Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
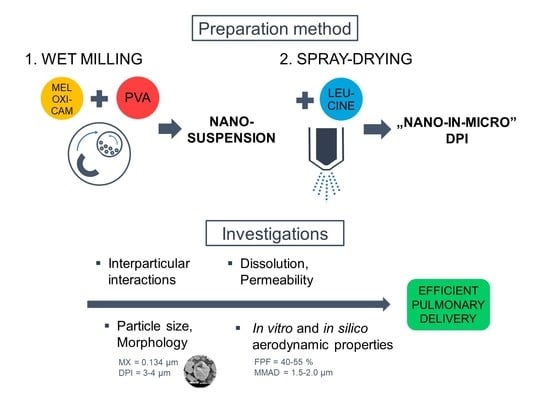
2.2. Preparation Method
2.2.1. Wet Milling
2.2.2. Co-Spray Drying
2.2.3. Physical Mixtures
2.3. Determination of Particle Size and Distribution
2.4. Investigation of Morphology
2.5. Density Measurement
2.6. Determination of the Interparticle Interactions
2.7. Structural Analysis
2.8. Thermoanalitycal Analysis
2.9. In Vitro Dissolution Test
2.10. In Vitro Diffusion Test
2.11. In Vitro Aerodynamic Measurements
2.12. In Silico Characterization
3. Results
3.1. Particle Size Distribution
3.2. Particle Morphology
3.3. Powder Rheology
3.4. Interparticular Interactions
3.5. X-ray Powder Diffraction Results
3.6. Thermoanalytical Results
3.7. In Vitro Dissolution Results
3.8. In Vitro Permeability Results
3.9. In Vitro Aerodynamic Results
3.10. In Silico Aerodynamic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sou, T.; Bergström, C.A.S. Contemporary formulation development for inhaled pharmaceuticals. J. Pharm. Sci. 2021, 110, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Darquenne, C. Aerosol deposition in health and disease. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruge, C.C.; Kirch, J.; Lehr, C.M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Kachrimanis, K.; Buckton, G.; Taylor, K.M.G. Preparation of respirable nanoparticle agglomerates of the low melting and ductile drug ibuprofen: Impact of formulation parameters. Powder Technol. 2017, 308, 123–134. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Meloxicam (accessed on 28 January 2021).
- Szabó-Révész, P. Modifying the physicochemical properties of NSAIDs for nasal and pulmonary administration. Drug Discov. Today Technol. 2018, 27, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafa, H.M.M.; Abdel-Wahab, M.H.; El-Shafeey, M.F.; Badary, O.A.; Hamada, F.M.A. Anti-fibrotic effect of meloxicam in a murine lung fibrosis model. Eur. J. Pharmacol. 2007, 564, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, H.; Kanazawa, K.; Ishida, T.; Oizumi, S.; Shinagawa, N.; Sukoh, N.; Harada, M.; Ogura, S.; Munakata, M.; Dosaka-Akita, H.; et al. Cyclooxygenase-2 inhibitors for non-small-cell lung cancer: A phase II trial and literature review. Mol. Clin. Oncol. 2014, 2, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.; Porter, S.; Rozenberg, D.; O’Connor, E.; Lee, T.; Balter, M.; Wentlandt, K. Chronic obstructive pulmonary disease: A palliative medicine review of the disease, its therapies, and drug interactions. J. Pain Symptom Manag. 2020, 60, 135–150. [Google Scholar] [CrossRef]
- Bartos, C.; Szabó-Révész, P.; Bartos, C.; Katona, G.; Jójárt-Laczkovich, O.; Ambrus, R. The effect of an optimized wet milling technology on the crystallinity, morphology and dissolution properties of micro- and nanonized meloxicam. Molecules 2016, 21, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomázi, A.; Buttini, F.; Ambrus, R.; Colombo, P.; Szabó-Révész, P. Effect of polymers for aerolization properties of mannitol-based microcomposites containing meloxicam. Eur. Polym. J. 2013, 49, 2518–2527. [Google Scholar] [CrossRef]
- Feng, A.L.; Boraey, M.A.; Gwin, M.A.; Finlay, P.R.; Kuehl, P.J.; Vehring, R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.K. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef]
- Chvatal, A.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Aerodynamic properties and in silico deposition of meloxicam potassium incorporated in a carrier-free DPI pulmonary system. Int. J. Pharm. 2017, 520, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, A.; Ambrus, R.; Party, P.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Fattal, E.; Tsapis, N. Formulation and comparison of spray dried non-porous and large porous particles containing meloxicam for pulmonary drug delivery. Int. J. Pharm. 2019, 559, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Seville, P.C.; Learoyd, T.P.; Li, H.Y.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van der Waals force in powder flowability and compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and HealthCare. 2.9. Bulk density and tapped density of powders. In European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2020; p. 359. [Google Scholar]
- Ambrus, R.; Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P. Novel dry powder inhaler formulation containing antibiotic using combined technology to improve aerodynamic properties. Eur. J. Pharm. Sci. 2018, 123, 20–27. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines and HealthCare. 2.9. Dissolution test for solid dosage forms. In European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2010; p. 228. [Google Scholar]
- Fröhlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Front. Pharmacol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parlati, C. Respirable Microparticles of Aminoglycoside Antibiotics for Pulmonary Administration. Ph.D. Thesis, University of Parma, Parma, Italy, 2008. [Google Scholar]
- May, S. Dissolution Testing of Powders for Inhalation. Ph.D. Thesis, Faculty of Natural Sciences and Technology, Saarbrücken, Germany, 2013; pp. 8–11. [Google Scholar]
- Boarder, M.; Dixon, J.; Newby, D.; Navti, P.; Zetterström, T. Pharmacology for Pharmacy and the Health Sciences; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Chvatal, A.; Alzhrani, R.; Tiwari, A.K.; Ambrus, R.; Szabó-Révész, P.; Boddu, S.H.S. Cytotoxicity of inhalable dry powders in A549 human lung cancer cell line. Farmacia 2018, 66, 172–175. [Google Scholar]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by design based formulation study of meloxicam-loaded polymeric micelles for intranasal administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and HealthCare. 2.9. Preparations for inhalation: Aerodynamic assessment of fine particles. In European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2005; p. 323. [Google Scholar]
- Buttini, F.; Colombo, G.; Kwok, P.C.L.; Wui, W.T. Aerodynamic assessment for inhalation products: Fundamentals and current pharmacopoeial methods. Inhal. Drug Deliv. Tech. Prod. 2013, 91–119. [Google Scholar] [CrossRef]
- Chapman, K.R.; Fogarty, C.M.; Peckitt, C.; Lassen, C.; Jadayel, D.; Dederichs, J.; Dalvi, M.; Kramer, B. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int. J. COPD 2011, 6, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Benke, E.; Farkas, Á.; Szabó-révész, P.; Ambrus, R. Development of an innovative, carrier-based dry powder inhalation formulation containing spray-dried meloxicam potassium to improve the in vitro and in silico aerodynamic properties. Pharmaceutics 2020, 12, 535. [Google Scholar] [CrossRef]
- Cunha, L.; Rodrigues, S.; da Costa, A.M.R.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable fucoidan microparticles combining two antitubercular drugs with potential application in pulmonary tuberculosis therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Raabe, O.G.; Yeh, H.; Schum, G.M.; Phalen, R.F. Tracheobronchial Geometry: Human, Dog, Rat, Hamster—A Compilation of Selected Data from the Project Respiratory Tract Deposition Models; Lovelace Foundation for Medical Education and Research: Albuquerque, NM, USA, 1976; Volume 741, pp. 1–11. [Google Scholar]
- Koblinger, L.; Hofmann, W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J. Aerosol Sci. 1990, 21, 661–674. [Google Scholar] [CrossRef]
- Farkas, Á.; Jókay, Á.; Füri, P.; Balásházy, I.; Müller, V.; Odler, B.; Horváth, A. Computer modelling as a tool in characterization and optimization of aerosol drug delivery. Aerosol Air Qual. Res. 2015, 15, 2466–2474. [Google Scholar] [CrossRef]
- Farkas, Á.; Jókay, Á.; Balásházy, I.; Füri, P.; Müller, V.; Tomisa, G.; Horváth, A. Numerical simulation of emitted particle characteristics and airway deposition distribution of Symbicort® Turbuhaler® dry powder fixed combination aerosol drug. Eur. J. Pharm. Sci. 2016, 93, 371–379. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Tan, G.; Gagenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A. Relationship between surface concentration of L -leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Focaroli, S.; Mah, P.T.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef]
- Bosquillon, C.; Lombry, C.; Préat, V.; Vanbever, R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release 2001, 70, 329–339. [Google Scholar] [CrossRef]
- Azari, F.; Ghanbarzadeh, S.; Safdari, R.; Yaqoubi, S.; Adibkia, K.; Hamishehkar, H. Development of a carrier free dry powder inhalation formulation of ketotifen for pulmonary drug delivery. Drug Res. 2020, 70, 26–32. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines and HealthCare. 2.9. Powder Flow. In European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2010; Volume 3, p. 346. [Google Scholar]
- Aytekin, Y.S.; Köktürk, M.; Zaczek, A.; Korter, T.M.; Heilweil, E.J.; Esenturk, O. Optical properties of Meloxicam in the far-infrared spectral region. Chem. Phys. 2018, 512, 36–43. [Google Scholar] [CrossRef]
- Najafabadi, A.R.; Gilani, K.; Barghi, M.; Rafiee-tehrani, M. The effect of vehicle on physical properties and aerosolisation behaviour of disodium cromoglycate microparticles spray dried alone or with l -leucine. Int. J. Pharm. 2004, 285, 97–108. [Google Scholar] [CrossRef]
- Williams, R.O.; Carvalho, T.C.; Peters, J.I. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Mitchell, J.; Newman, S.; Chan, H.K. In vitro and in vitro aspects of cascade impactor tests and inhaler performance: A review. AAPS PharmSciTech 2007, 8, 237–248. [Google Scholar] [CrossRef]







| Samples | MX (g/L) | PVA (g/L) | LEU (g/L) | Yield * (%) |
|---|---|---|---|---|
| nanoMX1_LEU0 | 4.00 | 0.90 | 0.00 | 45.41 ± 5.10 |
| nanoMX1_LEU0.5 | 4.00 | 0.90 | 2.00 | 57.56 ± 1.36 |
| nanoMX1_LEU1 | 4.00 | 0.90 | 4.00 | 58.43 ± 6.36 |
| Samples | MX (g) | PVA (g) | LEU (g) |
|---|---|---|---|
| pmMX1_LEU0 | 4.00 | 0.90 | 0.00 |
| pmMX1_LEU0.5 | 4.00 | 0.90 | 2.00 |
| pmMX1_LEU1 | 4.00 | 0.90 | 4.00 |
| ACI Stages | Cut-Off Diameter at 28.3 L/min (µm) |
|---|---|
| 0 | 9.0–10.0 |
| 1 | 5.8–9.0 |
| 2 | 4.7–5.8 |
| 3 | 3.3–4.7 |
| 4 | 2.1–3.3 |
| 5 | 1.1–2.1 |
| 6 | 0.7–1.1 |
| 7 | 0.4–0.7 |
| Filter | <0.4 |
| Samples | D[0.1] * (µm) | D[0.5] * (µm) | D[0.9] * (µm) | Span * | SSA * (m2/g) |
|---|---|---|---|---|---|
| raw MX | 2.719 ± 0.057 | 9.913 ± 0.371 | 29.49 ± 0.630 | 2.70 ± 0.043 | 1.09 ± 0.028 |
| MX suspension | 0.067 ± 0.001 | 0.138 ± 0.005 | 0.555 ± 0.310 | 3.584 ± 2.056 | 43.65 ± 5.318 |
| pmMX1_LEU0 | 3.073 ± 0.030 | 13.10 ± 0.500 | 349.92 ± 34.86 | 26.47 ± 1.649 | 0.88 ± 0.025 |
| pmMX1_LEU0.5 | 5.426 ± 0.631 | 91.22 ± 17.90 | 357.57 ± 168.2 | 3.86 ± 1.101 | 0.40 ± 0.066 |
| pmMX1_LEU1 | 7.983 ± 0.092 | 110.67 ± 0.261 | 353.25 ± 47.24 | 3.12 ± 0.433 | 0.27 ± 0.002 |
| nanoMX1_LEU0 | 1.497 ± 0.046 | 3.186 ± 0.019 | 6.481 ± 0.193 | 1.56 ± 0.068 | 2.22 ± 0.031 |
| nanoMX1_LEU0.5 | 1.834 ± 0.007 | 3.800 ± 0.014 | 7.389 ± 0.030 | 1.46 ± 0.004 | 1.88 ± 0.024 |
| nanoMX1_LEU1 | 1.977 ± 0.093 | 4.396 ± 0.032 | 8.903 ± 0.186 | 1.58 ± 0.075 | 1.71 ± 0.051 |
| Samples | D * (nm) | SEM Pictures | ||
|---|---|---|---|---|
| nanoMX1_LEU0 | 134.30 ± 23.07 |  |  |  |
| nanoMX1_LEU0.5 | 126.57 ± 27.26 |  |  |  |
| nanoMX1_LEU1 | 138.27 ± 42.57 |  |  |  |
| Samples | Bulk Density * (g/cm3) | Tapped Density * (g/cm3) | Hausner Ratio * | Carr Index * | Flowability |
|---|---|---|---|---|---|
| nanoMX1_LEU0 | 0.177 ± 0.020 | 0.262 ± 0.001 | 1.488 ± 0.048 | 32.39 ± 7.232 | Very poor |
| nanoMX1_LEU0.5 | 0.156 ± 0.009 | 0.274 ± 0.004 | 1.759 ± 0.084 | 43.09 ± 2.704 | Very, very poor |
| nanoMX1_LEU1 | 0.147 ± 0.013 | 0.204 ± 0.012 | 1.398 ± 0.209 | 27.65 ± 10.82 | Very poor |
| Samples | γd * [mN/m] | γp * [mN/m] | γ * [mN/m] | Wc * [mN/m] | Pol * [%] |
|---|---|---|---|---|---|
| MX | 45.49 ± 0.09 | 13.89 ± 0.13 | 59.38 ± 0.22 | 118.76 ± 0.44 | 23.39 ± 0.15 |
| PVA | 45.65 ± 0.10 | 36.89 ± 0.20 | 82.54 ± 0.30 | 165.08 ± 0.60 | 44.69 ± 0.11 |
| LEU | 30.00 ± 0.07 | 0.50 ± 0.17 | 30.50 ± 0.24 | 61.00 ± 0.48 | 1.639 ± 0.20 |
| pmMX1_LEU0 | 42.62 ± 0.12 | 30.65 ± 0.48 | 73.27 ± 0.60 | 146.54 ± 1.20 | 41.83 ± 0.56 |
| pmMX1_LEU0.5 | 36.57 ± 0.34 | 25.63 ± 0.27 | 62.20 ± 0.61 | 124.40 ± 1.22 | 41.21 ± 0.84 |
| pmMX1_LEU1 | 34.01 ± 0.55 | 16.57 ± 0.36 | 50.58 ± 0.91 | 101.16 ± 1.82 | 32.76 ± 0.44 |
| nanoMX1_LEU0 | 42.34 ± 0.08 | 31.03 ± 0.62 | 73.38 ± 0.70 | 146.76 ± 1.40 | 42.29 ± 0.44 |
| nanoMX1_LEU0.5 | 36.15 ± 0.95 | 25.69 ± 0.45 | 61.84 ± 0.51 | 123.68 ± 1.02 | 41.54 ± 1.07 |
| nanoMX1_LEU1 | 33.39 ± 0.86 | 16.59 ± 0.11 | 49.98 ± 0.97 | 99.96 ± 1.94 | 33.19 ± 0.43 |
| Samples | J (µg/cm2/h) | Kp (cm/h) |
|---|---|---|
| rawMX | 28.23 | 0.1394 |
| pmMX1_LEU0 | 34.69 | 0.2081 |
| pmMX1_LEU0.5 | 37.45 | 0.2247 |
| pmMX1_LEU1 | 33.25 | 0.1995 |
| nanoMX1_LEU0 | 61.80 | 0.3708 |
| nanoMX1_LEU0.5 | 86.90 | 0.5214 |
| nanoMX1_LEU1 | 73.58 | 0.4415 |
| Samples | MMAD * (µm) | FPD * (mg) | FPF * (%) | ED * (mg) | EF * (%) | Loaded API * (mg) | API content * (%) |
|---|---|---|---|---|---|---|---|
| nanoMX1_LEU0 | 2.33 ± 0.08 | 4.52 ± 0.33 | 75.67 ± 3.46 | 5.98 ± 0.22 | 72.42 ± 3.05 | 8.26 ± 0.14 | 93.81 ± 2.99 |
| nanoMX1_LEU0.5 | 1.74 ± 0.35 | 3.09 ± 0.31 | 72.81 ± 1.46 | 4.24 ± 0.34 | 83.47 ± 1.33 | 5.07 ± 0.33 | 55.48 ± 0.78 |
| nanoMX1_LEU1 | 1.55 ± 0.06 | 2.51 ± 0.04 | 73.63 ± 0.96 | 3.40 ± 0.10 | 75.22 ± 1.75 | 4.53 ± 0.23 | 51.46 ± 0.66 |
| Samples | Deposited Fraction * (%) | |||
|---|---|---|---|---|
| Extrathoracic | Lung | Bronchial | Acinar | |
| nanoMX1_LEU0 | 21.41 ± 2.79 | 46.73 ± 2.21 | 17.92 ± 2.93 | 28.81 ± 2.22 |
| nanoMX1_LEU0.5 | 14.45 ± 0.95 | 27.55 ± 0.99 | 10.72 ± 1.30 | 16.83 ± 1.34 |
| nanoMX1_LEU1 | 10.07 ± 0.47 | 22.44 ± 0.31 | 8.64 ± 0.54 | 13.80 ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics 2021, 13, 211. https://doi.org/10.3390/pharmaceutics13020211
Party P, Bartos C, Farkas Á, Szabó-Révész P, Ambrus R. Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics. 2021; 13(2):211. https://doi.org/10.3390/pharmaceutics13020211
Chicago/Turabian StyleParty, Petra, Csilla Bartos, Árpád Farkas, Piroska Szabó-Révész, and Rita Ambrus. 2021. "Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam" Pharmaceutics 13, no. 2: 211. https://doi.org/10.3390/pharmaceutics13020211
APA StyleParty, P., Bartos, C., Farkas, Á., Szabó-Révész, P., & Ambrus, R. (2021). Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics, 13(2), 211. https://doi.org/10.3390/pharmaceutics13020211