1. Introduction
Delivery of therapeutic compounds to the sub-cellular compartments where their targets reside is essential towards achieving maximal therapeutic efficacy [
1]. This is especially relevant for a majority of biological therapeutics that are aimed to elicit their action through interactions with molecular targets within cells. Among these, protein therapeutics are being widely developed due to the seminal roles proteins play as modulators of numerous physiological processes in the body [
2,
3]. However, while significant progress has been made towards the development of protein therapeutics for applications requiring interactions on cell surfaces or with secreted molecules (e.g., antibodies), some key challenges to the intracellular delivery of protein therapeutics still remain today [
4,
5].
A major hurdle to the delivery of proteins and other macromolecules inside cells is their large size and hydrophilicity that prevents their diffusion across lipid cell membranes [
3,
5]. While genetic approaches that rely on the expression of therapeutic antibodies within cells (e.g., intrabodies, nanobodies) can potentially overcome this limitation, they are met with challenges of their own such as integration into the genome, carcinogenicity, immunogenicity, etc. [
5,
6]. Alternatively, proteins can be conjugated with affinity molecules that can interact with specific cell receptors on the cell surface, leading to their internalization in endocytic vesicles [
3]. However, once they enter cells, they most often stay trapped in these vesicles, which leads to their lysosomal degradation [
5]. Endo-osmolytic agents have been incorporated in delivery systems to enable endosomal disruption and cargo release, yet the efficiency of such processes remains low and some of them are toxic to cells [
3,
5,
7]. Other approaches use non-endocytic mechanisms to deliver cargo to cells such as through conjugation with cell penetrating peptides (CPPs), also termed protein transduction domains (PTDs); however, these mostly suffer from toxicity, unclear mechanistic understanding, and low efficiency [
3,
5,
7,
8,
9]. Recent developments in the field have led to the design of less toxic and more stable cyclic CPPs, CPP prodrugs that can be programmed to release their cargo at specific tissue sites, as well as fusions with subcellular targeting moieties to achieve organelle targeting or with fusogenic peptides to achieve endosomal escape [
10]. In fact, CPPs are under testing in several clinical trials where the cargo is conjugated to the CPP or generated as a chimeric fusion [
10]. However, sparing some exceptions [
11,
12,
13], such strategies involve covalent attachment of targeting/delivery moieties to the protein cargo or development of chimeras, which can affect the protein folding, sites of interaction with substrates or partner proteins, and ultimately cargo activity, requiring synthesis and optimization on a case-by-case basis [
3,
5]. Additional strategies utilizing click chemistry have gained increasing attention due to their relative tunability based on the addition of modifiable units to the protein structure, which can then be conjugated to targeting polymers [
14]. However, while they can achieve intracellular targeting of cargo, similar concerns apply and these techniques do not offer a platform technology for protein delivery since each particular protein cargo requires their own residue-specific engineering/conjugating scheme. Some polymer-based systems for protein delivery use cationic polymer scaffolds that were traditionally used for gene delivery, with functionalization of groups such as boronate or guanidium on the polymer to increase attachment of protein cargo [
2]. However, their back-bones still raise toxicity concerns.
Utilization of other carriers systems as delivery vehicles may overcome these challenges [
15]. Fusogenic liposomes [
16], inorganic nanoparticles [
17], polymer-based nanoparticles [
18], solid lipid nanoparticles [
19], nucleic acids-based particles [
20,
21], and exosomes [
22] are examples of carrier systems that have been used to deliver a variety of protein therapeutics such as enzymes to lysosomes [
18,
23], transcription factors to the nucleus [
21], ribonucleoprotein complexes [
19], and protein therapeutics to the cytosol [
17,
19,
20]. Yet, these approaches face limitations such as loss of protein activity or stability during formulation, charge or size restrictions for certain cargo types, therapeutic loading limitations, toxicity of the carrier, inability to escape endosomes for several nanocarriers, or rapid clearance from the circulation [
2,
5,
18].
Hence, while a number of strategies are available to achieve intracellular delivery of proteins, the development of a non-covalent approach that can provide sufficient therapeutic load without affecting protein structure or activity, can be adapted to a variety of cargo and cell types, and can avoid cytotoxicity are limited [
11,
12,
13,
24,
25,
26], warranting the development of other delivery systems. Water-soluble polyphosphazenes (PZs) are hybrid organic-inorganic polyelectrolyte-type polymers that are attaining increasing recognition as multifunctional drug delivery vehicles [
27,
28,
29]. Their unique macromolecular structure consists of an alternating phosphorus and nitrogen backbone and organic side groups, which provides for hydrolytic degradability under physiological conditions [
30] and enables self-assembly with macromolecular drugs, including proteins, and some biological targets through non-covalent interactions [
27,
31,
32,
33,
34]. PZ polymers have been formulated mostly as vaccine adjuvants [
35] or in supramolecular assemblies including micelles, nanoparticles, hydrogels etc., hence they are flexible systems with multiple applications accorded by adjusting the side groups for different applications [
36].
We have reported the synthesis of two families of water-soluble PZs, which combine carboxylic acid functionalities with hydrophilic moieties, such as pyrrolidone (PZ-PYR) [
31,
37] and graft poly(ethylene glycol) (PEG) chains (PZ-PEG) [
32,
33]. One of the most important design features of these polymers is their excellent solubility in aqueous solutions at near physiological pH [
31,
32]. PZ polymers are miscible with water in a broad range of ratios and form clear, low viscosity solutions at concentrations ranging from 0.1 to 2 mg/mL, at which they have been used as injectable formulations [
27,
35]. We have shown these hydrolytically degradable carriers facilitate uptake of protein cargo by cells in culture, in particular, avidin used as a model protein [
31,
33]. However, their relative efficiency to deliver protein cargo to cells has not been compared, their behavior has not been explored comparatively in different cells types, e.g., control vs. diseased cells, and the mechanism for their uptake within cells has not been reported. Most importantly, their intracellular trafficking after entering cells remains largely unknown. Due to the presence of carboxylic groups in the polymer that could disrupt endosome membranes due to protonation at acidic pH conditions of the endosome [
38], it is possible that PZs could release endocytosed cargo to the cytosol. Yet, PZs have never been examined in this context.
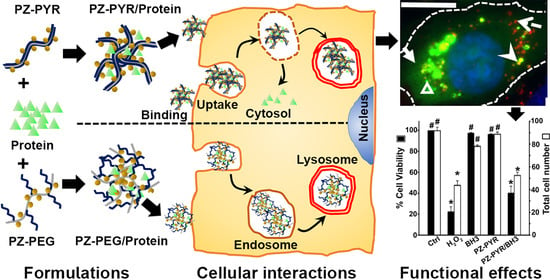
The results reported here demonstrate the differential behavior of each of these PZ polymers depending on the cell type investigated, their versatility regarding the uptake mechanisms they use to enter cells, and their ability to deliver active protein cargo to the cytosol, while being non-toxic.
2. Materials and Methods
Reagents. Cells used in this study were human oral adenosquamous carcinoma Cal27 cells from American Type Culture Collection (Manassas, VA, USA) and Human Umbilical Vein Endothelial Cells (HUVECs) from Lonza Walkersville, Inc (Walkersville, MD, USA). FITC-avidin lyophilized powder from egg white was from Sigma-Aldrich (St. Louis, MO, USA), biotinylated mouse IgG was from BD Biosciences (PharminGen, San Jose, CA, USA), Texas-Red dextran (10 kDa) was from Molecular Probes (Carlsbad, CA, USA), anti-Early Endosome Antigen-1 (EEA-1) antibody was from Sigma-Aldrich (St. Louis, MO, USA), Texas-red conjugated secondary antibodies were from Life Technologies (Carlsbad, CA, USA), anti-F-actin antibody was from Bioss antibodes (Woburn, MA, USA), Bax-BH3 peptide (STKKLSECLKRIGDELDSNM) was from AnaSpec Inc. (Fremont, CA, USA), phosphate buffered saline, pH 7.4 (PBS), and 4′, 6-diamidino-2-phenylindole (DAPI) from Invitrogen (Carlsbad, CA, USA). LIVE/DEAD Mammalian Kit was from molecular Probes (Eugene, OR, USA). Medium M199 was from Invitrogen (Carlsbad, CA, USA) and Dulbecco’s Modified Eagle’s Medium from GibcoBRL (Grand Island, NY, USA).
Cell Culture. HUVECs were cultured in M199 supplemented with 15% fetal bovine serum, 2 mM l-glutamine, 15 µg/mL endothelial cell growth supplement, 100 µg/mL heparin, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cal27 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Both cell types were seeded on gelatin-coated glass coverslips and grown to confluence at 37 °C, 5% CO2, and 95% relative humidity.
Preparation of PZ Polymers and PZ/protein Complexes. The polymers used in this study were (a) PZs containing 70% (mol) carboxylic acid and 30% (mol) pyrrolidone side groups, i.e., poly[(carboxylatoethylphenoxy)(3-(2-oxo-1-pyrrolidinyl)propylamino)phosphazene], herein called PZ-PYR, or (b) PZ containing 84% (mol) carboxylic acid and 16% (mol) graft 5 kDa polyethylene glycol (PEG) side groups, i.e., poly[di(carboxylatoethylphenoxy)phosphazene]-graft-poly(ethylene glycol), herein called PZ-PEG (
Figure 1A). They were synthesized via macromolecular substitution route as described previously [
28,
29,
34].
PZ-PYR or PZ-PEG solutions were then vortexed for 2 min and mixed at 0.6 mg/mL polymer and 0.3 mg/mL protein cargos, including FITC-labeled avidin as a model protein, anti F-actin antibody, or Bax-BH3 peptide as active cargos. The complexes were vortexed for 2 min, complete cell medium was added to reach a concentration of 0.2 mg/mL polymer and 0.1 mg/mL protein, then suspensions were vortexed again for 2 min and used for studies.
Characterization of PZ/protein Complexes. Asymmetric Flow Field Flow Fractionation (AF4) characterization was conducted using Postnova AF2000 MT series instrument (Postnova Analytics, Landsberg, Germany) equipped with UV-Vis detector (SPD-20A/20AV, Shimadzu Scientific Instruments, Columbia, MD, USA) and regenerated cellulose membrane (10 kDa molecular weight cutoff, Postnova Analytics, Landsberg, Germany). 25 mM phosphate buffer, pH 7.4 was employed as an eluent. The collected data was processed using AF2000 software (Postnova Analytics, Landsberg, Germany). This technique allows separation of analytes by their size through applying perpendicular flow of mobile phase against the semi-permeable membrane in the analytical cartridge [
39]. Although somewhat similar to size exclusion chromatography, AF4 allows characterization of analytes of up to microns in size and minimizes non-specific interactions with a stationary state [
39].
Dynamic Light Scattering. DLS analysis was conducted using Malvern Zetasizer Nano series, ZEN3600 instrument and Malvern Zetasizer 7.10 software (Malvern Instruments Ltd., Worcestershire, UK). Samples were prepared in 25 mM phosphate buffer, pH 7.4.
Binding. Cells grown on coverslips in 24-well plates were incubated with control FITC-avidin, PZ-PYR/FITC-avidin or PZ-PEG/FITC-avidin (0.1 mg/mL FITC-avidin) at 37 °C in complete medium for 0.5, 2 or 5 h. Cells were then washed to remove unbound materials, fixed with 2% paraformaldehyde, nuclei stained with DAPI, and samples mounted on slides with Mowiol and imaged using an Olympus IX81 microscope (Olympus, Inc., Center Valley, PA, USA), 60× oil immersion objective (UPlanApo, Olympus, Inc., Center Valley, PA, USA), ORCA-ER camera (Hamamatsu Corporation, Bridgewater, NJ), and SlideBook™ 4.2 software (Intelligent Imaging Innovations, Denver, CO, USA). Fluorescence images were taken under the green and blue channels to monitor FITC and DAPI respectively, and bright field images provided visualization of cells and cell-cell borders. The FITC-avidin cargo associated with cells was quantified as mean and sum intensity using respective grayscale images and corrected for background intensity, using Image-Pro® v6.3 (Media Cybernetics, Bethesda, MD, USA).
Uptake. Cells were incubated with control FITC-avidin, PZ-PYR/FITC-avidin or PZ-PEG/FITC-avidin (green; 0.1 mg/mL FITC-avidin) for the indicated times. To elucidate the uptake mechanism, experiments were conducted at 4 °C vs. 37 °C, in control medium or in the presence of endocytosis inhibitors, including 1 μg/mL filipin to inhibit caveoli, 50 μM monodansylcadaverine (MDC) to inhibit clathrin-dependent endocytosis, or 0.5 μM wortmannin to inhibit macropinocytosis. Additionally, experiments were conducted in the presence of 0.1 mg/mL non-fluorescent avidin or 0.2 mg/mL PZ polymer to block potential binding sites on cells. Both inhibitors and blockers were applied to cells 30 min prior to polymer/protein complexes and then kept during the polymer/protein incubation. Cells were then washed to remove unbound materials, fixed with 2% paraformaldehyde and incubated with mouse IgG conjugated to both biotin and Texas Red. This provided co-staining of green avidin located at the cell surface in red (green + red = yellow), while internalized avidin would not be accessible to this IgG and appeared green. Cell nuclei were stained in blue with DAPI. Samples were mounted onto slides and imaged by fluorescence microscopy as described above. The total number of vesicles per cell, internalized vesicles, and percentage of internalization over total vesicles counted were quantified using a custom macro in Image Pro
® (Media Cybernetics, Bethesda, MD, USA) described elsewhere [
40].
Subcellular Localization. HUVECs were incubated with PZ-PYR/FITC-avidin or PZ-PEG/FITC-avidin (0.2 mg/mL polymer, 0.1 mg/mL protein) for 1 h followed by fixation (1 h trafficking) or then washed to remove non-bound materials and incubated for additional 4 h in complex-free medium to follow the trafficking of pre-bound materials (1 h pulse + 4 h chase = 5 h trafficking). Cells were then fixed, washed, permeabilized, and incubated with anti-Early Endosome Antigen-1 (EEA-1) antibody for endosome colocalization, followed by Texas red-secondary antibody and DAPI. To stain lysosomes, HUVECs were first incubated with 1 mg/mL 10 kDa Texas-red dextran for 45 min, followed by incubation for another 45 min in dye-free medium, then incubation with polymer/protein complexes as described for endosomes, fixation, and fluorescence microscopy imaging. By this technique, the green FITC-avidin colocalizing with red endosomes or lysosomes would appear yellow, which was quantified using a custom macro in Image-Pro 6.3, as described [
41,
42].
Functional Protein Delivery. First, cytosolic delivery of a cell-impermeable antibody was studied. For this, HUVECs were left untreated or incubated at 37 °C with 0.1 mg/mL anti-F-actin alone or complexed with PZ-PYR (0.2 mg/mL polymer) for 1 h pulse, followed by 4 h chase as described above. Then, cells were washed, fixed, permeabilized, and incubated with Texas red-secondary antibody to visualize anti-F-actin. As a positive control, cells were fixed and permeabilized prior to incubation with anti-F-actin, followed by Texas red-secondary antibody. Fluorescence images were taken under the red channel at 60× magnification to image whether anti-F-actin would have bound and thus would have revealed filamentous actin in the cytosol. To determine the mechanism, similar experiments were conducted where HUVECs were pre-treated for 30 min with 300 μM chloroquine (to alkalinize endo-lysosomes) or 0.1 μM latrunculin (to inhibit polymerization of actin into filaments), then incubated with PZ-PYR/anti-F-actin in the presence of these agents.
Second, intracellular delivery of a pro-apoptotic Bax-BH3 peptide was investigated. HUVECs were left untreated or incubated at 37 °C for 1 h pulse with 0.2 mg/mL PZ-PYR alone, 0.1 mg/mL Bax-BH3 peptide alone, PZ-PYR/BH3 peptide (0.2 mg/mL polymer, 0.1 mg/mL protein), or 5 mM hydrogen peroxide (H2O2) known to damage cells, followed by 4 h chase as described above. Cells were then washed, incubated with LIVE/DEAD assay components containing 0.1 μM calcein AM and 1 μM ethidium homodimer for 30 min. Live fluorescence images were taken under the green and red channels at 20× magnification to monitor live (calcein positive; green) and dead (ethidium positive; red) cells, respectively. They were quantified using Image-Pro 6.3 to calculate the percentage of live (viable) cells from the total cells counted.
Polymer Cytotoxicity. HUVECs were left untreated or incubated for 1 h at 37 °C with 0.2 mg/mL PZ-PYR or 5 mM H2O2 control, followed by a 4 h chase as described above. Cells were then washed, incubated with LIVE/DEAD assay components, and the percentage of live (viable) cells and the number of cells were determined as indicated above.
Furthermore, the integrity of the cell membrane was assessed using the Pierce LDH Cytotoxicity Assay Kit (Thermo Scientific, Asheville, MA, USA). HUVECs grown on 96-well plates were left untreated or incubated for 1 h at 37 °C with 0.2 mg/mL PZ-PYR or 0.1% Triton (known to permeabilize cell membranes), followed by 4 h chase as above. Then, the release of intracellular LDH to the cell medium was determined after separation by centrifugation and by measuring LDH catalytic activity for 30 min per manufacturer’s protocol. Absorbance at 490 nm was measured using SpectraMax M2 Plate Reader and analyzed with SoftMax® Pro Software (both from Molecular Devices, San Jose, CA, USA).
Finally, apoptosis was examined using the Caspase 3/7 Glo assay (Promega, Madison, WI, USA). For this, HUVECs grown on 96-well plates were left untreated or incubated for 1 h with 0.2 mg/mL PZ-PYR or 1 μM staurosporine (known to induce apoptosis). Then, caspase 3/7 revealing reagents were added to cells and measured by luminescence using SpectraMax M2 Plate Reader (Molecular Devices, San Jose, CA, USA) following the manufacturer’s protocol.
Statistics. For microscopy, two independent experiments, each one with two independent replicates (total of n = 4 wells/condition) were analyzed cell-by-cell, for a total of n ≥ 100 cells per condition, randomly selected throughout the whole slide area. For cytotoxicity tests, two independent experiments with 4 replicates/each were conducted. Data were calculated as mean ± standard error of the mean (SEM). Statistical significance for two-way comparisons was determined using Student’s t-test, p < 0.05.
4. Discussion
Delivery of protein therapeutics to intracellular sites remains a major challenge. Strategies that take advantage of nanomaterials offer an attractive alternative towards this goal, but many of these systems suffer from toxicity, restrictions of cargo size and type, or involve modification of the protein cargo resulting in detrimental structural and/or activity changes; thus, a platform-technology that can be broadly applicable to different cargos remains elusive [
3,
5,
7,
17]. As a result, only a handful of protein therapeutics whose targets are intracellular have been found to be effective [
24,
25]. Most clinical trials being conducted relate to custom-designed, covalently-conjugated, protein-cargo systems based on CPPs, which have been researched for over 30 years and represent the most commonly studied intracellular delivery system [
10]. Focusing on this challenge, in this study we have investigated PZ polymers since they are biodegradable [
31,
32], can bind a variety of proteins through non-covalent interactions [
29,
34], and can be further functionalized to tune parameters such as extending in vivo half-life using PEG [
32].
As demonstrated here, both PZ-PYR and PZ-PEG facilitated interaction of protein cargo, i.e., FITC-avidin used as a model protein, with cells (
Figure 2). This was expected based on the characteristics of these polymers. Since PZ polymers interact with proteins and other molecules (e.g., lipids, polysaccharides, etc.) via non-covalent electrostatic and hydrogen bonding [
27,
28,
34], it is expected that these polymers would interact with such elements on the cell surface, facilitating protein delivery. Interestingly, in studies to determine the mechanism of interaction with cells, it was observed that both avidin and polymer pre-incubated with cells and present during the treatment with polymer/protein complexes decreased uptake of the complex by cells, with avidin exerting a more acute influence (
Figure 4A). This can be explained based on the loading capacity of avidin in the polymer and the fact that this interaction must reach a dynamic equilibrium. When non-fluorescent avidin was used as a competitor, it possibly displaced or exchanged with FITC-avidin from the complex. Since polymer/non-fluorescent avidin would not be visible by fluorescence microscopy and freed FITC-avidin would not efficiently interact with cells, this would explain the observed result. When PZ-PYR was used as a competitor, FITC-avidin from the PZ-PYR/FITC-avidin complex may also exchange into the competitor polymer, so that the same total amount of FITC-avidin is loaded in a greater amount of polymer and this “loading dilution” effect would not affect cell interaction but lower the amount of cargo that was delivered. Alternatively or simultaneously, protein-free PZ-PYR may be interacting with the cell surface similarly as PZ-PYR/FITC-avidin does, blocking and/or competing off binding of PZ-PYR/FITC-avidin.
Importantly, data revealed that these polymer/protein complexes facilitated the rapid interaction and internalization of FITC-avidin in cells, although the efficiency of these processes depended on the particular polymer and cell type examined (
Figure 2 and
Figure 3). In general, PZ-PEG was slower and reached lower levels than PZ-PYR for these processes. This finding is not surprising since PEG chains on carriers are known to interfere with cell surface interactions, possibly owing to the formation of a hydration layer and steric hindrances [
46]. However, interestingly, slower and lower binding/uptake of PZ-PEG/FITC-avidin was much more noticeable in control endothelial cells than adenocarcinoma cells. This result can be speculatively explained by the mechanism followed by PZ polymers to enter cells (
Figure 4B,C). This was governed by an active process (
Figure 4B), but no specific pathway was found to be involved, since none of the endocytosis inhibitors used hindered uptake by cells. This means these complexes do not actively induce endocytosis, but rather piggyback into the cell by any endocytic route the cell is using at the moment. Since PZ polymers could interact with elements of the cell membrane through the non-covalent interactions described above, then as a cell performs endocytosis to uptake molecules from the cell medium or recycle its membrane components, PZ/protein complexes would simply enter the cell attached to the membrane at sites where endocytic vesicles are being formed. As such, blocking clathrin, caveolar, or macropinocytic pathways individually would not necessarily block polymer/protein uptake since they could piggyback along the pathways that are left uninhibited, in a compensatory-like manner. This is in contrast to polymers that bear functional groups such as guanidium and boronate on cationic backbones to act as protein cargo “glue” and increase stability of the complexes [
2]. For instance, guanidyl-modified polyethyleneimine polymers have also been shown to be effective for intracellular protein delivery through interaction with membrane phospholipids, but do not utilize compensatory mechanisms in case of pathway inhibition [
24]. Coming back to the question on why PZ-PEG/FITC-avidin was slower and reached lower levels in endothelial cells compared to adenocarcinoma cells, this could be explained if the former cell type had a lower endocytic activity compared to the latter cell type. In fact, enhanced endocytosis is a feature recognized in many cancer types [
47]. This phenomenon applies to all drug delivery systems, including CPPs [
12], because different cell types exhibit different sizes/surface areas and different shapes/morphology, as well as a different endocytic activity and/or use different endocytic pathways at different extents depending on their biological function, the signals they receive from the environment, their metabolic rate, and/or their pathophysiological states [
47,
48]. This is true even for drug delivery systems targeted to specific cell-surface receptors, e.g., the same ICAM-1 targeted polymer nanoparticles have been shown to traffic mainly to lysosomes in neuronal-like cells [
49], fibroblasts [
50], or endothelial cells grown without a basolateral free-surface [
50], while they mainly traffic across the cell body via transcytosis in endothelial cells or epithelial cells grown with a basolateral free-surface [
51,
52]. Similarly, endocytic pathways can be altered, either decreased or increased, depending on the pathophysiological state of cells [
48], e.g., uptake via clathrin or caveolar pathways, but not the CAM pathway, was decreased in fibroblasts affected by various genetic diseases called lysosomal storage disorders [
41]. Therefore, based on this differential cellular interaction/internalization efficacy of PZ-PYR and PZ-PEG, each of these polymers could offer different advantages depending on the cell target intended for a particular therapeutic application.
Following entry into cells by endocytosis, protein delivery systems are often retained in the endo-lysosomal compartment and cannot escape into the cytosol to traffic to or reach other intracellular targets [
2,
5,
8,
24,
45]. With regard to the subsequent endo-lysosomal trafficking of internalized PZ complexes, we again saw a difference in the kinetics of the process between the two polymers, with the PZ-PYR/FITC-avidin trafficking rapidly to the lysosomal compartment, as evidenced by a low colocalization with endosomes at 1 h or 5 h, while their localization with lysosomes was slightly higher compared to endosomes and sustained over this time (
Figure 5A,B). PZ-PEG/FITC-avidin was more significantly found in endosomes by 1 h, then it accumulated in the lysosomes over time. This would be expected from the interaction/uptake kinetics observed for these two polymers. However, interestingly, when observing the internalized fraction of PZ-PEG/FITC-avidin (
Figure 3A,C), it seems that all of the internalized cargo was retained in the endo-lysosomal compartment (
Figure 5C) at 1 h and 5 h. For PZ-PYR/FITC-avidin, although there was some colocalization with the endo-lysosomal compartment, this was a much smaller fraction compared to what was internalized, e.g. only ~20% of the internalized cargo was present in the endo-lysosomal compartment (
Figure 3C vs.
Figure 5C), indicating that the remaining fraction escaped this compartment. A closer look at the internalization kinetics revealed that there is ~30% loss of the internalized PZ-PYR/FITC-avidin from 2 to 5 h (opposed to ~96% loss of PZ-PEG/FITC-avidin), indicating that the remaining 50% possibly escaped into the cytosol. The fact that significant reduction of PZ-PEG/FITC-avidin is found over time (
Figure 3C) suggests there is time-dependent degradation of this complex, possibly as a consequence of its endo-lysosomal retention. This differential ability of PZ-PYR compared to PZ-PEG to enable endo-lysosomal escape may be speculatively explained considering PEG may be shielding PZ functional groups, preventing the interaction of the polymer with endo-lysosomal membranes, just as observed for interaction with the cell membrane (
Figure 2 and
Figure 3). The increased punctate-like distribution observed for PZ-PEG/FITC-avidin vs. the more diffuse distribution of PZ-PYR/FITC-avidin (pictures in
Figure 2A,B) supports their respective more vesicular vs. more cytoplasmic distribution, respectively.
Consequently, PZ-PYR was examined for its ability to facilitate cytoplasmic delivery of active protein cargos into cells (
Figure 6). Data on PZ-PYR-based delivery of a large protein, anti-F-actin, demonstrated release of this antibody into the cytosol and binding to filamentous actin (
Figure 6A). Expectedly, said delivery was not as prominent as when the antibody was applied on permeabilized cells, as this represents a scenario where cell membranes do not constitute a barrier for antibody penetration. In fact, this suggests that PZ-PYR did not permeabilize the cell membrane, in agreement with its capacity for cytosolic delivery from endo-lysosomal compartments after internalization. This was verified by the fact that chloroquine, a mild base that prevents endo-lysosomal acidification, hindered cytosolic delivery of anti-F-actin antibody (
Figure 6A). Further, data on the Bax-BH3 peptide showed ≈60% reduction in cell viability which would only be possible if the peptide escaped from the endo-lysosomal compartment into the cytosol and then bound its molecular target on the cytosol-facing outer membrane of mitochondria (
Figure 6B). Such functional experiments are more reliable and preferable compared to only tracing a labeled cargo using flow cytometry or microscopy [
53]. The effect observed was similar to other studies where the peptide was microinjected into cells [
43] or introduced by conjugation with cell penetrating peptides [
54]. However, most such membrane permeabilizing methods are limiting due to toxicity, while PZ-PYR used here did not alter the cell membrane or viability (
Figure 7), as demonstrated by the fact that ethidium homodimer used in the Live/Dead assay did not penetrate into cells, cells maintained their metabolic activity since calcein AM was cleaved demonstrating intracellular esterase activity (
Figure 7A), LDH was not released from cells, and the polymer did not induce apoptosis as observed by a lack of induction of effector caspase 3 and 7 (
Figure 7B,C).
Hence, PZ/protein complexes represent a platform-based delivery system that is non-toxic, can form complexes with different types of proteins, ranging in molecular weights from 2 kDa to 150 kDa, carry them into cells and deliver them to the endo-lysosomal route (PZ-PEG) or the cytosol (PZ-PYR;
Figure 5), maintaining their activity (
Figure 6). Other delivery systems that have been investigated for their ability to non-covalently assemble into supramolecular complexes broadly applicable across different protein cargo types are cationic polymers functionalized with guanidium [
24], polymeric protein transduction domain mimics (PTDMs) [
25], fluoroamphiphilic polymers [
26], Pep-1 [
13], CPP adaptors [
11], which have been found to be efficient for in vitro cytosolic delivery using reporter molecules. Of particular note is that the polymer:cargo ratio for this PZ delivery system ranged from 2:1 to 2.5:1, significantly higher than CPPs that use a 10:1 CPP:cargo ratio [
12], and most other systems designed to carry proteins, are not aimed for intracellular delivery [
4,
27,
55]. Therefore, these PZ polymers will add to a very narrow repertoire of polymers that can spontaneously self-assemble with a number of different types of cargo such as peptides, proteins and antibodies, using a simple mixing protocol, and deliver them to different cell types. The in vivo pharmacokinetics of these polymers, the use of targeting moieties to enhance their tissue specificity, and the incorporation of sub-cellular targeting ligands will be explored in future studies.