidoid-poly(DL-lactic-co-glycolic acid) hybrid nanoparticles for highly efficient and safe gene silencing: A quality by design-based approach. Eur. J. Pharm. Biopharm. 2017, 120, 22–33. [Google Scholar] [CrossRef]
- Keil, T.W.; Baldassi, D.; Merkel, O.M. T-cell targeted pulmonary siRNA delivery for the treatment of asthma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1634. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.D.; Clarke, A.R. Gene manipulation through the use of small interfering RNA (siRNA): From in vitro to in vivo applications. Adv. Drug Del. Rev. 2007, 59, 87–100. [Google Scholar] [CrossRef]
- Shaffer, C. Mist begins to clear for lung delivery of RNA. Nat. Biotechnol. 2020, 38, 1110–1112. [Google Scholar] [CrossRef]
- Chow, M.Y.; Qiu, Y.; Lam, J.K. Inhaled RNA therapy: From promise to reality. Trends Pharmacol. Sci. 2020, 41, 715–729. [Google Scholar] [CrossRef]
- Mei, D.; Tan, W.S.D.; Tay, Y.; Mukhopadhyay, A.; Wong, W.S.F. Therapeutic RNA Strategies for Chronic Obstructive Pulmonary Disease. Trends Pharmacol. Sci. 2020, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Sel, S.; Henke, W.; Dietrich, A.; Herz, U.; Renz, H. Treatment of allergic asthma by targeting transcription factors using nucleic-acid based technologies. Curr. Pharm. Des. 2006, 12, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Chellappan, D.K.; Wich, P.R.; Hansbro, N.G.; Hansbro, P.M.; Dua, K. miRNA nanotherapeutics: Potential and challenges in respiratory disorders. Future Med. Chem. 2020, 12, 987–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.; Pekoz, A.Y.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int. J. Pharm. 2019, 569, 118524. [Google Scholar] [CrossRef] [PubMed]
- O‘Leary, L.; Sevinç, K.; Papazoglou, I.M.; Tildy, B.; Detillieux, K.; Halayko, A.J.; Chung, K.F.; Perry, M.M. Airway smooth muscle inflammation is regulated by micro RNA-145 in COPD. FEBS Lett. 2016, 590, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vencken, S.; Foged, C.; Ramsey, J.M.; Sweeney, L.; Cryan, S.-A.; MacLoughlin, R.J.; Greene, C.M. Nebulised lipid–polymer hybrid nanoparticles for the delivery of a therapeutic anti-inflammatory microRNA to bronchial epithelial cells. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef]
- Keller, A.; Ludwig, N.; Fehlmann, T.; Kahraman, M.; Backes, C.; Kern, F.; Vogelmeier, C.F.; Diener, C.; Fischer, U.; Biertz, F.; et al. Low miR-150–5p and miR-320b Expression Predicts Reduced Survival of COPD Patients. Cells 2019, 8, 1162. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Sun, J.; Wu, D.; Lin, D.; Sun, D.; Li, Q.; Chen, J.; Niu, H.; He, P.; Ding, Y. MicroRNA-186 is associated with hypoxia-inducible factor-1α expression in chronic obstructive pulmonary disease. Mol. Genet. Genom. Med. 2019, 7, e531. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.; Peinado, V.I.; Ramírez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barberà, J.A. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Musri, M.M.; Coll-Bonfill, N.; Maron, B.A.; Peinado, V.I.; Wang, R.S.; Altirriba, J.; Blanco, I.; Oldham, W.M.; Tura-Ceide, O.; García-Lucio, J.; et al. MicroRNA Dysregulation in Pulmonary Arteries from Chronic Obstructive Pulmonary Disease. Relationships with Vascular Remodeling. Am. J. Respir. Cell Mol. Biol. 2018, 59, 490–499. [Google Scholar] [CrossRef]
- Ikari, J.; Smith, L.M.; Nelson, A.J.; Iwasawa, S.; Gunji, Y.; Farid, M.; Wang, X.; Basma, H.; Feghali-Bostwick, C.; Liu, X.; et al. Effect of culture conditions on microRNA expression in primary adult control and COPD lung fibroblasts in vitro. Vitro Cell Dev. Biol. Anim. 2015, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Tang, W.; Guo, J.; Sun, S. miR-483–5p plays a protective role in chronic obstructive pulmonary disease. Int. J. Mol. Med. 2017, 40, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Xu, J.; Liu, H.; Zhao, Z. Peripheral leukocyte microRNAs as novel biomarkers for COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conickx, G.; Mestdagh, P.; Avila Cobos, F.; Verhamme, F.M.; Maes, T.; Vanaudenaerde, B.M.; Seys, L.J.; Lahousse, L.; Kim, R.Y.; Hsu, A.C.; et al. MicroRNA Profiling Reveals a Role for MicroRNA-218–5p in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 43–56. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Wu, Y.; Lin, M.J.; Bian, T.; Xiao, Y.L.; Qin, C. LncRNA-MEG3 functions as a competing endogenous RNA to regulate Treg/Th17 balance in patients with asthma by targeting microRNA-17/ RORγt. Biomed. Pharmacother. 2019, 111, 386–394. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, X.Y.; Li, N.; Zhao, L.M.; Guo, Y.L.; Li, X.S.; Tian, C.J.; Cheng, D.J.; Chen, Z.C.; Zhang, L.X. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018, 212, 93–101. [Google Scholar] [CrossRef]
- Tasena, H.; Faiz, A.; Timens, W.; Noordhoek, J.; Hylkema, M.N.; Gosens, R.; Hiemstra, P.S.; Spira, A.; Postma, D.S.; Tew, G.W. microRNA–mRNA regulatory networks underlying chronic mucus hypersecretion in COPD. Eur. Respir. J. 2018, 52, 1701556. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Hung, G.; Xiao, X.; Peralta, R.; Bhattacharjee, G.; Murray, S.; Norris, D.; Guo, S.; Monia, B.P. Characterization of target mRNA reduction through in situ RNA hybridization in multiple organ systems following systemic antisense treatment in animals. Nucleic Acid Ther. 2013, 23, 369–378. [Google Scholar] [CrossRef]
- Falcoz, C.; Oliver, R.; McDowall, J.E.; Ventresca, P.; Bye, A.; Daley-Yates, P.T. Bioavailability of orally administered micronised fluticasone propionate. Clin. Pharmacokinet. 2000, 39 (Suppl. S1), 9–15. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming nanomedicine manufacturing toward Quality by Design and microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef]
- Chan, H.K.; Kwok, P.C. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Stute, P.; Neulen, J.; Wildt, L. The impact of micronized progesterone on the endometrium: A systematic review. Climacteric 2016, 19, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Malcolmson, R.J.; Embleton, J.K. Dry powder formulations for pulmonary delivery. Drug Deliv. Technol. 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Steckel, H.; Brandes, H.G. A novel spray-drying technique to produce low density particles for pulmonary delivery. Int. J. Pharm. 2004, 278, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Sosnowski, T.R.; Gradoń, L. Conception, preparation and properties of functional carrier particles for pulmonary drug delivery. Int. J. Pharm. 2012, 433, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Saboti, D.; Maver, U.; Chan, H.-K.; Planinšek, O. Novel budesonide particles for dry powder inhalation prepared using a microfluidic reactor coupled with ultrasonic spray freeze drying. J. Pharm. Sci. 2017, 106, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Thakkar, F.M.V.; Soni, T.; Gohel, M.; Gandhi, T. Supercritical fluid technology: A promising approach to enhance the drug solubility. J. Pharm. Sci. Res. 2009, 1, 1. [Google Scholar]
- Xu, P.Y.; Kankala, R.K.; Pan, Y.J.; Yuan, H.; Wang, S.B.; Chen, A.Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018, 13, 4685–4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankala, R.K.; Lin, X.F.; Song, H.-F.; Wang, S.-B.; Yang, D.-Y.; Zhang, Y.S.; Chen, A.-Z. Supercritical Fluid-Assisted Decoration of Nanoparticles on Porous Microcontainers for Codelivery of Therapeutics and Inhalation Therapy of Diabetes. ACS Biomater. Sci. Eng. 2018, 4, 4225–4235. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Mack, P.; Williams, S.; Fromen, C.; Shen, T.; Tully, J.; Pillai, J.; Kuehl, P.; Napier, M.; Desimone, J.M.; et al. Microfabricated engineered particle systems for respiratory drug delivery and other pharmaceutical applications. J. Drug Deliv. 2012, 2012, 941243. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.F.; Oliver, A.J.; Ioannou, C.; Billiard, J.; Dennison, J.; Van Den Berg, F.; Yang, S.; Chandrasekaran, V.; Young, G.C.; Lahiry, A. A novel inhaled dry-powder formulation of ribavirin allows for efficient lung delivery in healthy participants and those with chronic obstructive pulmonary disease in a phase 1 study. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Chan, H.K.; Gengenbach, T.; Denman, J.A. Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2017, 119, 224–234. [Google Scholar] [CrossRef]
- Le Clair, D.A.; Cranston, E.D.; Xing, Z.; Thompson, M.R. Optimization of Spray Drying Conditions for Yield, Particle Size and Biological Activity of Thermally Stable Viral Vectors. Pharm. Res. 2016, 33, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.H.; Choi, Y.H. Physico-chemical properties of protein-bound polysaccharide from Agaricus blazei Murill prepared by ultrafiltration and spray drying process. Int. J. Food Sci. Technol. 2007, 42, 1–8. [Google Scholar] [CrossRef]
- Hickey, A.J.; Mansour, H.M.; Telko, M.J.; Xu, Z.; Smyth, H.D.; Mulder, T.; McLean, R.; Langridge, J.; Papadopoulos, D. Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J. Pharm. Sci. 2007, 96, 1282–1301. [Google Scholar] [CrossRef]
- Zhu, K.; Tan, R.B.; Ng, W.K.; Shen, S.; Zhou, Q.; Heng, P.W. Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler. J. Aerosol Sci. 2008, 39, 510–524. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Schmidt, S.T.; Christensen, D.; Larsen, N.B.; Hinrichs, W.L.J.; Andersen, P.; Rantanen, J.; Nielsen, H.M.R.; Yang, M.; Foged, C. Designing CAF-adjuvanted dry powder vaccines: Spray drying preserves the adjuvant activity of CAF01. J. Control. Release 2013, 167, 256–264. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhao, M.; Liu, G.; Tang, X. Physical characteristics and aerosolization performance of insulin dry powders for inhalation prepared by a spray drying method. J. Pharm. Pharmacol. 2007, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Xu, Y.; Qu, D.-S.; Li, H.-Y. The influence of amino acids on aztreonam spray-dried powders for inhalation. Asian J. Pharm. 2015, 10, 541–548. [Google Scholar] [CrossRef] [Green Version]
- BÜCHI Labortechnik AG. Trainings-Papers Spray Drying Brouch; BUCHI BÜCHI Labortechnik AG: Flawil, Switzerland, 2002. [Google Scholar]
- Ståhl, K.; Claesson, M.; Lilliehorn, P.; Lindén, H.; Bäckström, K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int. J. Pharm. 2002, 233, 227–237. [Google Scholar] [CrossRef]
- Li, X.; Mansour, H.M. Physicochemical characterization and water vapor sorption of organic solution advanced spray-dried inhalable trehalose microparticles and nanoparticles for targeted dry powder pulmonary inhalation delivery. AAPS PharmSciTech 2011, 12, 1420–1430. [Google Scholar] [CrossRef] [Green Version]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Edwards, D.A.; Hanes, J.; Caponetti, G.; Hrkach, J.; Ben-Jebria, A.; Eskew, M.L.; Mintzes, J.; Deaver, D.; Lotan, N.; Langer, R. Large porous particles for pulmonary drug delivery. Science 1997, 276, 1868–1871. [Google Scholar] [CrossRef] [Green Version]
- Bot, A.I.; Tarara, T.E.; Smith, D.J.; Bot, S.R.; Woods, C.M.; Weers, J.G. Novel lipid-based hollow-porous microparticles as a platform for immunoglobulin delivery to the respiratory tract. Pharm. Res. 2000, 17, 275–283. [Google Scholar] [CrossRef]
- Nolan, L.M.; Tajber, L.; McDonald, B.F.; Barham, A.S.; Corrigan, O.I.; Healy, A.M. Excipient-free nanoporous microparticles of budesonide for pulmonary delivery. Eur. J. Pharm. Sci. 2009, 37, 593–602. [Google Scholar] [CrossRef]
- Weers, J.; Tarara, T. The PulmoSphere™ platform for pulmonary drug delivery. Ther. Deliv. 2014, 5, 277–295. [Google Scholar] [CrossRef]
- Visser, J. Van der Waals and other cohesive forces affecting powder fluidization. Powder Technol. 1989, 58, 1–10. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Feng, A.L.; Boraey, M.A.; Gwin, M.A.; Finlay, P.R.; Kuehl, P.J.; Vehring, R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.-K. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef]
- Lu, W.; Rades, T.; Rantanen, J.; Chan, H.-K.; Yang, M. Amino acids as stabilizers for spray-dried simvastatin powder for inhalation. Int. J. Pharm. 2019, 572, 118724. [Google Scholar] [CrossRef]
- Gilani, K.; Najafabadi, A.R.; Barghi, M.; Rafiee-Tehrani, M. The effect of water to ethanol feed ratio on physical properties and aerosolization behavior of spray dried cromolyn sodium particles. J. Pharm. Sci. 2005, 94, 1048–1059. [Google Scholar] [CrossRef]
- Chew, N.Y.; Tang, P.; Chan, H.K.; Raper, J.A. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 2005, 22, 148–152. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R. Pollen shape particles for pulmonary drug delivery: In vitro study of flow and deposition properties. In Proceedings of the 13th International Conference on Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1434–1437. [Google Scholar]
- Fults, K.A.; Miller, I.F.; Hickey, A.J. Effect of particle morphology on emitted dose of fatty acid-treated disodium cromoglycate powder aerosols. Pharm. Dev. Technol. 1997, 2, 67–79. [Google Scholar] [CrossRef]
- Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Zeng, X.M.; Martin, G.P.; Marriott, C.; Pritchard, J. The influence of carrier morphology on drug delivery by dry powder inhalers. Int. J. Pharm. 2000, 200, 93–106. [Google Scholar] [CrossRef]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q.T. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Dormenval, C.; Lokras, A.; Cano-Garcia, G.; Wadhwa, A.; Thanki, K.; Rose, F.; Thakur, A.; Franzyk, H.; Foged, C. Identification of Factors of Importance for Spray Drying of Small Interfering RNA-Loaded Lipidoid-Polymer Hybrid Nanoparticles for Inhalation. Pharm. Res. 2019, 36, 142. [Google Scholar] [CrossRef]
- Farkas, D.R.; Hindle, M.; Longest, P.W. Characterization of a New High-Dose Dry Powder Inhaler (DPI) Based on a Fluidized Bed Design. Ann. Biomed. Eng. 2015, 43, 2804–2815. [Google Scholar] [CrossRef] [Green Version]
- Chow, M.Y.T.; Qiu, Y.; Liao, Q.; Kwok, P.C.L.; Chow, S.F.; Chan, H.K.; Lam, J.K.W. High siRNA loading powder for inhalation prepared by co-spray drying with human serum albumin. Int. J. Pharm. 2019, 572, 118818. [Google Scholar] [CrossRef]
- Ito, T.; Okuda, T.; Takayama, R.; Okamoto, H. Establishment of an Evaluation Method for Gene Silencing by Serial Pulmonary Administration of siRNA and pDNA Powders: Naked siRNA Inhalation Powder Suppresses Luciferase Gene Expression in the Lung. J. Pharm. Sci. 2019, 108, 2661–2667. [Google Scholar] [CrossRef]
- Liang, W.; Chow, M.Y.; Lau, P.N.; Zhou, Q.T.; Kwok, P.C.; Leung, G.P.; Mason, A.J.; Chan, H.K.; Poon, L.L.; Lam, J.K. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol. Pharm. 2015, 12, 910–921. [Google Scholar] [CrossRef]
- Wu, J.; Wu, L.; Wan, F.; Rantanen, J.; Cun, D.; Yang, M. Effect of thermal and shear stresses in the spray drying process on the stability of siRNA dry powders. Int. J. Pharm. 2019, 566, 32–39. [Google Scholar] [CrossRef]
- Liang, W.; Chow, M.Y.; Chow, S.F.; Chan, H.-K.; Kwok, P.C.; Lam, J.K. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. Int. J. Pharm. 2018, 552, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kito, D.; Oiwa, A.; Fukushima, M.; Hira, D.; Okamoto, H. Gene silencing in a mouse lung metastasis model by an inhalable dry small interfering RNA powder prepared using the supercritical carbon dioxide technique. Biol. Pharm. Bull. 2013, 36, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, M.Y.; Qiu, Y.; Lo, F.F.; Lin, H.H.; Chan, H.K.; Kwok, P.C.; Lam, J.K. Inhaled powder formulation of naked siRNA using spray drying technology with L-leucine as dispersion enhancer. Int. J. Pharm. 2017, 530, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Polach, K.J.; Matar, M.; Rice, J.; Slobodkin, G.; Sparks, J.; Congo, R.; Rea-Ramsey, A.; McClure, D.; Brunhoeber, E.; Krampert, M.; et al. Delivery of siRNA to the mouse lung via a functionalized lipopolyamine. Mol. Ther. 2012, 20, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel, A.; Bigey, P.; Dhotel, H.; Scherman, D.; Escriou, V. Reduced in vitro and in vivo toxicity of siRNA-lipoplexes with addition of polyglutamate. J. Control. Release 2013, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Busignies, V.; Arruda, D.C.; Charrueau, C.; Ribeiro, M.C.S.; Lachages, A.M.; Malachias, A.; Finet, S.; Rehman, A.U.; Bigey, P.; Tchoreloff, P.; et al. Compression of Vectors for Small Interfering RNAs Delivery: Toward Oral Administration of siRNA Lipoplexes in Tablet Forms. Mol. Pharm. 2020, 17, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kim, N.H.; Nadithe, V.; Schalk, D.; Thakur, A.; Kılıç, A.; Lum, L.G.; Bassett, D.J.; Merkel, O.M. Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. J. Control. Release 2016, 229, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Ko, K.S.; Park, J.S.; Kim, Y.H.; Kim, S.W.; Lee, M. Dexamethasone conjugated poly (amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int. J. Pharm. 2006, 320, 171–178. [Google Scholar] [CrossRef]
- McKiernan, P.J.; Cunningham, O.; Greene, C.M.; Cryan, S.-A. Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int. J. Nanomed. 2013, 8, 3907–3915. [Google Scholar]
- Bielski, E.; Zhong, Q.; Mirza, H.; Brown, M.; Molla, A.; Carvajal, T.; da Rocha, S.R. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int. J. Pharm. 2017, 527, 171–183. [Google Scholar] [CrossRef]
- Okuda, T.; Morishita, M.; Mizutani, K.; Shibayama, A.; Okazaki, M.; Okamoto, H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J. Control. Release 2018, 279, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Cun, D.; Maltesen, M.J.; Frokjaer, S.; Nielsen, H.M.; Foged, C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J. Control. Release 2010, 142, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, O.; Nassar, T.; Benhamron, S.; Lazarovici, P.; Benita, S.; Yavin, E. Solid nano-in-nanoparticles for potential delivery of siRNA. J. Control. Release 2017, 257, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liu, Y.; Tang, Y.; Chen, J.; Li, S.; Pu, J.; Han, L. GABAB receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2018, 179, 135–144. [Google Scholar] [CrossRef]
- Mohamed, A.; Kunda, N.K.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Polymeric nanoparticles for the delivery of miRNA to treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Qiu, Y.; Chow, M.Y.; Liang, W.; Chung, W.W.; Mak, J.C.; Lam, J.K. From pulmonary surfactant, synthetic kl4 peptide as effective siRNA delivery vector for pulmonary delivery. Mol. Pharm. 2017, 14, 4606–4617. [Google Scholar] [CrossRef]
- Moschos, S.A.; Jones, S.W.; Perry, M.M.; Williams, A.E.; Erjefalt, J.S.; Turner, J.J.; Barnes, P.J.; Sproat, B.S.; Gait, M.J.; Lindsay, M.A. Lung delivery studies using siRNA conjugated to TAT (48−60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug. Chem. 2007, 18, 1450–1459. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Man, R.C.H.; Liao, Q.; Kung, K.L.K.; Chow, M.Y.T.; Lam, J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release 2019, 314, 102–115. [Google Scholar] [CrossRef]
- Lam, J.K.; Liang, W.; Chan, H.K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012, 64, 1–15. [Google Scholar] [CrossRef]
- Bohr, A.; Water, J.; Beck-Broichsitter, M.; Yang, M. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr. Pharm. Des. 2015, 21, 5829–5844. [Google Scholar] [CrossRef]
- Dua, K.; Wadhwa, R.; Singhvi, G.; Rapalli, V.; Shukla, S.D.; Shastri, M.D.; Gupta, G.; Satija, S.; Mehta, M.; Khurana, N.; et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev. Res. 2019, 80, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Griesenbach, U.; Kitson, C.; Garcia, E.S.; Farley, R.; Singh, C.; Somerton, L.; Painter, H.; Smith, R.L.; Gill, D.R.; Hyde, S.C. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir. Res. 2006, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Yıldız-Peköz, A.; Ehrhardt, C. Advances in Pulmonary Drug Delivery. Pharmaceutics 2020, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Kadam, S.; Pawar, A.; Bothiraja, C. Dendrimers for pulmonary delivery: Current perspectives and future challenges. New J. Chem. 2019, 43, 8396–8409. [Google Scholar] [CrossRef]
- Jensen, D.K.; Jensen, L.B.; Koocheki, S.; Bengtson, L.; Cun, D.; Nielsen, H.M.; Foged, C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release 2012, 157, 141–148. [Google Scholar] [CrossRef]
- Thanki, K.; van Eetvelde, D.; Geyer, A.; Fraire, J.; Hendrix, R.; Van Eygen, H.; Putteman, E.; Sami, H.; de Souza Carvalho-Wodarz, C.; Franzyk, H.; et al. Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration. J. Control. Release 2019, 310, 82–93. [Google Scholar] [CrossRef]
- Kumar, P.; Ban, H.S.; Kim, S.S.; Wu, H.; Pearson, T.; Greiner, D.L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T Cell-Specific siRNA Delivery Suppresses HIV-1 Infection in Humanized Mice. Cell 2008, 134, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Das, S.C.; Stewart, P.J. The influence of lung surfactant liquid crystalline nanostructures on respiratory drug delivery. Int. J. Pharm. 2016, 514, 465–474. [Google Scholar] [CrossRef]
- De Backer, L.; Naessens, T.; De Koker, S.; Zagato, E.; Demeester, J.; Grooten, J.; De Smedt, S.C.; Raemdonck, K. Hybrid pulmonary surfactant-coated nanogels mediate efficient in vivo delivery of siRNA to murine alveolar macrophages. J. Control. Release 2015, 217, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baoum, A.; Ovcharenko, D.; Berkland, C. Calcium condensed cell penetrating peptide complexes offer highly efficient, low toxicity gene silencing. Int. J. Pharm. 2012, 427, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Chen, W.L.; You, B.G.; Liu, Y.; Yang, S.D.; Li, J.Z.; Zhu, W.J.; Zhou, X.F.; Liu, C.; Zhang, X.N. Multifunctional nanoparticles co-delivering EZH2 siRNA and etoposide for synergistic therapy of orthotopic non-small-cell lung tumor. J. Control. Release 2017, 268, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Bonini, S.; Seeger, W.; Belvisi, M.G.; Ward, B.; Holmes, A. Barriers to new drug development in respiratory disease. Eur. Respir. J. 2015, 45, 1197–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Mode of Action | Representative Drugs | Adverse Effects | Ref. | |
|---|---|---|---|---|
| Corticosteroids | Corticosteroids bind to GRs in the target cell cytosol. The corticosteroid–GR complex binds to specific sequences on the upstream regulatory elements of target genes after translocation into the nucleus. GRs also interact with protein transcription factors and coactivator molecules in the nucleus, which regulate gene expression at a transcriptional level. | Oral corticosteroids (e.g., prednisone and prednisolone). | Fluid retention, increased appetite, weight gain, osteoporosis, capillary fragility, hypertension, peptic ulceration, diabetes mellitus, cataract, and psychosis (long-term oral corticosteroid therapy). Fail to reduce disease progression or mortality in COPD patients. High doses of ICSs increase the risk of pneumonia in most patients with COPD. | [14] |
| ICSs, e.g., beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone, and mometasone. | ||||
| β2 adrenoceptor agonists | Act via specific receptors (ADRβ2), which are localized mainly on airway smooth muscle cells. Binding to ADRβ2 by agonists causes activation of the Gs-adenylyl cyclase ecAMPePKA pathway, leading to bronchial smooth muscle relaxation. | SABA, e.g., salbutamol, and terbutaline. | Lack of selectivity for the β2 AR, resulting in “off-target” effects mediated by either α or β1 AR, or ill-defined β2 AR-mediated effects that appear to involve either β2 AR desensitization, or exacerbation of airway inflammation and its consequences. | [19,20] |
| LABA, e.g., formoterol, and salmeterol. | ||||
| Muscarinic receptor antagonists | Inhibit acetylcholine stimulation of muscarinic receptors, block airway smooth muscle contraction, and vagally induce increased mucus secretion. | SAMA, e.g., ipratropium bromide, and oxitropium bromide. | Dry mouth, bitter taste, and urinary retention. Systemic adverse effects are uncommon. | [21] |
| LAMA, e.g., aclidinium bromide, tiotropium bromide, glycopyrronium bromide, and umeclidinium bromide. | ||||
| Antileuko-trienes | Inhibit 5-lipoxygenase, prevent leukotriene synthesis, inhibit LTD4 binding to its receptor, and prevent its action. | Oral. Leukotriene-receptor antagonist zafirlukast. Leukotriene-synthesis inhibitor zileuton antileukotriene, montelukast. | Uncommon. | [22] |
| Antibiotics | Inhibit NF-κB and other transcription factors, resulting in reduction of chronic inflammation. The precise mechanism of action has not yet been determined. | Macrolides, e.g., erythromycin, azithromycin, and telithromycin. | Nausea and diarrhea are the most common gastrointestinal adverse effects. Macrolides prolong the corrected QT intervals on electrocardiograms, which increases the risk of torsades de pointes, potentially resulting in ventricular fibrillation and sudden death. Telithromycin rarely causes liver injury, with high morbidity and mortality rates. | [23,24] |
| Cromones | Delay activation of chloride channels in cell membranes. Inhibit cell activation. Inhibit both antigen- and exercise-induced asthma. | E.g., inhaled sodium cromoglicate, and nedocromil sodium. | Poorly absorbed, and serious adverse effects are rare. | [25] |
| Novel Inhaled Medicine | Drug | Mechanisms/Effects | Administration Route | ClinicalTrials.gov Identifier | Phase | Main Finding | Ref. |
|---|---|---|---|---|---|---|---|
| Antioxidants | NAC/glutamines | A reactive oxygen species scavenger and precursor of reducing glutathione | Oral | NCT01136239 | Phase 4 | Completed. High-dose NAC (600 mg bid) reduced COPD exacerbations and improved small airways function. | [51,52,53,54] |
| NCT00184977 | Phase 4 | Completed. Reduced the degree of deterioration in GOLDII-III COPD patients; high-dose NAC was not significantly beneficial in low-risk patients (600 mg/day) | |||||
| NCT00476736 | Phase 4 | Unknown. High-dose NAC (1200 mg daily) reduced gas trapping and improved exercise endurance in patients with emphysematous COPD. | |||||
| Catalase/SOD/GPx | Reduce ROS | Anti-inflammatory effects on smoking-induced lung inflammation in animal models | [55] | ||||
| Sulforaphane | Increases the gene expression of Nrf2 | Oral | NCT01335971 | Phase 2 | Completed. Sulforaphanetrial in COPD patients did not induce the expression of Nrf2 genes or affect the level of other antioxidants | [56] | |
| Protease inhibitors (MMPs) | AZ11557272 | MMP-9/12 inhibitor. Ameliorates emphysema | Ameliorate morphological emphysema, small airways remodeling, and the functional consequences of these lesions in a non-murine species | [57] | |||
| Multiple MMPs (MMP-1, -2, -3, -8, -9, and -10); cytokines (IL-6 and IL-8) | Degrade the extracellular matrix and drive tissue remodeling | NCT01701869 | Completed. MMP-3, -7, -8, -9, -10, and -12 concentrations closely associated with CT markers of small airways disease. Emphysema severity was also associated with MMP-3, -7 and -10. No strong relationships between MMPs and the bronchial wall thickness of larger airways | [58] | |||
| AZD1236 | MMP-9/12 inhibitor. Inhibits emphysema | Oral | NCT00758706 | Phase 2 | Completed. No clinical efficacy of AZD1236 was demonstrated | [59] | |
| FP-025 | MMP-12 inhibitor. Allergen-induced airway responses, inflammation, and remodeling | NCT03858686 | Phase 2 | Recruiting | |||
| Protease inhibitors (neutrophil elastase) | Sivelestat (ONO-5046) | Protect the lungs from tissue damage and control the exuberant inflammatory response | NCT00417326 | Phase 2 | Completed. ONO-5046 approved in Japan for the treatment of pneumonia and respiratory failure | ||
| AZD9668 combined with budesonide/formoterol | Reversible and selective inhibitor of neutrophil elastase | Oral | NCT01023516 | Phase 2 | Completed. AZD9668 did not significantly improve respiratory signs and symptoms | [60] | |
| Alvelestat (MPH966) | Bronchiolitis obliterans syndrome | NCT02669251 | Phases 1 and 2 | Recruiting | |||
| Cytokines/chemokines receptor inhibitors | Canakinumab | Inhibition of IL-1β, for inflammation and cardiovascular risk | Subcutaneous | NCT02272946 | Phase 2 | Recruiting | |
| Tocilizumab | Inhibition of IL-6, for rheumatoid arthritis and inflammation diseases | Subcutaneous | NCT03288584 | Phase 2 | Recruiting. Improved endothelial function led to a greater increase of effective myocardial function and reduced the inflammatory burden and oxidative stress | [61,62] | |
| Infliximab | Inhibition of TNF-α | NCT00056264 | Phase 3 | Completed. Patients with moderate to severe COPD did not benefit from treatment | [63] | ||
| Etanercept | Inhibition of TNF-α | Subcutaneous | NCT00789997 | Phases 2 and 3 | Completed. Etanercept is not more efficacious than prednisone for the treatment of COPD deterioration | ||
| Mepolizumab | Inhibition of IL-5 | Intravenous or subcutaneous | NCT01691521 | Phase 3 | Completed. Significantly reduced asthma exacerbations | [64] | |
| Reslizumab | Inhibition of IL-5 | Intravenous | NCT01287039 and NCT01285323 | Phase 3 | Completed. The use of reslizumab in patients with asthma and elevated blood eosinophil counts | [65,66] | |
| Benralizumab | Inhibition of IL-5 | Subcutaneous | NCT01238861 | Phase 2 | Completed. Benralizumab seems to reduce asthma exacerbations in adults with uncontrolled eosinophilic asthma and baseline blood eosinophils | [67] | |
| Dupilumab | Anti-IL-4 receptor α monoclonal antibody, inhibits IL-4 and IL-13 signaling | Subcutaneous | NCT01854047 | Phase 2 | Completed. Benefit patients with uncontrolled persistent asthma | [68,69,70,71] | |
| Dupilumab | NCT01312961 | Phase 2 | Completed. In patients with persistent, moderate-to-severe asthma, dupilumab therapy was associated with fewer asthma exacerbations with improved lung function and reduced Th2-associated inflammatory markers | ||||
| Dupilumab | NCT02414854 | Phase 3 | Completed. Dupilumab reduced severe exacerbation rates, improved FEV1 and asthma control, and suppressed type 2 inflammatory biomarkers in patients with uncontrolled, moderate-to-severe asthma with or without evidence of allergic asthma | ||||
| Cytokines/chemokines receptor inhibitors | SB010 (DNAzyme) | Therapeutic targeting of GATA3, which is an important transcription factor of the Th2 pathway | Inhalation | NCT01743768 | Phase 2 | Completed. Treatment with SB010 significantly attenuated late and early asthmatic responses after allergen provocation in patients with allergic asthma. Biomarker analysis showed attenuation of Th2-regulated inflammatory responses | [72] |
| Brodalumab (AMG 827) | Blocking IL-17 receptor signaling | Inhalation | NCT01199289 | Phase 2 | Completed. Ineffective in patients with severe asthma, although the subjects were not selected for neutrophilic inflammation | [73] | |
| AZD4818 | Inhibition of CCR1 | Inhalation | NCT00629239 | Phase 2 | Completed. Inhaled AZD4818 did not indicate a beneficial treatment effect | [74] | |
| AZD2423 | Inhibition of CCR1 | NCT01215279 | phase 2 | Completed | |||
| Navarixin (MK-7123) | Inhibition of CXCR2 | NCT01006616 and NCT00441701 | Phase 2 | Terminated. Improvement in FEV1 | [75] | ||
| AZD5069 | CXCR2 antagonist | Oral | NCT01233232 | Phase 2 | Completed. No safety issues and no increase in infection rates in dosage group compared with placebo | [76] | |
| Navarixin (SCH527123) | Binds with high affinities to human CXCR1 and CXCR2, which are the receptors for ligands including IL-8, GRO-α, and CXCL5 | Oral | NCT01006161 | Phase 2 | Withdrawn | [77] | |
| NCT01068145 | Phase 1 | Terminated. SCH527123 caused significant attenuation of ozone-induced airway neutrophilia in healthy subjects | |||||
| Cytokines/chemokines receptor inhibitors | BIIL 284 | Inhibition of LTB4 receptor | NCT02249247 and NCT02249338 | Phase 2 | Completed. No data published | ||
| Zileuton | Inhibition of 5-LO | NCT00493974 | Phase 3 | Terminated (lack of feasibility due to low recruitment). No significant improvement in the treatment of COPD patients with acute exacerbations | [78] | ||
| Inflammasome inhibitors | Canakinumab | A human anti-IL-1β monoclonal antibody | Intravenous infusion | NCT00581945 | Phases 1 and 2 | Completed. No statistical differences in FEV1 and FVC among canakinumab-treated and placebo-treated COPD patients | |
| MEDI8968 | Inhibits IL receptor 1 (IL-1α and IL-1β) | Intravenous infusion | NCT01448850 | Phase 2 | Completed. MEDI8968 did not produce statistically significant improvements in AECOPD rate, lung function, or quality of life | [79] | |
| MEDI2338 | A human anti-IL-18 monoclonal antibody | Intravenous infusion | NCT01322594 | Phase 1 | Completed. No statistical differences were observed between treated and placebo COPD patients | ||
| Kinase inhibitors (p38 MAPK inhibitors) | BIRB-796 and dexamethasone | Inhibit p38 MAPK | p38 MAPK activation in alveolar macrophages is corticosteroid-insensitive; combining a p38 MAPK inhibitor with a corticosteroid synergistically enhanced the anti-inflammatory effects on LPS-mediated cytokine production by alveolar macrophages from patients with COPD | [80] | |||
| Dilmapimod (SB-681323) | Inhibits p38 MAPK | Oral | NCT00564746, NCT00380133, NCT00439881 | Phase 1 | Completed. No results published | [79] | |
| NCT00144859 | Phase 2 | Completed. Inhibited TNF-α production after a single oral dose | |||||
| Losmapimod (GW856553) | Inhibits p38 MAPK | oral | NCT02993757 | Phase 2 | Completed | [81,82,83] | |
| NCT01541852 | Phase 2 | Completed. Discontinued: not effective in COPD | |||||
| NCT00642148 | Phase 2 | Completed. No significant effects on lung function or sputum neutrophils | |||||
| Acumapimod (BCT197) | Inhibits p38 MAPK | Oral | NCT0133209 | Phase 2 | Completed. Well tolerated. Repeated single-dose acumapimod showed a clinically relevant improvement in FEV1 over placebo on day 8 | [84] | |
| PH-797804 | Inhibits p38 MAPK | Oral | NCT00559910 | Phase 2 | Completed. Significantly improved lung function and dyspnoea in moderate-to-severe COPD but was discontinued | [85] | |
| p38α-ASO | Reduces p38α MAPK mRNA expression | Inhalation | The ASO significantly reduced OVA-induced increases in total cell counts, eosinophil counts, and IL-4, IL-5, and IL-13 levels in bronchoalveolar lavage fluid | [86] | |||
| PF-03715455 | Inhibits p38 MAPK | Inhalation | NCT02219048 and NCT02366637 | Phase 2 | Terminated | ||
| AZD7624 | Inhibits p38 MAPK | Inhalation | NCT02238483 and NCT02753764 | Phase 2 | Discontinued. Not effective in the treatment of COPD and asthma | [87] | |
| RV568 | Inhibits p38MAPK pathway | Inhalation | NCT01661244, NCT01867762 and NCT01475292 | Phases 1 and 2 | Completed. Significantly increased FEV1 and reduced sputum malondialdehyde and serum myeloperoxidase in COPD patients | ||
| Kinase inhibitors (P13K inhibitors) | Nemiralisib (GSK2269557) | Inhibits PI3Kδ | Inhalation | NCT02294734 | Phase 2 | Completed. Effective as placebo on FEV1 | [88,89] |
| NCT02130635 | Phase 2 | Completed. Acceptable safety profile for progression to larger study | |||||
| NCT02522299 | Phase 2 | Completed. Did not significantly improve FEV1 and the use of rescue medication in patients with acute exacerbation | |||||
| NCT03345407 | Phase 2 | Terminated. Unfavorable benefit–risk profile | |||||
| NCT03189589 | Phase 1 | Completed. Progression to phase 2 study supported | |||||
| TG100-115 | Selectively blocks PI3Kγ and PI3Kδ | Inhalation | Inhibited pulmonary neutrophils induced by intranasal LPS and smoke in mice with COPD | [90] | |||
| RV1729 | Inhibits PI3Kδ | NCT02140346 | Phase 1 | Completed. Limited efficacy data have been collected | |||
| AS605240 | Inhibits PI3Kγ | Oral | - | - | Prevented pulmonary fibrosis by suppressing inflammatory cell recruitment and production of inflammatory cytokines in bleomycin-induced pulmonary fibrosis | [91] | |
| RNA therapeutics | ALN-RSV01 | Regulating protein expression that is mediated by siRNA | Nasal spray | NCT00496821 NCT01065935 | Phase 2 | Completed. ALN-RSV01 has significant antiviral activity against human RSV infection | [92,93] |
| MRT5005 | mRNA encoding fully functional CFTR protein | Nebulization | NCT03375047 | Phases 1 and 2 | Recruiting. A marked improvement of lung function in patients after single dose at the mid-dose (8–16 mg) level | [94] | |
| Eluforsen | Single-stranded RNA ASO targeting CFTR | Intranasal in phase 1; Inhalation in phase 2 | NCT02564354 and NCT02532764 | Phases 1 and 2 | Completed. Safe, well tolerated, and improved respiratory symptoms |
| Type | Carrier | Drug | Key Excipients | Method of Preparation | z-Average (A) and MMAD (B) | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Microparticles | - | siRNA | Mannitol and HSA | Spray drying | B: 1.3–1.4 µm | Bioactivity of siRNA was preserved in RAW264.7 cells | [211] |
| - | eGFP siRNA | Mannitol | Spray drying | - | HPLC was used to evaluate chemical stability of siRNA; the thermal and shear stress of the spray drying could influence the chemical integrity of siRNA | [214] | |
| - | MCP-1 siRNA | Mannitol | Spray freeze drying | B: 4.0–4.5 µm | Biologically active in RAW264.7 (mouse macrophage-like cells) | [215] | |
| siRNA | Chitosan and mannitol | Supercritical carbon dioxide technique | B: 10–20 µm | Biologically active in mice bearing colon 26/Luc cells | [216] | ||
| - | siRNA targeting IL-10 | L-leucine and mannitol | Spray drying | B: 1.35–1.99 µm | Examined the integrity of siRNA by gel retardation assay | [217] | |
| Nanoembedded microparticles | Liposome | siRNA targeting GFP, CD31, CD45, and Tie-2 | Lipopolyamine (Staramine) | Film-rehydration method | - | Slower clearance rate from the lung tissue and gene knockdown in the lungs of normal mice. | [218] |
| siRNA specific to luciferase | 1. DMAPAP and DOPE 2. Trehalose or trehalose with mannitol | 1. Film-rehydration method [219] 2. Spray freeze drying | - | After compression into tablets, siRNAs retained more than 60% of their gene-silencing efficacy. | [220] | ||
| Polyplexes | siRNA | Tf-PEI (molar ratio of Tf to PEI was 1.5:1) | Incubated after mixing Tf-PEI with siRNA | A: 72–197 nm | Successful gene silencing in a murine model of allergic asthma. Well tolerated in healthy animals and no toxicity | [221] | |
| VDBP- siRNA | DEXA-PEI | Incubated after mixing VDBP- siRNA with DEXA-PEI. Modified from [222] | - | Reduced goblet cell hyperplasia, ovalbumin sensitization, challenge-induced enhancement of airway inflammation, expressions of interleukin-4 (IL-4), IL-13, and eosinophil mobilizing chemokine (CCL11) | [137] | ||
| MiR-126 | PEI or chitosan | Mixed and left on ice for 30 min | A: From 100 to 1000 nm, depending on the N/P ratio and diluent solvents | miRNA uptake is highly polymer-dependent; no direct correlation between the levels of miRNA and the downstream gene knockdown | [223] | ||
| Dendrimer | TNF-α siRNA | 1. PAMAM 2. Trehalose and inulin | 1. Bulk mixing and microfluidics 2. Spray drying | A: 87–103 nm B: ~5 µm | The integrity and gene silencing efficiency of siRNA was preserved | [136] | |
| eGFP siRNA | 1. PAMAM 2. Mannitol | 1. Vortex and incubate 2. Spray drying | A: 120–400 nm B: 3.8 ± 0.2 μm | Dendriplexes with the dendrimers containing the highest surface density of TPP and at N/P 30 showed the best gene knockdown efficiency | [224] | ||
| Polymeric nanoparticles | siRNA | 1. PEI 2. L-leucine | 1. Incubated after mixing siRNA with PEI 2. Spray freeze drying | A: ~190 nm B: ~10 µm | In vivo pulmonary gene silencing | [225] | |
| siRNA | 1. PLGA (molar ratio of 75:25, Mw: 20 kDa); 2. Trehalose, lactose, and mannitol. | 1. Modified-SESD; 2. Spray drying | B: 4.99 ± 0.15 µm | Biologically active of spray-dried siRNA using H1299 cells | [226] | ||
| siRNA | 1. PLGA (molar ratio of 50:50, Mw: 100 kDa), DOTAP and HSA | 1. Desolvation 2. Spray drying | A: 100 nm B: 580–770 nm by SEM | Biologically active of spray-dried siRNA using A549 cells | [227] | ||
| Survivin siRNA | 1. Bac-TMC, TPP 2. Mannitol | 1. Formed spontaneously by electrostatic interaction 2. Spray drying | A: 232 nm B: 3.64 ± 0.06 µm | Biologically active of spray-dried siRNA using A549 cells | [228] | ||
| LPNs | eGFP siRNA | 1. PLGA, DOTAP, PVA 2. Mannitol | 1. DESE 2. Spray drying | A: 261.1 nm B: 3.69 ± 0.18 µm | Preserve the integrity of the siRNA and the gene silencing activity of the siRNA-loaded PLGA nanoparticles. | [90] | |
| TNF-α siRNA | 1. Penta-substituted lipidoid, PLGA, PVA 2. Mannitol | 1. DESE 2. Spray drying | A: 197.1 ± 3.0 nm B: 3.3 ± 0.2 µm | The chemical stability of the siRNA was preserved upon spray drying with high loading. Data collected for mRNA expression after transfection in RAW 264.7 cells showed efficient gene silencing after spray drying | [138,209] | ||
| miR-146a | PGA-co-PDL and DOTAP | Oil-in-water (o/w) single emulsion method | A: 244.8 ± 4.4 nm | miR-146a retained biological activity in vitro with 40% reduced IRAK1 expression and reduced IL-8 promoter reporter GFP | [229] | ||
| miR-17 | 1. PLGA and DOTAP 2. Trehalose | 1. DESE 2. Freeze drying | A: 208.0 ± 16.7 nm B: 4.20 ± 0.05 µm (nebulization) | Downregulated LPS-induced IL-8 secretion by >40% in bronchial epithelial cells | [148] | ||
| Nanoembedded Microparticles | Surfactant protein-based nanoparticles | siRNA | SP-B mimic, synthetic KL4 peptide | Incubated after mixing siRNA with KL14 | A: 280–460 nm | It mediated siRNA transfection effectively in vitro in human lung epithelial cells, A549 cells, and BEAS-2B cells | [230] |
| CPP based nanoparticles | siRNA | Cholesterol, TAT (48–60) | - | - | siRNA-mediated mRNA knockdown of p38 MAPK in mouse lung | [231] | |
| mRNA | 1. PEG12KL4 2. Mannitol | 1. Incubated after mixing siRNA with PEG12KL4 2. Spray drying or spray freeze drying | A: 467.9 ± 24.9 nm B: less than 5 µm | Effective transfection in the lung when administered intratracheally either as liquid or powder, with low risk of inflammatory response and toxicity | [232] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Thakur, A.; Zhang, Y.; Foged, C. Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects. Pharmaceutics 2021, 13, 177. https://doi.org/10.3390/pharmaceutics13020177
Xu Y, Thakur A, Zhang Y, Foged C. Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects. Pharmaceutics. 2021; 13(2):177. https://doi.org/10.3390/pharmaceutics13020177
Chicago/Turabian StyleXu, You, Aneesh Thakur, Yibang Zhang, and Camilla Foged. 2021. "Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects" Pharmaceutics 13, no. 2: 177. https://doi.org/10.3390/pharmaceutics13020177
APA StyleXu, Y., Thakur, A., Zhang, Y., & Foged, C. (2021). Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects. Pharmaceutics, 13(2), 177. https://doi.org/10.3390/pharmaceutics13020177








