Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants
Abstract
:1. Introduction
2. Results
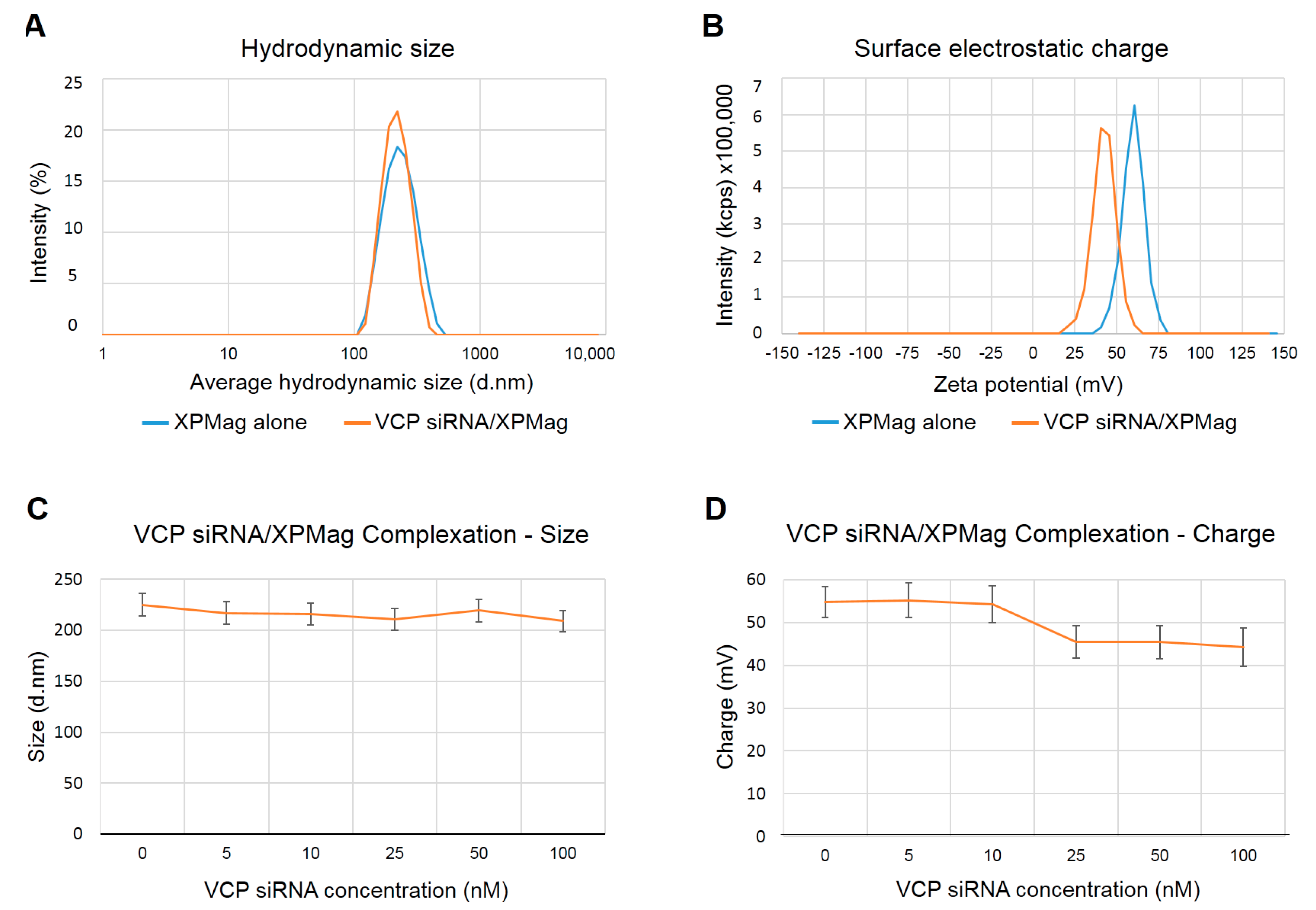
2.1. Characterization of VCP siRNA/XPMag Complexes
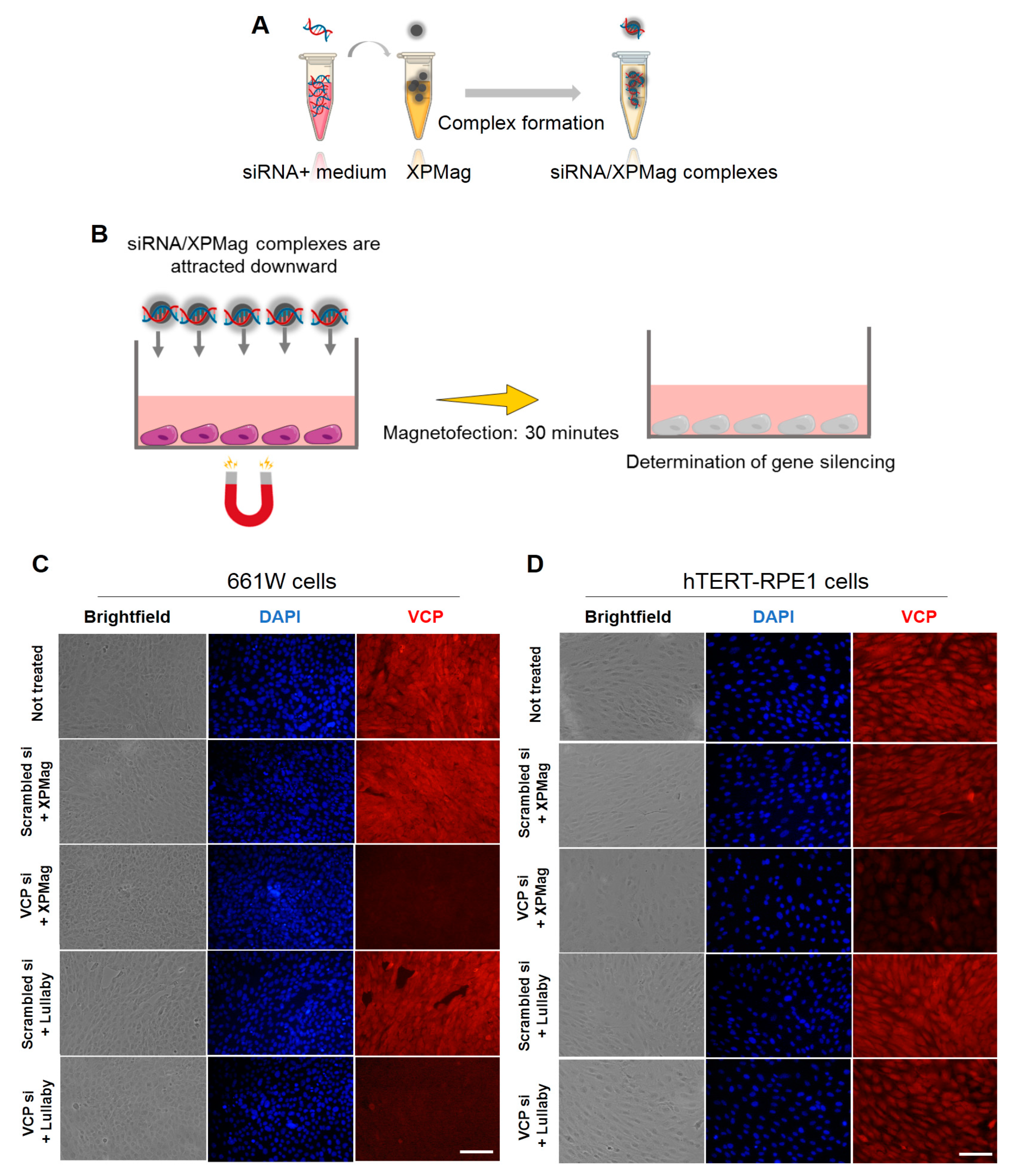
2.2. Classic Magnetofection to Silence VCP in Retinal Cell Lines
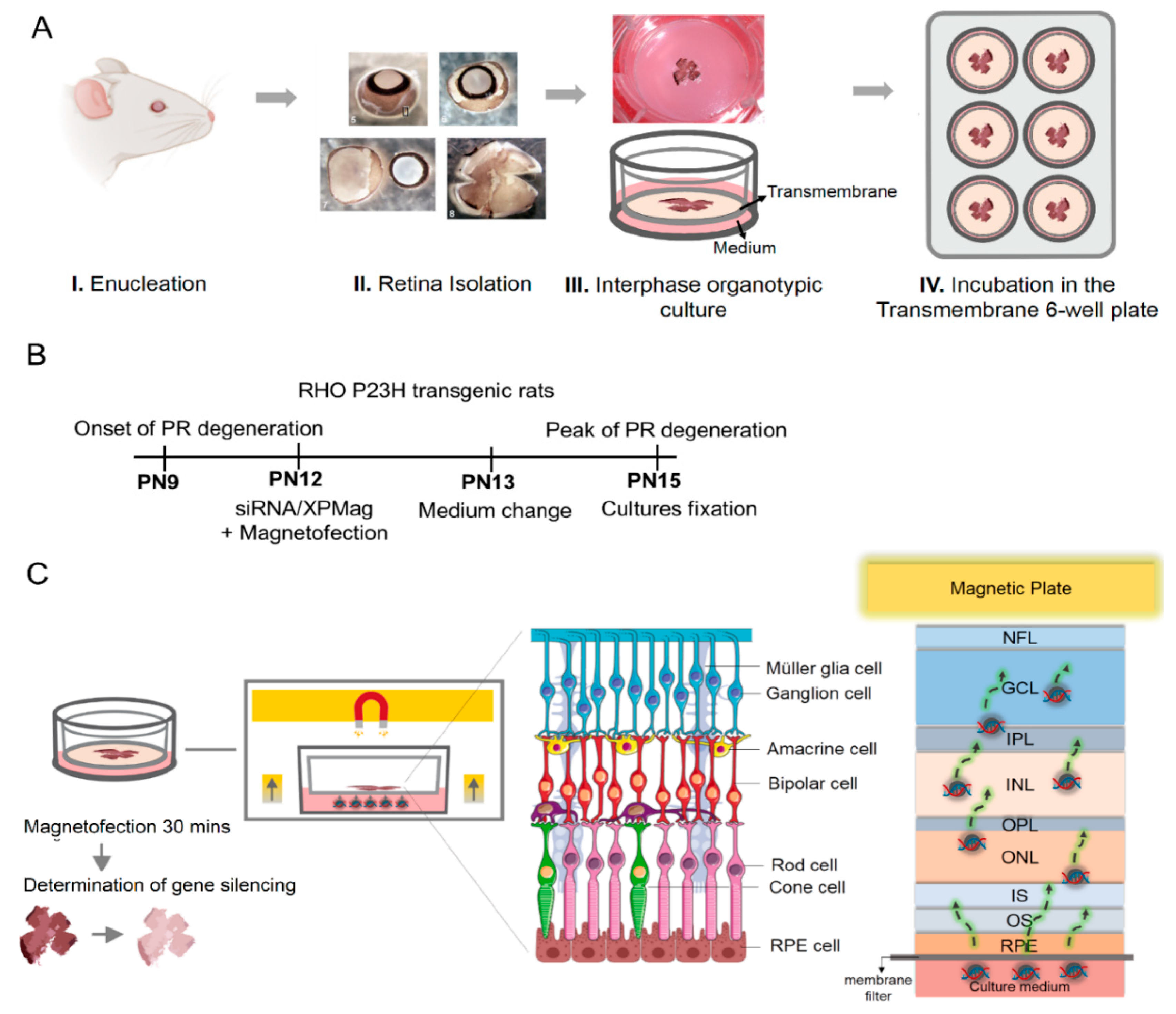
2.3. VCP Silencing in Retina Organotypic Cultures of RHO P23H Transgenic Rats by Reverse Magnetofection
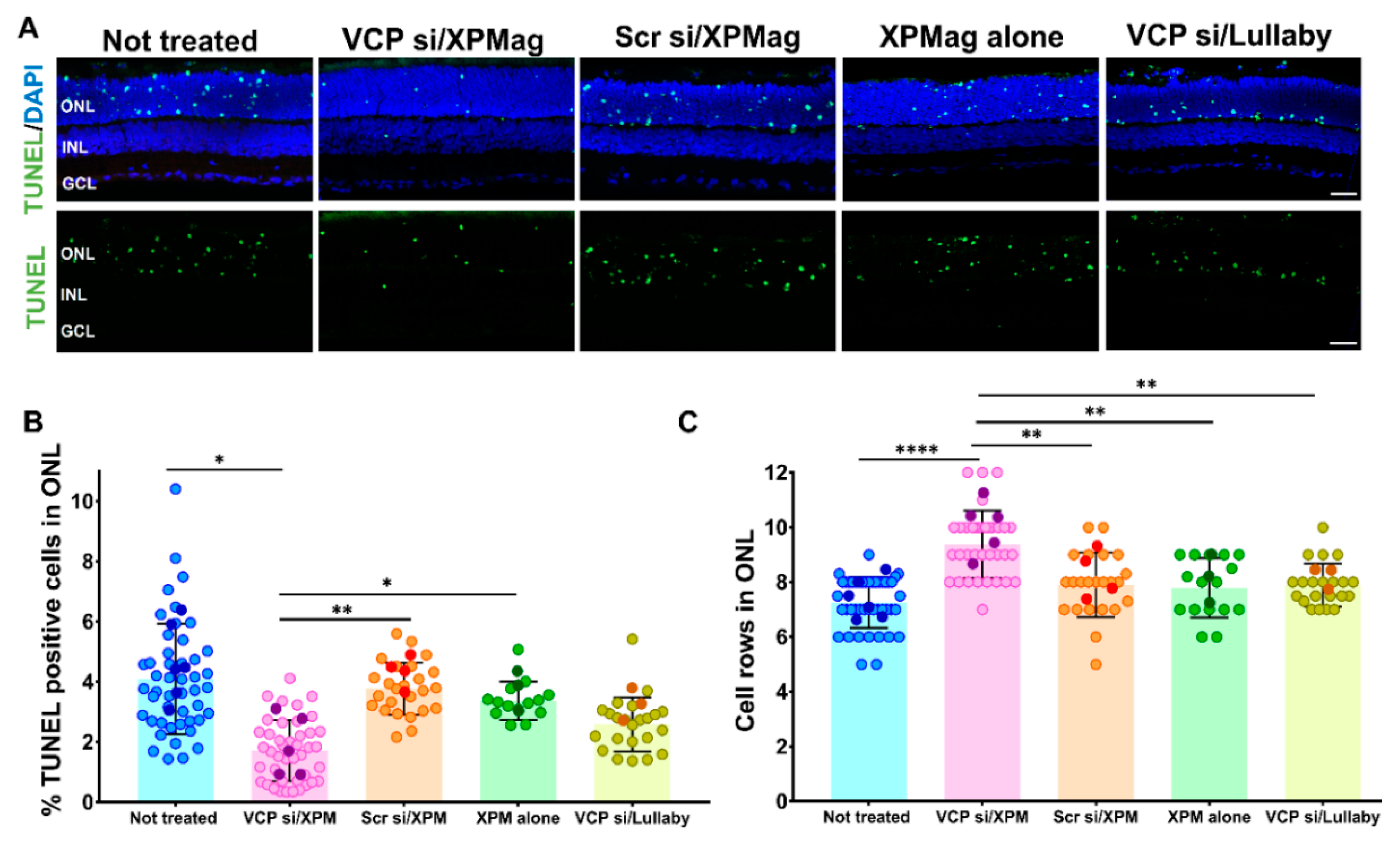
2.4. Reverse Magnetofection Is Well Tolerated, and VCP Silencing Shows a Neuroprotective Effect in RHO P23H Organotypic Cultures
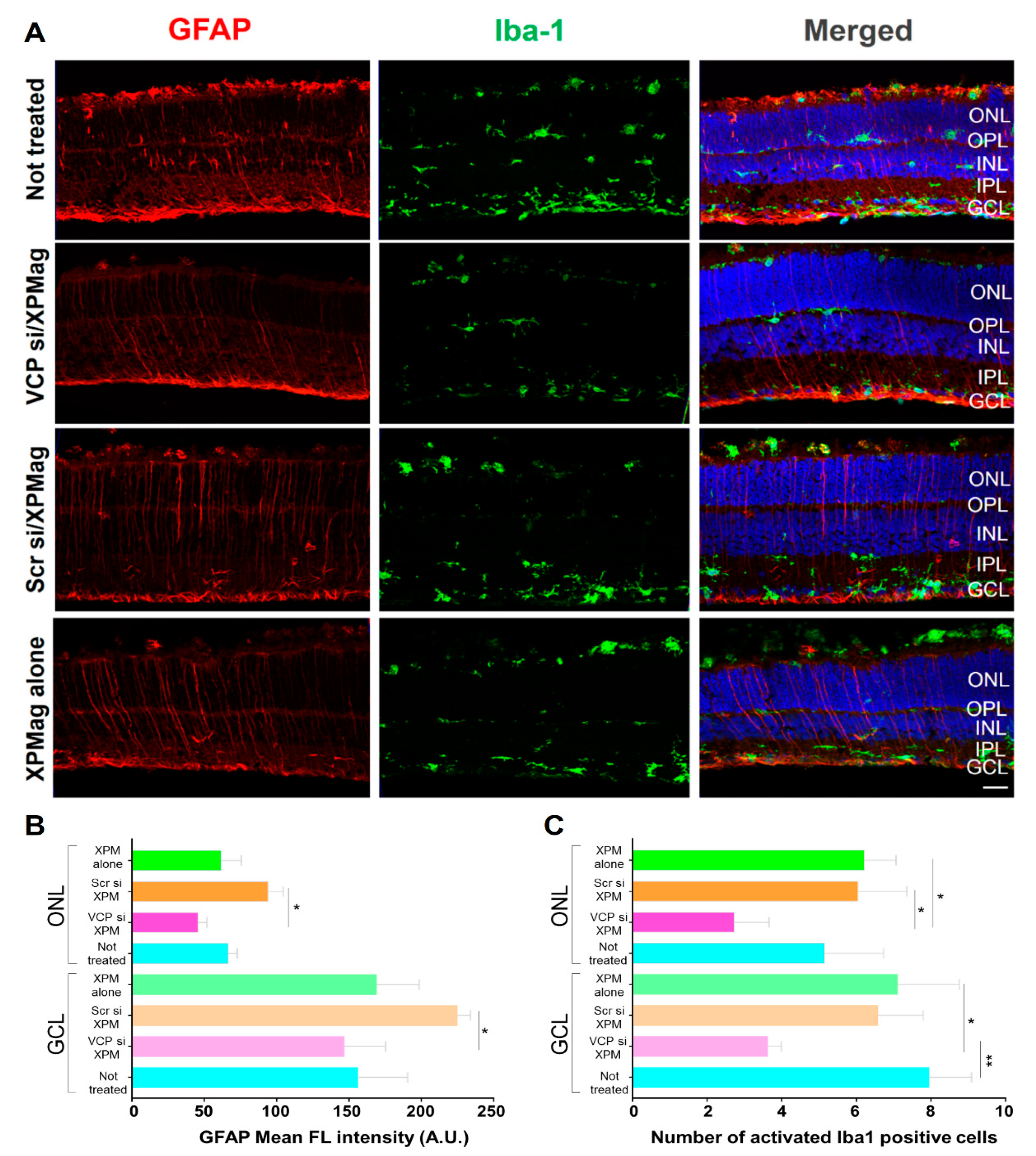
2.5. Effects of Reverse Magnetofection on Astrocytes, Müller Cells, and Microglial Cells in RHO P23H Retina Organotypic Cultures
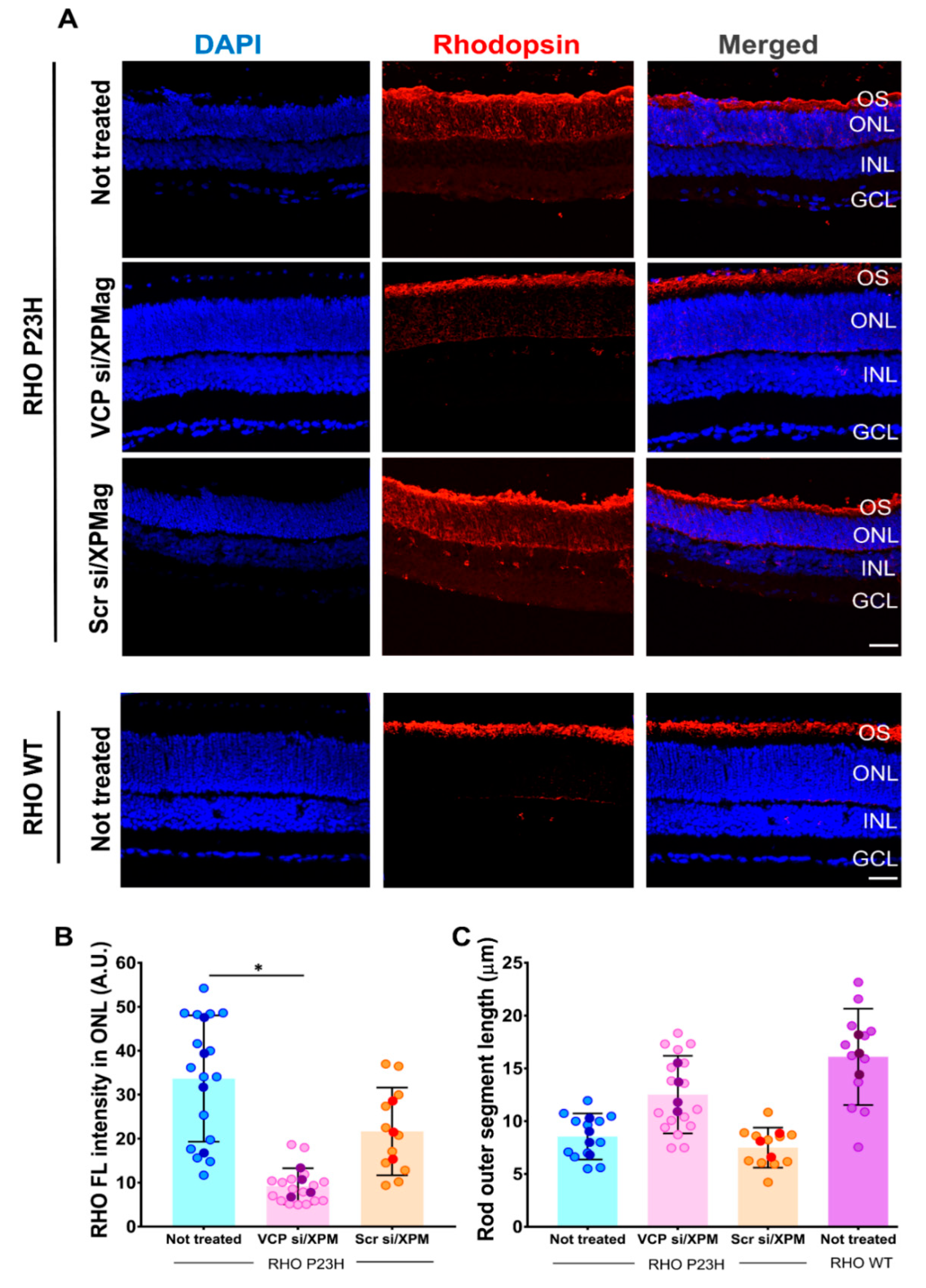
2.6. VCP Silencing through Reverse Magnetofection Restores RHO Intracellular Distribution
3. Discussion
4. Material and Methods
4.1. Study Approval and Animals
4.2. XPMag Synthesis and Characterization of VCP siRNA/XPMag Complex
4.3. Transmission Electron Microscopy (TEM)
4.4. Cell Culture and Transfections
4.5. Organotypic Culture of Explants
4.6. Classical Magnetofection of VCP siRNA to Immortalized Mouse 661W Retinal and Human hTERT-RPE1 Cell Lines
4.7. Classic-, Reverse Magnetofection and Lipofection of VCP siRNA in RHO P23H Rat Retinal Explants
4.8. Evaluation of VCP Silencing by Immunocytochemistry (ICC) in Retinal Cell Lines
4.9. Evaluation of VCP Silencing by Western Blotting in Human hTERT-RPE1 Cell Line
4.10. Preparation of Retinal Sections and Immunohistochemistry for Frozen Retinal Slides
4.11. TUNEL Assay
4.12. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman-Aranguez, A.; Loma, P.; Pintor, J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013, 170, 730–747. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Titze-de-Almeida, R.; David, C.; Titze-de-Almeida, S.S. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm. Res. 2017, 34, 1339–1363. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Martínez, T.; González, M.V.; Roehl, I.; Wright, N.; Pañeda, C.; Jiménez, A.I. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol. Ther. 2014, 22, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Solano, E.C.R.; Kornbrust, D.J.; Beaudry, A.; Foy, J.W.D.; Schneider, D.J.; Thompson, J.D. Toxicological and pharmacokinetic properties of QPI-1007, a chemically modified synthetic siRNA targeting caspase 2 mRNA, following intravitreal injection. Nucleic Acid Ther. 2014, 24, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Dejneka, N.S.; Wan, S.; Bond, O.S.; Kornbrust, D.J.; Reich, S.J. Ocular biodistribution of bevasiranib following a single intravitreal injection to rabbit eyes. Mol. Vis. 2008, 14, 997–1005. [Google Scholar]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T. Risks of intravitreous injection: A comprehensive review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Seigel, G.M. The golden age of retinal cell culture. Mol. Vis. 1999, 5, 4. [Google Scholar]
- Arango-Gonzalez, B.; Szabó, A.; Pinzon-Duarte, G.; Lukáts, Á.; Guenther, E.; Kohler, K. In vivo and in vitro Development of S- and M-Cones in Rat Retina. Investig. Opthalmol. Vis. Sci. 2010, 51, 5320. [Google Scholar] [CrossRef] [Green Version]
- del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Sekimukai, D.; Honda, S.; Negi, A. RNA interference for apoptosis signal-regulating kinase-1 (ASK-1) rescues photoreceptor death in the rd1 mouse. Mol. Vis. 2009, 15, 1764–1773. [Google Scholar]
- Pitkänen, L.; Ruponen, M.; Nieminen, J.; Urtti, A. Vitreous is a barrier in non-viral gene transfer by cationic lipids and polymers. Pharm. Res. 2003, 20, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Meacham, J.M.; Durvasula, K.; Degertekin, F.L.; Fedorov, A.G. Physical Methods for Intracellular Delivery: Practical Aspects from Laboratory Use to Industrial-Scale Processing. J. Lab. Autom. 2014, 19, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, Y.; Satoh, S.; Otsu, M.; Sasaki, E.; Okada, T.; Watanabe, S. In vitro cell subtype-specific transduction of adeno-associated virus in mouse and marmoset retinal explant culture. Biochimie 2012, 94, 2716–2722. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Kageyama, R. Retrovirus-mediated gene transfer to retinal explants. Methods 2002, 28, 387–395. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-Progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiger, S.P.; Bowne, S.J.; Sullivan, L.S. Genes and Mutations Causing Autosomal Dominant Retinitis Pigmentosa. Cold Spring Harb. Perspect. Med. 2014, 5, a017129. [Google Scholar] [CrossRef] [Green Version]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Griciuc, A.; Aron, L.; Roux, M.J.; Klein, R.; Giangrande, A.; Ueffing, M. Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in Drosophila. PLoS Genet. 2010, 6, e1001075. [Google Scholar] [CrossRef] [Green Version]
- Arango-Gonzalez, B.; Sen, M.; Guarascio, R.; Ziaka, K.; del Amo, E.M.; Hau, K.; Poultney, H.; Asfahani, R.; Urtti, A.; Chou, T.-F.; et al. Inhibition of VCP preserves retinal structure and function in autosomal dominant retinal degeneration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bassetto, M.; Sen, M.; Gonzalez, B.A.; Ueffing, M.; Poulhes, F.; Zelphati, O. Synthesis and application of magnetic nanoparticles (MNPs) in siRNA delivery to the retina. Acta Ophthalmol. 2019, 97. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 44–58. ISBN 9780081005637. [Google Scholar]
- Kaur, J.; Mencl, S.; Sahaboglu, A.; Farinelli, P.; van Veen, T.; Zrenner, E.; Ekström, P.; Paquet-Durand, F.; Arango-Gonzalez, B. Calpain and PARP Activation during Photoreceptor Cell Death in P23H and S334ter Rhodopsin Mutant Rats. PLoS ONE 2011, 6, e22181. [Google Scholar] [CrossRef] [Green Version]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef]
- Monai, N.; Yamauchi, K.; Tanabu, R.; Gonome, T.; Ishiguro, S.I.; Nakazawa, M. Characterization of photoreceptor degeneration in the rhodopsin P23H transgenic rat line 2 using optical coherence tomography. PLoS ONE 2018, 13, 0193778. [Google Scholar] [CrossRef] [Green Version]
- Krötz, F.; de Wit, C.; Sohn, H.Y.; Zahler, S.; Gloe, T.; Pohl, U.; Plank, C. Magnetofection--a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol. Ther. 2003, 7, 700–710. [Google Scholar] [CrossRef]
- Plank, C.; Anton, M.; Rudolph, C.; Rosenecker, J.; Krötz, F. Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opin. Biol. Ther. 2003, 3, 745–758. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M.; Nishikawa, S.; Steinberg, R.H.; Naash, M.I.; Duncan, J.L.; Trautmann, N.; Matthes, M.T.; Yasumura, D.; Lau-Villacorta, C.; Chen, J.; et al. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp. Eye Res. 2018, 167, 56–90. [Google Scholar] [CrossRef]
- Meyer, H.; Weihl, C.C. The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 2014, 127, 3877–3883. [Google Scholar] [CrossRef] [Green Version]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosche, J.; Grimm, D.; Clemens, N.; Reichenbach, A. Retinal light damage vs. normal aging of rats: Altered morphology, intermediate filament expression, and nuclear organization of Müller (glial) cells. J. Hirnforsch. 1997, 38, 459–470. [Google Scholar] [PubMed]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller Cell Alterations During Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell. Neurosci. 2015, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Mertsch, K.; Hanisch, U.; Kettenmann, H. Characterization of Microglial Cells and Their Response to Stimulation in an Organotypic Retinal Culture System. J. Comp. Neurol. Comp. Neurol. 2001, 227, 217–227. [Google Scholar] [CrossRef]
- Brigitte, M.; Wagner, F.; Lorenz, B.; Stieger, K. Organotypic Cultures of Adult Mouse Retina: Morphologic Changes and Gene Expression. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1930–1940. [Google Scholar] [CrossRef] [Green Version]
- Sakami, S.; Kolesnikov, A.V.; Kefalov, V.J.; Palczewski, K. P23H opsin knock-in mice reveal a novel step in retinal rod disc morphogenesis. Hum. Mol. Genet. 2014, 23, 1723–1741. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Hüttinger, C.; Hirschberger, J.; Jahnke, A.; Köstlin, R.; Brill, T.; Plank, C.; Küchenhoff, H.; Krieger, S.; Schillinger, U. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: A phase I trial. J. Gene Med. 2008, 10, 655–667. [Google Scholar] [CrossRef]
- Singh, J.; Mohanty, I.; Rattan, S. In vivo magnetofection: A novel approach for targeted topical delivery of nucleic acids for rectoanal motility disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G109–G118. [Google Scholar] [CrossRef]
- Ohashi, K.; Enomoto, T.; Joki, Y.; Shibata, R.; Ogura, Y.; Kataoka, Y.; Shimizu, Y.; Kambara, T.; Uemura, Y.; Yuasa, D.; et al. Neuron-derived neurotrophic factor functions as a novel modulator that enhances endothelial cell function and revascularization processes. J. Biol. Chem. 2014, 289, 14132–14144. [Google Scholar] [CrossRef] [Green Version]
- Titze de Almeida, S.; Horst, C.; Soto-Sánchez, C.; Fernandez, E.; Titze de Almeida, R. Delivery of miRNA-Targeted Oligonucleotides in the Rat Striatum by Magnetofection with Neuromag®. Molecules 2018, 23, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092. [Google Scholar] [CrossRef] [Green Version]
- Prow, T.W.; Imran Bhutto, S.Y.K. Ocular Nanoparticle Toxicity and Transfection of the Retina and Retinal Pigment Epithelium. Nanomedicine 2008, 4, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dengler, M.; Saatchi, K.; Dailey, J.P.; Matsubara, J.; Mikelberg, F.S.; Häfeli, U.O.; Yeung, S.N. Targeted delivery of magnetic cobalt nanoparticles to the eye following systemic administration. AIP Conf. Proc. 2010, 1311, 329–336. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.R.; Jiang, H.; Shen, M.; Cui, L.; Bhattacharya, S.K. Detection of magnetic particles in live DBA/2J mouse eyes using magnetomotive optical coherence tomography. Eye Contact Lens 2010, 36, 346–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanai, A.; Häfeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused Magnetic Stem Cell Targeting to the Retina Using Superparamagnetic Iron Oxide Nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef]
- Turchinovich, A.; Zoidl, G.; Dermietzel, R. Non-viral siRNA delivery into the mouse retina in vivo. BMC Ophthalmol. 2010, 10. [Google Scholar] [CrossRef]
- Andersen, E. Magnetic resonance imaging—Safety and health issues. AAOHN J. 2007, 55, 137–139. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Vedula, A.; Dubovy, S.R.; Goldberg, J.L. Evaluation of magnetic micro- and nanoparticle toxicity to ocular tissues. PLoS ONE 2011, 6, 0017452. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Padgett, K.R.; Rodriguez, J.E.; Goldberg, J.L. Investigation of nanoparticles using magnetic resonance imaging after intravitreal injection. Clin. Exp. Ophthalmol. 2012, 40, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic non-viral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambhatla, L.; Chiu, C.-P.; Glickman, R.D.; Rowe-Rendleman, C. In vitro differentiation capacity of telomerase immortalized human RPE cells. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1622–1630. [Google Scholar]
- Kuznetsova, A.V.; Kurinov, A.M.; Aleksandrova, M.A. Cell Models to Study Regulation of Cell Transformation in Pathologies of Retinal Pigment Epithelium. J. Ophthalmol. 2014, 2014, 801787. [Google Scholar] [CrossRef]
- Caffé, A.R.; Ahuja, P.; Holmqvist, B.; Azadi, S.; Forsell, J.; Holmqvist, I.; Söderpalm, A.K.; Van Veen, T. Mouse retina explants after long-term culture in serum free medium. J. Chem. Neuroanat. 2002, 22, 263–273. [Google Scholar] [CrossRef]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, M.; Bassetto, M.; Poulhes, F.; Zelphati, O.; Ueffing, M.; Arango-Gonzalez, B. Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics 2021, 13, 225. https://doi.org/10.3390/pharmaceutics13020225
Sen M, Bassetto M, Poulhes F, Zelphati O, Ueffing M, Arango-Gonzalez B. Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics. 2021; 13(2):225. https://doi.org/10.3390/pharmaceutics13020225
Chicago/Turabian StyleSen, Merve, Marco Bassetto, Florent Poulhes, Olivier Zelphati, Marius Ueffing, and Blanca Arango-Gonzalez. 2021. "Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants" Pharmaceutics 13, no. 2: 225. https://doi.org/10.3390/pharmaceutics13020225
APA StyleSen, M., Bassetto, M., Poulhes, F., Zelphati, O., Ueffing, M., & Arango-Gonzalez, B. (2021). Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics, 13(2), 225. https://doi.org/10.3390/pharmaceutics13020225






